Introduction
The International Council for Harmonisation (ICH) emerged in 1990 as a collaborative effort between regulatory bodies and the pharmaceutical industry from the United States, Europe, and Japan. The aim was to streamline regulatory requirements and technical standards for the development and registration of pharmaceutical products. By harmonizing guidelines, ICH facilitates a more predictable regulatory environment, enabling pharmaceutical companies to navigate the drug development process more efficiently and bring life-saving medications to patients sooner.
In addition to fostering international cooperation, the ICH delves into the granular aspects of pharmaceutical development and registration, providing a systemic approach to assessing the potential for drug interactions and ensuring the safety and efficacy of medications. Recent developments, such as the ICH M14 initiative and the increasing average length of clinical trials, highlight the ongoing relevance of ICH in the pharmaceutical landscape. With its mission to protect and promote public health and safety, the ICH continues to be a cornerstone of the global effort to enhance the quality, safety, and efficacy of pharmaceuticals.
History and Formation of ICH
Founded in 1990, the International Council for Harmonization (ICH) arose as a cooperative endeavor between governing bodies and the medicine industry from the United States, Europe, and Japan, with the goal of simplifying the maze of requirements and technical standards for the development and registration of medical products. The establishment of ICH was a response to the growing complexities and discrepancies in drug development regulations, which were creating barriers to the introduction of new and effective treatments to the market. By synchronizing guidelines, ICH enables a more foreseeable governing environment, allowing drug companies to navigate the drug development process more efficiently and bring life-saving medications to patients sooner.
Besides promoting global collaboration, the ICH explores the detailed facets of drug development and registration, including in vitro assessments, clinical trials, and predictive modeling. It provides a systemic approach to assessing the potential for drug interactions, which is crucial for the safety and efficacy of medications, especially for individuals taking multiple drugs simultaneously. This comprehensive approach to drug evaluation and risk management is reflected in the Ich's guidance, which serves as a touchstone for scientists, reviewers, policymakers, and healthcare providers alike.
Recent developments underscore the ICH's continued relevance and dynamism in the pharmaceutical landscape. For instance, the ICH M14 initiative is set to introduce a multidisciplinary guideline to harmonize post-approval non-interventional studies using real-world data by June 2025. This is a testament to the Ich's recognition of the increasing availability of real-world data and its potential to revolutionize postmarketing pharmacovigilance and safety evaluations, addressing inefficiencies faced by both sponsors and regulators.
