Introduction
Clinical trials are a vital component of medical research, driving advancements in treatments and therapies. However, they are not without challenges. From the complex web of costs to patient recruitment and retention, clinical trial companies must navigate a multifaceted landscape to ensure success.
In this article, we will explore the various factors that impact the costs of clinical trials, such as patient logistics, investigator fees, study design, and regulatory compliance. We will also delve into the implications of these cost factors on trial outcomes and the broader healthcare landscape. Join us as we uncover the intricacies of clinical trial budgeting and its significance in advancing medical knowledge and improving patient outcomes.
Background and Objectives
The landscape of clinical trials is rapidly evolving, underscored by the sophisticated interplay of technology, economics, and emerging medical needs. Recent case studies, like that of Spinal Cord Stimulation (SCS) to treat chronic pain, highlight the economic implications and the transformative potential of such trials. Chronic pain, affecting over 100 million Americans, has prompted a shift from medication-dependence to diverse therapeutic strategies, including SCS.
This modality not only demonstrated efficacy in managing pain but also showed a reduction in opioid usage and costs post-therapy, thus reshaping both clinical outcomes and economic benchmarks.
Advancements in predictive analytics, like the HINT and SPOT algorithms from Jimeng Sun's lab, are revolutionizing trial design by forecasting success probabilities and optimizing trial parameters, thereby potentially reducing costs and increasing trial efficacy. In parallel, the financial landscape of pharmaceuticals presents challenges, as evidenced by the median cost of new drug launches increasing by 35% in 2023 compared to the previous year, according to Reuters. This underscores the need for clinical trial companies to meticulously plan and allocate resources.
Clinical trials not only evaluate new treatments but also provide critical insights into diseases and patient experiences. The journey of a patient from rural Pennsylvania to an international clinical trial in Turkey encapsulates the complexities involved in such life-saving research endeavors. This scenario raises questions about logistics, language barriers, and the broader impact of clinical trials on participants' lives.
Such considerations are fundamental to the planning and execution of clinical trials, which must factor in the participant's needs and the overarching goal of improving health outcomes.
Reflecting on the multitude of decisions preceding a trial's outcome, experts emphasize the importance of strategic planning. As articulated by industry professionals, optimizing each decision along the clinical trial process can have a ripple effect, enhancing the overall quality and success of the trial. The criticality of these decisions, informed by extensive data analysis and experience, cannot be understated as companies navigate the intricate web of clinical research.
In summary, clinical trial companies must contend with a complex array of factors, from economic considerations to technological innovations and patient-centric logistics. The success of these enterprises hinges on their ability to adapt, innovate, and meticulously plan every facet of the trial, ensuring that the path to new medical advances is both scientifically sound and economically viable.
Target Audience and Their Needs
Clinical trial companies, along with pharmaceutical firms, researchers, and other key players in the healthcare sector, must navigate complex challenges as they drive the progress of clinical trials. A pivotal aspect of this journey is comprehending the intricate web of costs involved in clinical trials to enable strategic decision-making and resource optimization. For instance, the compelling scenario of a patient from rural Pennsylvania facing the prospect of joining a clinical trial in Turkey illustrates the logistical hurdles and costs of cross-border participation.
Such cases underscore the necessity for trial organizers to address questions on visa acquisition, language barriers, and travel coordination, all of which can significantly impact the overall budget and feasibility of clinical studies.
Moreover, the importance of clinical trials in validating the safety and efficacy of new treatments cannot be overstated—they are the cornerstone of advancing medical breakthroughs. Yet, the clinical trial landscape is fraught with obstacles, as evidenced by statistics revealing that nearly 80% of studies do not conclude on schedule, often delayed by six months or more. The struggle to retain enough patients is a major contributing factor, with 85% of studies falling short of enrollment targets, profoundly affecting the financial viability of the trials.
Insights from industry experts, like a biomedical engineer with over a decade of experience in managing clinical studies for medical devices, highlight the critical need for meticulous planning and execution. Quotes from these professionals reveal that many of the decisions made early on in the trial design could benefit from more rigorous scrutiny and optimization. This forward-thinking approach can lead to more robust trial outcomes and mitigate the risk of costly delays or missteps.
