Introduction
The International Council for Harmonisation (ICH) has played a pivotal role since its establishment in 1990, bringing together regulatory authorities and industry professionals from Europe, Japan, and the USA to streamline regulatory procedures in the pharmaceutical industry. This joint initiative aims to expedite global drug development, registration, and monitoring, ensuring the safety of patients managing multiple medications. The ICH's comprehensive activities cover everything from in vitro evaluations to risk management, guiding scientists, regulatory reviewers, and healthcare providers through a systemic approach to drug interactions.
Its influence extends beyond borders, with mutual recognition agreements and exemptions reflecting a trend towards international cooperation. Through its harmonization efforts, the ICH brings safe and effective medical devices to the global market, aligning with the goals of other international entities. The impact of its work is evident in the number of citations from various countries, indicating broad international engagement with the guidelines it establishes.
With its mission to harmonize regulatory standards, the ICH contributes to the development of safe and innovative medical products, fostering quicker access to treatments and promoting public health and safety.
History and Establishment of ICH
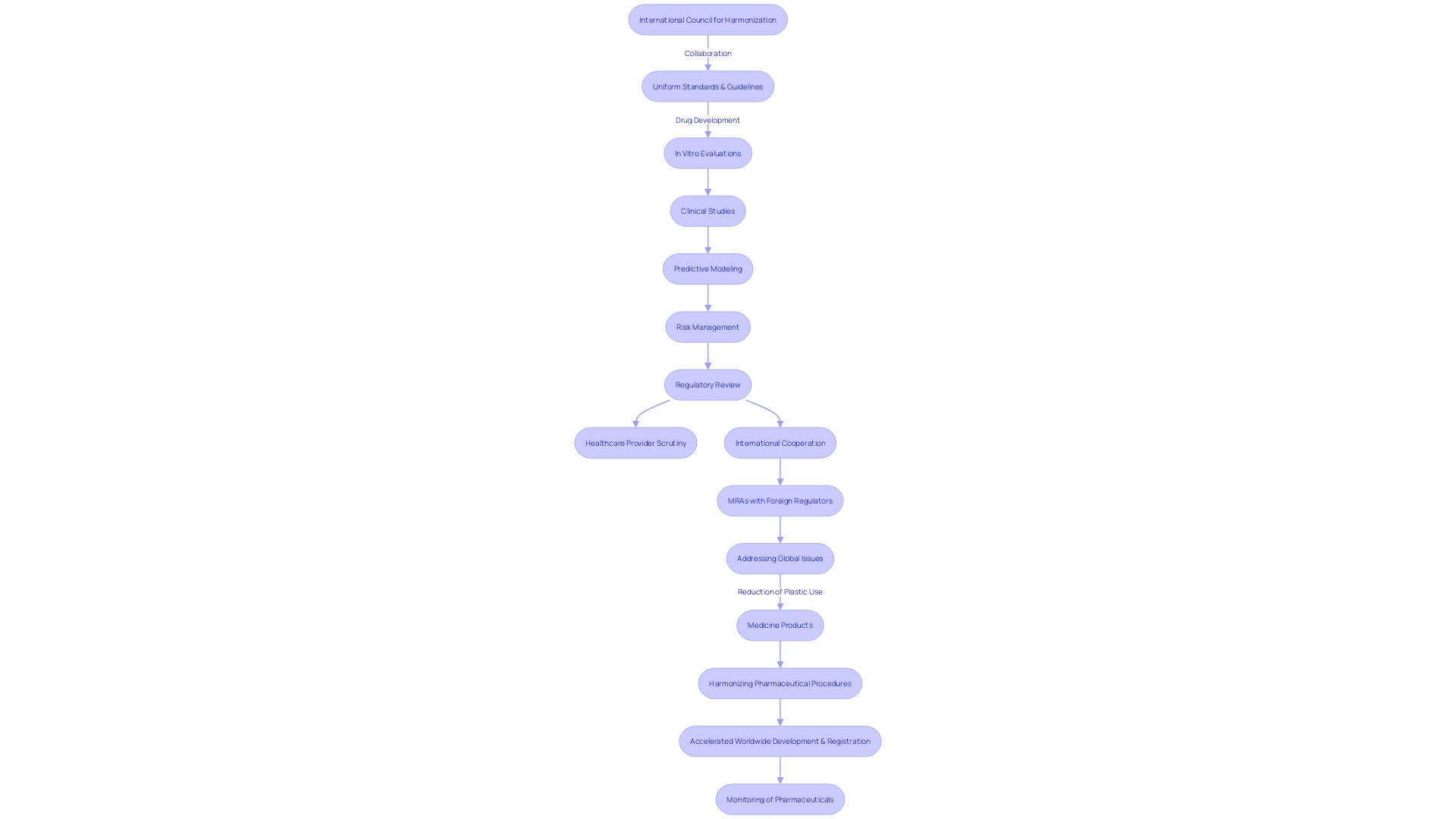
The International Council for Harmonization (ICH) is crucial in establishing uniform standards and guidelines for the medicine industry, a foundation laid since its inception in 1990. This collaborative effort brings together authorities and industry experts from Europe, Japan, and the USA, with the goal of simplifying procedures and accelerating the worldwide development, registration, and monitoring of pharmaceuticals. The Ich's influence extends to a comprehensive suite of activities, from in vitro evaluations and clinical studies to predictive modeling and risk management, ensuring medication safety for patients managing multiple medications. It guides scientists, regulatory reviewers, and healthcare providers through a systemic approach to scrutinize drug interactions throughout the drug development and regulatory assessment phases. The Ich's role in harmonization is further underscored by the FDA's recent exemptions for Medical Device Reporting, reflecting a trend towards leveraging real-world data and fostering international cooperation, as seen in the mutual recognition agreements (MRAs) with foreign regulators. These MRAs have significant implications for inspection processes and the broader medicine trade, including efforts to mitigate non-tariff barriers and accommodate international negotiations, such as those addressing the reduction of plastic use in medicine products. The Ich's efforts are instrumental in bringing safe and effective medical devices to the global market, in line with the shared objectives of international entities like the IMDRF and APEC LSIF Regulatory Harmonization Steering Committee, and are recognized in the number of citations from various countries, indicating a broad international engagement with the guidelines it helps to establish.
Objectives and Mission of ICH