As the average length of clinical trials has increased over the years, with Phase 3 trials now taking an average of 44 months and Phase 2 trials 41 months, the role of the ICH in promoting efficient clinical development has become even more critical. With up to 80 percent of clinical trials failing to finish on time, and with the competitive pressure to be the first to market, the Ich's work in harmonization is invaluable to the biopharmaceutical industry's quest to expedite the delivery of new treatments to market, particularly in a landscape where regulatory decisions have significant commercial implications.
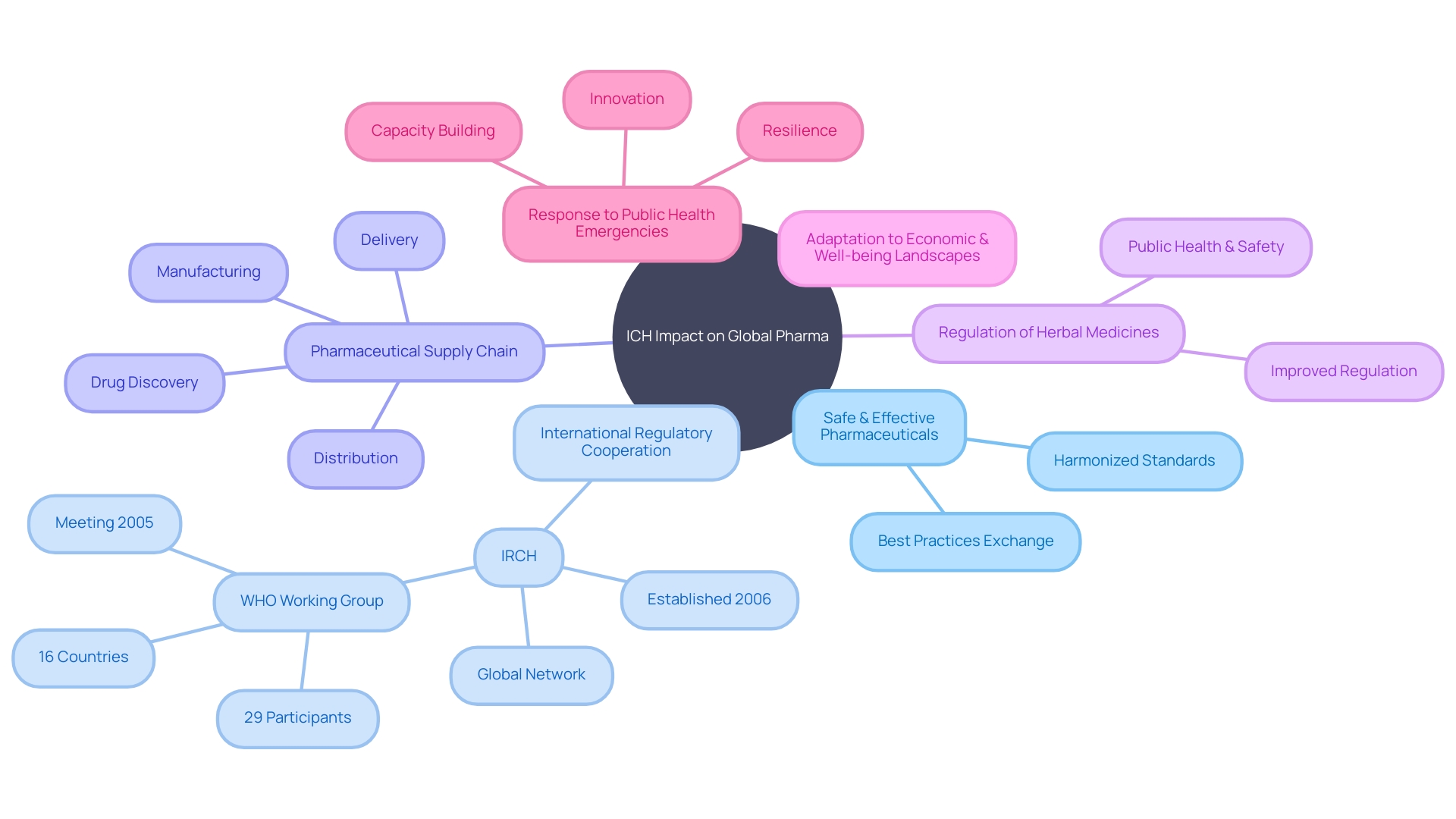
The ICH's mission, to safeguard and advance public well-being and safety through enhanced regulation, is demonstrated by the creation of networks like the International Regulatory Cooperation for Herbal Medicines (IRCH), which was established in 2006. The IRCH's goal to enhance control for herbal medicines is a reflection of the broader objectives of the ICHâto ensure that medicines, whether conventional or alternative, are effectively regulated to safeguard public well-being.
With a focus on the future, the ICH remains a foundation of the global endeavor to improve the quality, safety, and effectiveness of medications, thus supporting the well-being and welfare of individuals worldwide.
Mission and Objectives of ICH
The International Council for Harmonization (ICH) plays a crucial role in the intricate ecosystem of global well-being, striving to ensure the availability of safe, effective, and high-quality pharmaceuticals to patients around the world. Through the development of harmonized guidelines and standards, ICH facilitates the exchange of scientific information and expertise, enhancing international regulatory cooperation. A testament to the importance of such collaboration can be seen in the work of the International Regulatory Cooperation for Herbal Medicines (IRCH), which, since its establishment in 2006, has been dedicated to improving the regulation of herbal medicines to safeguard public health.
With the pharmaceutical supply chain intricately linked from drug discovery to delivery, Ich's objectives resonate strongly. The journey of a medicine from conception to the hands of a patient involves rigorous processes, including sourcing, manufacturing, distribution, and delivery, all underpinned by stringent regulatory oversight. Companies like Viatris exemplify this commitment to safety and compliance, operating extensive networks and partnerships to ensure supply chain resilience and continuity. Furthermore, the worldwide exchange of best practices and tools, as described in the Toolkit for medical product quality and supply chain security, emphasizes the importance of harmonized standards that can adapt to the diverse economic and well-being landscapes of different regions.
In light of recent global challenges, the ability to respond to public health emergencies and maintain strategic autonomy has been recognized as crucial. The COVID-19 pandemic highlighted the importance of a strong, research-based drug infrastructure, as demonstrated by Europe's crucial role in vaccine development and distribution. This success depended on previous investments and research within the region, a testament to the foresight of frameworks that ICH seeks to harmonize on a global scale. As such, Ich's mission extends beyond guidelines, impacting the very resilience of healthcare systems and their capacity to innovate and respond to emergent needs.