The FDA's ongoing efforts to streamline clinical research, harmonize regulations, and safeguard participant rights further emphasize the dynamic nature of clinical trials. These initiatives aim to enhance study design, ensure the reliability of data, and facilitate the development of medical products. As the landscape evolves, clinical trial companies must remain adaptive and well-informed to successfully navigate the complexities of clinical research and contribute to the transformative impact on healthcare and patient lives.
Key Cost Factors in Clinical Trials
Clinical trial companies are pivotal in advancing medical treatments and therapies. They must navigate a complex array of costs that are essential for the successful execution of clinical trials. Patient recruitment and retention, for instance, is not just a matter of finding the right participants; it also encompasses the logistics and ethical considerations of enrolling individuals from diverse geographical locations, possibly as far-reaching as a patient in rural Pennsylvania considering a trial in Turkey.
This includes managing cross-border travel and the nuances of international regulations, which add to the overall cost and complexity.
Investigator fees are another significant expense, rewarding the expertise and time of investigators and study site staff. The development of a study protocol, which requires meticulous planning and input from various stakeholders, including physicians trained in novel therapies such as Spinal Cord Stimulation (SCS), is another cost-intensive area. SCS, for example, has not only proven effective in managing chronic pain but has also shown to reduce the long-term costs associated with pain management by decreasing or eliminating the need for opioids.
Data collection and management incur costs through the need for specialized tools and resources to handle the vast amounts of data generated, especially with the increasing use of connected devices and wearables in trials. The use of advanced algorithms, like the HINT and SPOT systems developed by Jimeng Sun's lab, can significantly affect trial outcomes by predicting success rates and optimizing trial designs, which can ultimately be a cost-saving measure.
Regulatory compliance, including adherence to ethical standards and obtaining necessary approvals, represents a substantial portion of a trial's budget. With less than half of new products offering significant therapeutic advancements according to HAS and GBA, the push for added value in drug development is stronger than ever, underscoring the importance of cost-effective trial designs.
In summary, while the costs associated with conducting clinical trials are considerable, the strategic use of technology, data, and innovative study designs can not only streamline processes but also potentially reduce costs in the long run, contributing to the overarching goal of improving patient outcomes and advancing medical knowledge.
Case Study Overview
As we delve into the financial aspects of a clinical study led by a prominent clinical trial company, it's crucial to understand the various cost factors that play a pivotal role in the execution and success of such trials. The trial in question, focused on testing the effectiveness of a new medication for a particular health condition, serves as a case-in-point for assessing the economic stakes involved. Clinical trial companies, such as CMIC Group, a trailblazer with over three decades of experience in the Contract Research Organization (CRO) sector in Japan, offer comprehensive services across the pharmaceutical value chain.
These services include contract development, manufacturing, healthcare solutions, and more, all tailored to meet the specific needs of different stages in drug development. The case of CMIC illustrates the complexity and breadth of services that such organizations provide to ensure advancements in medical research and patient care.
Recent findings from a study conducted in Canada, which examined around 6,720 clinical trials between 2009 and 2019, highlight the criticality of trial registration and reporting. It was found that only 59% of these trials were registered before participant enrollment, and a significant 32% failed to report results or publish findings. This lack of transparency underscores the challenges faced by clinical trial companies in maintaining rigorous standards of reporting and compliance.
Such issues not only have implications for the credibility of the trials but also for patient outcomes and the advancement of medical science.
Reflecting on the experiences of patients, one must consider the logistical hurdles faced by individuals involved in international clinical trials. For instance, a patient from rural Pennsylvania with an ultra-rare disease, presented with the chance to partake in a clinical trial in Turkey, confronts daunting challenges related to travel, visa procurement, and language barriers. These personal stories and statistics illustrate the multifaceted nature of clinical trials and the imperative for companies to address both the scientific and human elements of their work.
As companies like COMIC Group demonstrate, the integration of a broad array of services can provide robust support for pharmaceutical advancements, ultimately contributing to positive changes in healthcare and people's lives.