The International Council for Harmonization (ICH) is pivotal in advancing the safety, efficacy, and quality of medical products across the globe. By promoting global cooperation, ICH accelerates the accessibility of medicines to patients by simplifying the processes for the development and post-market activities of pharmaceuticals. The harmonization efforts include a comprehensive approach involving in vitro evaluations, clinical studies, predictive modeling, study interpretation, risk assessment, and risk management. This partnership involves a variety of stakeholders, including scientists from pharmaceutical companies, reviewers overseeing regulations, policymakers, and healthcare providers, guaranteeing a comprehensive approach to evaluating interactions between substances during development.
The work of ICH is not limited to chemical drugs but also extends to herbal medicines through the International Regulatory Cooperation for Herbal Medicines (IRCH), established in 2006. This worldwide network consists of authorities committed to safeguarding public health by improving herbal medicine regulations.
Recent advancements in regulatory science have been marked by the development of frameworks to support digital health technologies (DHTs) and computational models in regulatory submissions, showcasing the progressive nature of regulatory harmonization.
Statistics from the FDA's Center for Drug Evaluation and Research (CDER) illustrate the tangible outcomes of such harmonization, with a range of new medications and biological products, including innovative treatments that have never been used in clinical practice, being approved annually. This is made possible by CDER's deep understanding of the science behind new products, necessary study designs, and the conditions they aim to treat.
The impact of ICH's work is further emphasized by expansions in facilities like Schnelldorf, which aim to meet the growing global demand for critical drugs by increasing capacity and capabilities, thus ensuring that medicines reach patients more efficiently.
In essence, ICH's mission to harmonize standards is a cornerstone in the development of safe and effective medical products, enabling quicker access to treatments and fostering innovation in public health and safety.
Membership and Structure of ICH
The International Council for Harmonization (ICH) is a significant entity in the sector, including authorities from major regions such as the European Union, Japan, and the United States, as well as observers from various countries. These regulatory bodies are represented through their respective agencies tasked with the oversight of medicinal products. Moreover, the pharmaceutical industry plays a crucial role as a member of ICH, with representation from trade associations and organizations dedicated to the field. ICH operates through a series of working groups and expert panels, each focusing on specific aspects of medication development, ranging from initial registration to post-approval processes. These groups work together to improve and synchronize requirements, with the ultimate goal of ensuring the safety, quality, and effectiveness of medicines, while also promoting global drug development efficiencies and reducing repetition in clinical trials.