Detailed Breakdown of Costs
Clinical trial companies are tasked with the multifaceted challenge of conducting and managing clinical trials which are the heartbeat of medical advancements. One fundamental aspect is patient recruitment and retention, which is notably resource-intensive. XYZ Clinical Research, for instance, has channeled significant investment into patient recruitment through diverse strategies such as advertising, community outreach, and forging partnerships with healthcare providers.
The commitment of resources extends to the meticulous screening and retention of eligible participants, which inevitably consumes a major slice of the trial budget.
Engaging experienced investigators is another pivot around which clinical trials revolve. These professionals are entrusted with the pivotal role of supervising the trial process. XYZ Clinical Research, acknowledging their expertise and the criticality of their role, ensures appropriate compensation for their contributions.
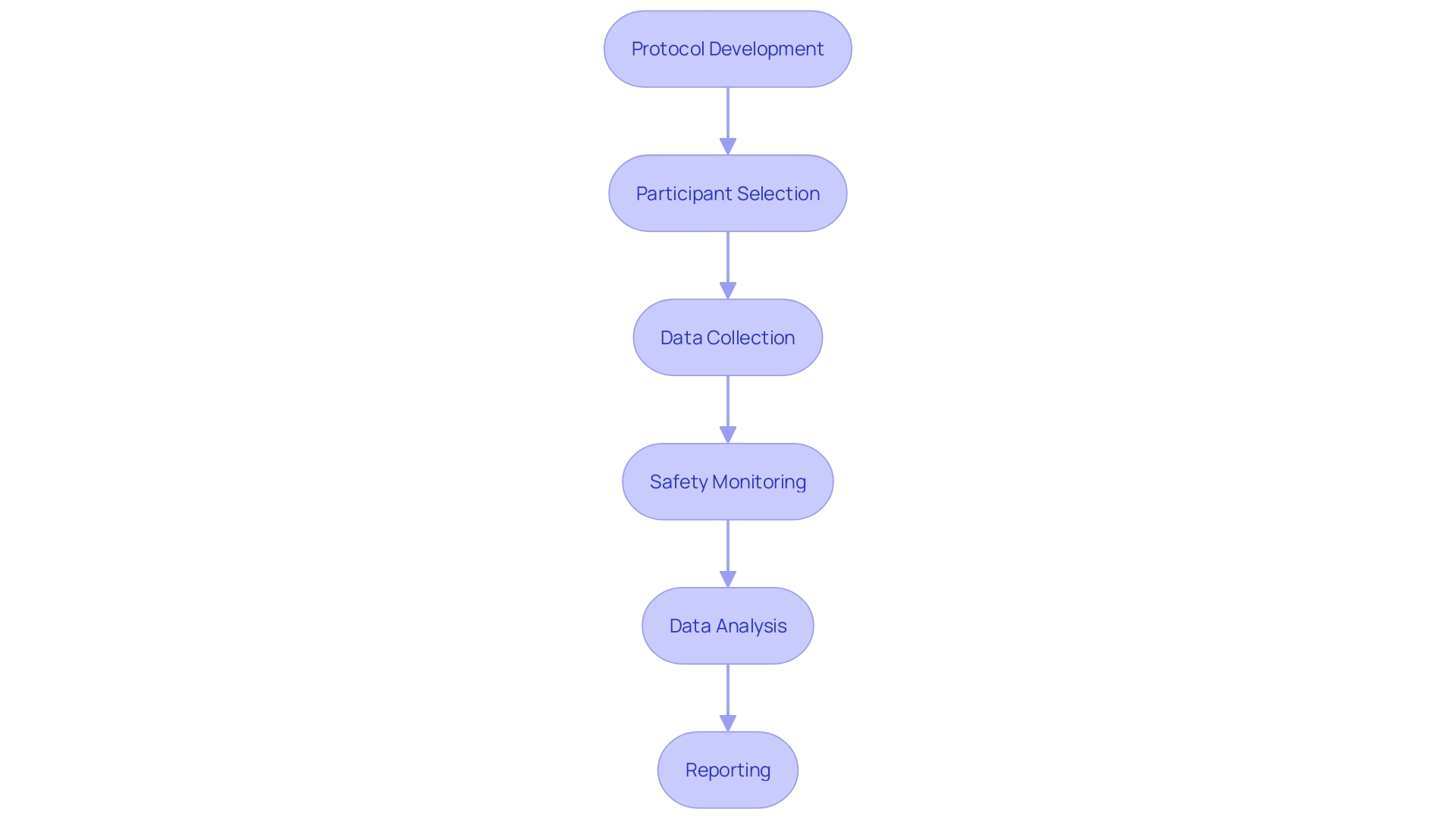
The blueprint of a clinical trial lies in its study design and protocol development—a task that demands the collective expertise of medical professionals, statisticians, and regulatory specialists. XYZ Clinical Research's multidisciplinary approach to developing these protocols underscores the complexity and substantial financial investment involved in crafting a robust framework for the trial.
Data stands as the cornerstone of clinical trials, necessitating sophisticated electronic data capture systems and skilled personnel for its collection and management. The expenses here are not just in the acquisition of the software but also encompass training and salaries for the personnel responsible for maintaining the integrity and confidentiality of trial data.
Lastly, regulatory compliance cannot be overstated. XYZ Clinical Research dedicates a portion of its resources to navigating the labyrinth of regulatory requirements, obtaining the necessary approvals, and facilitating regulatory submissions, inspections, and audits—all crucial steps that ensure the trial's adherence to the highest standards of safety and ethics.
Impact of Cost Factors on Trial Outcomes
XYZ Clinical Research's clinical trial was a testament to how strategic investments in various facets of a trial can optimize outcomes. A concerted effort in patient recruitment and retention bolstered the statistical power of the trial by achieving a robust sample size. Seasoned investigators were pivotal in executing the trial to the highest standards, ensuring protocol adherence and quality outcomes.
A multi-skilled team's adeptness in study design and protocol development underpinned the trial's scientific integrity. Moreover, meticulous data management practices paved the way for precise data analysis and interpretation. Upholding regulatory compliance was not only about meeting standards but also about preserving the trial's integrity and bolstering its credibility in the scientific community.
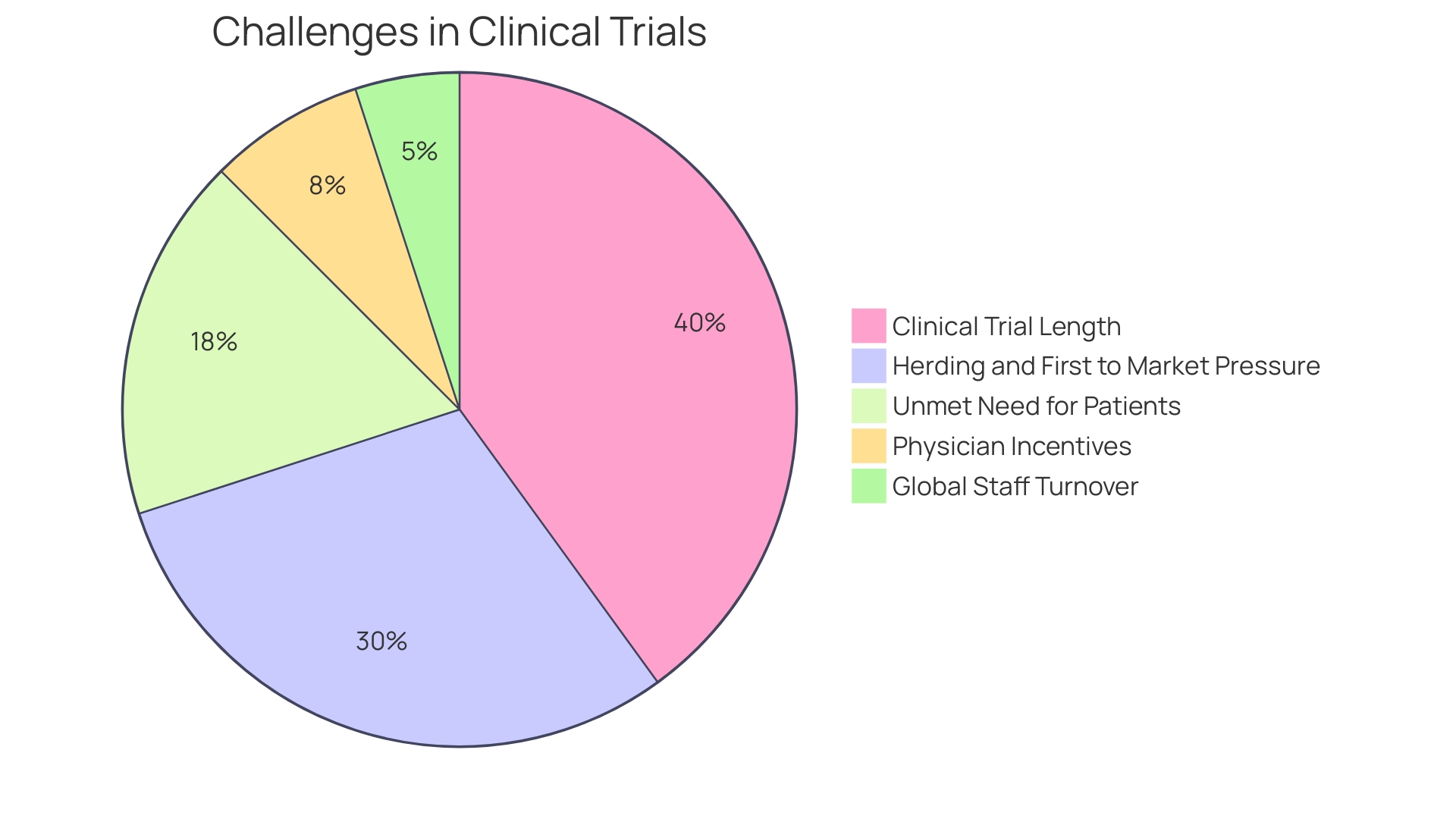
Recent trends in the industry underscore the significance of such strategic investments. McKinsey analysis reveals a worrying trend of clinical trials not finishing on time, with up to 80 percent facing delays. This is particularly concerning given the increasing pressure on biopharma companies to expedite clinical development and be first to market.
The passage of legislation like the US Inflation Reduction Act further amplifies the stakes by influencing drug pricing and market dynamics. With more than 6,000 rare diseases lacking approved therapies and several cancers having low five-year survival rates, the urgency for efficient and successful clinical trials is palpable.
In light of these challenges, innovative approaches such as digital therapeutics are emerging, offering potential cost savings and improved management of chronic diseases and neurological conditions. However, as this field is still in its infancy regarding clinical trial testing, its full potential remains to be harnessed.
The landscape of clinical trials is evolving, and as it does, so must the strategies of clinical research organizations. Adapting to the shifting regulatory, economic, and technological paradigms will be crucial for the advancement of medical knowledge and patient outcomes.
SEO Considerations and Keywords
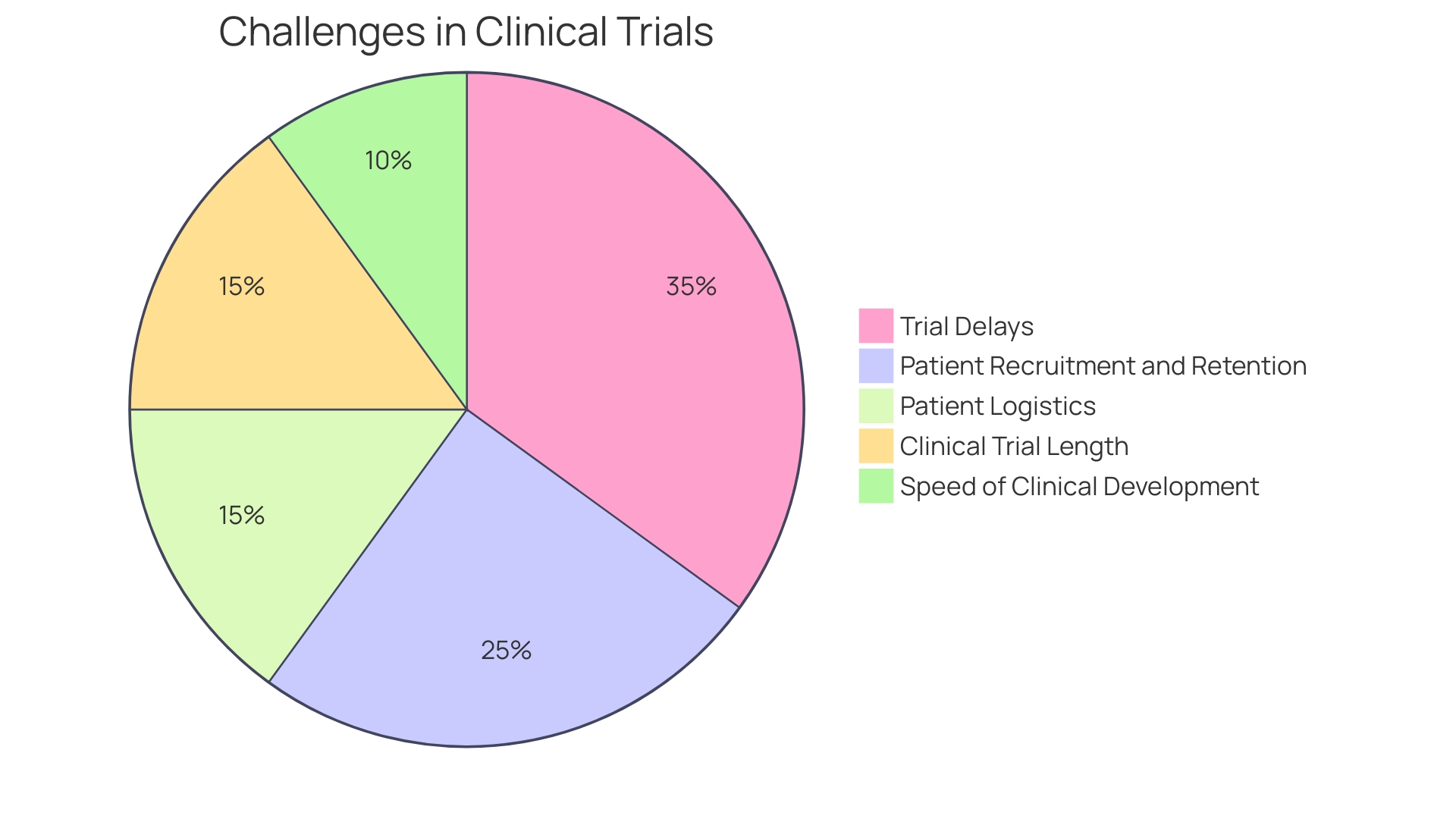