In the broader context, initiatives like the International Regulatory Cooperation for Herbal Medicines (IRCH), established in 2006, illustrate the global effort to enhance regulation for the benefit of public health. The IRCH, with its mission aligned with that of ICH, aims to safeguard public health through improved oversight of herbal medicines, welcoming membership from national control bodies and international entities. This cooperative approach to regulation underscores the importance of harmonization across different types of medicinal products and the need for consistent, reliable standards that can be applied internationally. Such collaborative networks are crucial for adjusting to the changing landscape of medical research and ensuring that frameworks keep up with technological advancements and emerging healthcare challenges.
Harmonization Process and Guidelines
The International Council for Harmonization (ICH) is committed to the harmonization of development guidelines, a process that is crucial for promoting consistency and efficiency in the global medical landscape. By using a collaborative approach, specialists from governing bodies and the medicine industry come together to address common obstacles and create guidelines covering aspects such as quality, safety, effectiveness, and diverse multidisciplinary fields of medicine advancement. These guidelines serve as a blueprint for aligning requirements and industry practices, thereby streamlining the development and approval process internationally.
One instance of the ICH's influence is observed in the conversations of interaction programs, where specialists clarify how elements from in vitro assessments to risk control come together to guarantee the secure and efficient use of medications in polypharmacy situations. In addition, the Ich's efforts encourage strategic intellectual property research, enabling industry professionals to swiftly uncover patterns in competitors' formulations and pinpoint potential areas for innovation.
Furthermore, the ICH's commitment to transparency and collaboration is exemplified by its multidisciplinary guidelines, such as the anticipated ICH M14, aimed at harmonizing post-approval non-interventional studies with real-world data. This initiative addresses the need for internationally standardized guidance, thereby eliminating inefficiencies for governing bodies and drug developers alike.
Statistics from the International Regulatory Cooperation for Herbal Medicines (IRCH) demonstrate the value of such harmonization efforts. Since its inception in 2006, the IRCH has fostered a network of regulators focused on the safety and efficacy of herbal medicines, showcasing the potential for harmonized guidelines to protect public health on a global scale.
The ICH's role is further underscored by the evolving landscape of pharmaceutical manufacturing. Recent guidance issued by authorities facilitates sponsors in evaluating manufacturing changes for therapies, ensuring that product quality remains unaffected post-alteration. Furthermore, the ICH offers a framework for credible computational models supporting submissions to authorities, a testament to its progressive approach to science in regulation.
The ICH's endeavors resonate with the principles outlined for the governance of digital platforms, where multiple stakeholders, including states, civil society, and digital companies, share responsibilities to foster an open, secure environment. In the context of medical regulation, the ICH embodies these principles by promoting an environment where transparency, accountability, and adherence to international standards are paramount.
Quotes from experts in the field further affirm the Ich's mission. They emphasize the rigorous scientific and data-driven processes that underpin regulation, emphasizing the organization's commitment to ensuring the safety, quality, and efficacy of pharmaceutical products. Through the Ich's guidelines, regulatory authorities and industry professionals are equipped with the tools to navigate the complexities of drug development, reflecting a shared dedication to public health and innovation.
Conclusion
In conclusion, the International Council for Harmonisation (ICH) has been instrumental in streamlining regulatory procedures in the pharmaceutical industry since its establishment in 1990. By bringing together regulatory authorities and industry professionals from Europe, Japan, and the USA, the ICH aims to expedite global drug development, registration, and monitoring.
The ICH's comprehensive activities cover a wide range of areas, from in vitro evaluations to risk management, ensuring the safety of patients managing multiple medications. Through its harmonization efforts, the ICH contributes to the development of safe and innovative medical products, fostering quicker access to treatments and promoting public health and safety.
The ICH's influence extends beyond borders, with mutual recognition agreements and exemptions reflecting a trend towards international cooperation. Its work is not limited to chemical drugs but also includes herbal medicines, showcasing its dedication to protecting public health.
Operating through working groups and expert panels, the ICH collaborates to refine and harmonize regulatory requirements, ensuring consistent standards across different regions. The organization's commitment to transparency and collaboration is exemplified by its multidisciplinary guidelines, which address emerging challenges such as the use of real-world data and computational models in regulatory submissions.
The tangible outcomes of the ICH's harmonization efforts can be seen in the approval of new drugs and biological products, as well as advancements in facilities to meet the growing global demand for critical drugs. The ICH's work aligns with the objectives of other international entities and underscores the importance of collaboration and consistent standards in the global pharmaceutical landscape.
In summary, the ICH plays a pivotal role in the development of safe and effective medical products. By providing accurate and detailed information, the organization contributes to the advancement of public health and the well-being of patients worldwide.