In the realm of medical research, clinical trial companies emerge as pivotal entities, orchestrating the complex symphony of drug development. However, the process is fraught with intricate challenges, most notably in patient recruitment and retention. Nearly 80% of clinical trials do not conclude on time, with a staggering 85% failing to maintain adequate patient numbers.
This not only delays potential breakthroughs but also incurs significant financial burdens on sponsors and clinical research organizations (CROs). The intricacies of patient participation are exemplified by a Pennsylvania patient needing to navigate international travel for a trial in Turkey, highlighting the logistical hurdles that can deter trial involvement.
Moreover, the clinical trial landscape is witnessing an extension in the duration of studies, with Phase 3 trials now averaging 44 months, up from 41 months in the earlier part of the decade, and Phase 2 trials extending similarly. This trend underscores the urgency for biopharma companies to expedite clinical development amidst the competitive race to be first to market, especially in a climate where indications are heavily influenced by regulatory changes, such as those from the US Inflation Reduction Act.
To connect with a broader audience and enhance the visibility of clinical trials, a targeted keyword strategy is indispensable. Keywords must be meticulously chosen for their volume, relevance, and difficulty, considering terms like 'clinical trial cost factors' and 'patient recruitment in clinical trials'. These keywords act as digital signposts, guiding interested parties through the virtual expanse to the pivotal information on clinical trial budgeting, regulatory compliance, and data management.
The selection of such keywords must resonate with the local context and user intent, ensuring that the information reaches those in search of answers, whether they are researchers, sponsors, or potential participants seeking to navigate the healthcare landscape.
References and Further Reading
Navigating the Cost and Complexity of Clinical Trials
Clinical trials are a pivotal stage in the development of new treatments, but they come with substantial financial and logistical complexities. A comprehensive analysis of these trials illuminates the multifaceted nature of clinical trial expenses. Such an analysis is not only essential for budget optimization but also crucial in understanding the broader economic impact of bringing new therapies to market.
The escalating costs associated with clinical trials are a reflection of their growing size and intricacy. eRoom's law highlights a concerning trend: despite the doubling of components on integrated circuits every two years, the cost of drug development inversely increases, with R&D efficiency declining over the years. This phenomenon is particularly pronounced in clinical trials, which now consume approximately half of the total time and budget required to bring a new medication to fruition.
The sobering reality is that only a fraction of drugs that enter phase I trials receive approval, emphasizing the high-stakes nature of clinical research.
Recent news underscores this challenge, with reports indicating that many cancer drugs approved in Europe showed minimal evidence of added clinical benefit, particularly those fast-tracked to approval. The financial implications are significant, with the median cost of newly launched drugs in 2023 registering a 35% increase compared to the preceding year.
The success of a clinical trial is not solely contingent on its budget but also on its design and execution. Advanced algorithms, such as HINT and SPOT, offer predictive insights into trial outcomes by evaluating variables like drug molecules, patient eligibility criteria, and historical data. These tools can inform pivotal decisions, potentially redirecting the course of a trial and enhancing its probability of success.
Even with sophisticated predictive models, the practical aspects of trial participation can pose daunting challenges for patients, particularly in the case of international trials. The logistics of cross-border travel, including visa procurement and navigating foreign languages and bureaucracies, can be formidable barriers. This highlights the necessity for clinical trial companies to consider patient accessibility and support as part of their planning.
The industry's focus on speed and efficiency is reflected in recent statistics, revealing that clinical trials have lengthened in duration, with a majority failing to conclude on time. The competitive landscape, characterized by 'herding' behavior among biopharma companies, further intensifies the race to market, with the first approved product often reaping disproportionate rewards.
The US Inflation Reduction Act's implications on drug pricing strategies and the prioritization of indications also play a crucial role in the clinical trial landscape. For patients, especially those with rare diseases or aggressive cancers, the urgency for effective treatments remains high, underlining the critical importance of efficient and successful clinical trial conduct.
Despite the challenges, the United States continues to be a key player in clinical trials, hosting a significant portion of global Phase 3 trials. The country's healthcare system, however, presents its own set of obstacles, with incentive models that may dissuade physician participation in research. The turnover of healthcare staff, including principal investigators and trial coordinators, has reached unprecedented levels, signaling a need for a re evaluation of clinical trial infrastructure and support systems.
As the clinical trial landscape evolves, companies must adapt by investing more thoughtfully in trial design, execution, and patient support, ensuring that each step is optimized to overcome the hurdles that have historically plagued the drug development process.
Conclusion
In conclusion, clinical trial companies face a complex landscape that impacts the costs of clinical trials. Factors such as patient logistics, investigator fees, study design, and regulatory compliance all play a crucial role in determining trial success. Patient recruitment and retention, international trial logistics, and ethical considerations significantly impact budgets.
Investigator fees and study protocol development ensure scientific integrity. Data collection and management require specialized resources, and regulatory compliance is essential.
Strategic investments in trial aspects optimize outcomes and advance medical knowledge. Patient recruitment, experienced investigators, robust study design, meticulous data management, and regulatory compliance are crucial for success. Challenges include trial delays and pressure to expedite clinical development amidst regulatory changes.
A targeted keyword strategy is vital for enhancing trial visibility and reaching a broader audience. Carefully chosen keywords guide interested parties to relevant information on trial budgeting, compliance, and data management.
Clinical trial companies must adapt to the evolving research landscape by investing thoughtfully in trial design, execution, and patient support. Navigating complexities and making strategic decisions contribute to medical knowledge advancement and improved patient outcomes.