Membership and Governance Structure
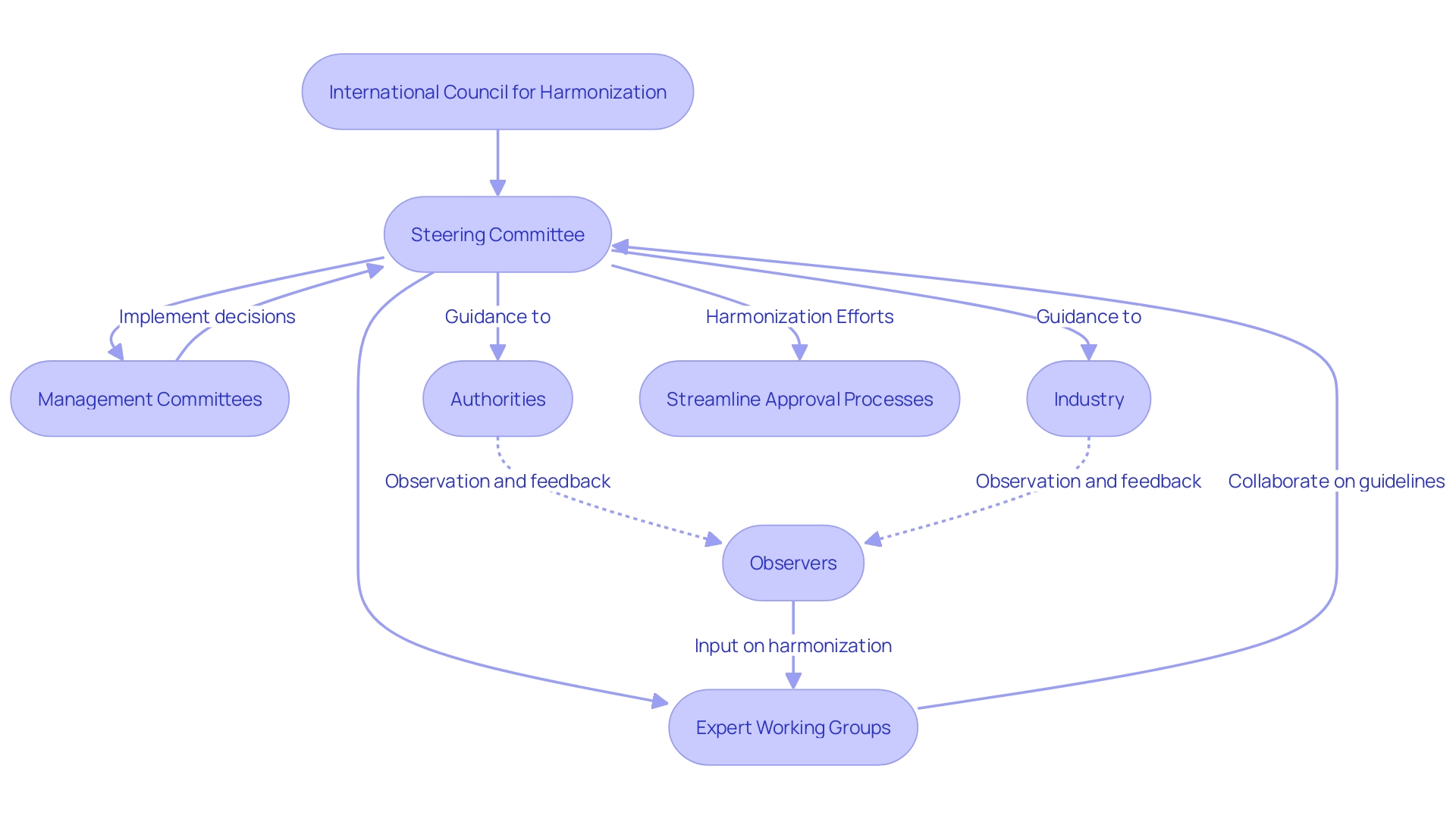
The International Council for Harmonization (ICH) is a crucial entity with a strategic governance framework composed of authorities, the medicine industry, and observers. With governing bodies from various nations at its core, ICH ensures the creation of universally accepted guidelines, enabling a harmonious approach to the evaluation of medical devices and pharmaceuticals. Industry associations play a vital role in shaping these standards, ensuring they are practical and relevant to market needs. Observers, including non-profit organizations, contribute diverse perspectives to ICH deliberations.
ICH's governance is upheld by multiple committees, such as the Steering Committee, Expert Working Groups, and Management Committees. These groups are instrumental in steering the harmonization efforts, addressing the complexities of regulatory practices across different jurisdictions. The objective is to streamline the approval processes while maintaining high safety and efficacy standards, thereby facilitating quicker access to medical innovations worldwide. This collaborative model echoes the need for uniform assessment criteria, as emphasized in recent discussions by the HTAi Medical Devices Interest Group, to avoid market fragmentation.
Given the ever-changing global landscape, the Ich's role is more relevant than ever. Events like Canada's comprehensive evaluation of government actions during crises, and the establishment of research-practice models for heritage properties, underscore the importance of adaptable and inclusive frameworks in decision-making processes. The ICH, by engaging a broad spectrum of stakeholders, mirrors the Human Rights Council's practice of inclusive, state-driven reviews, ensuring that diverse experiences and challenges inform the development of global standards. The Council's commitment to harmonization not only aligns with the principles of the Universal Declaration of Human Rights but also addresses the shared interests of manufacturers, users, and assessment bodies in having consistent evaluation criteria for health and wellness technologies.
Process of Harmonization
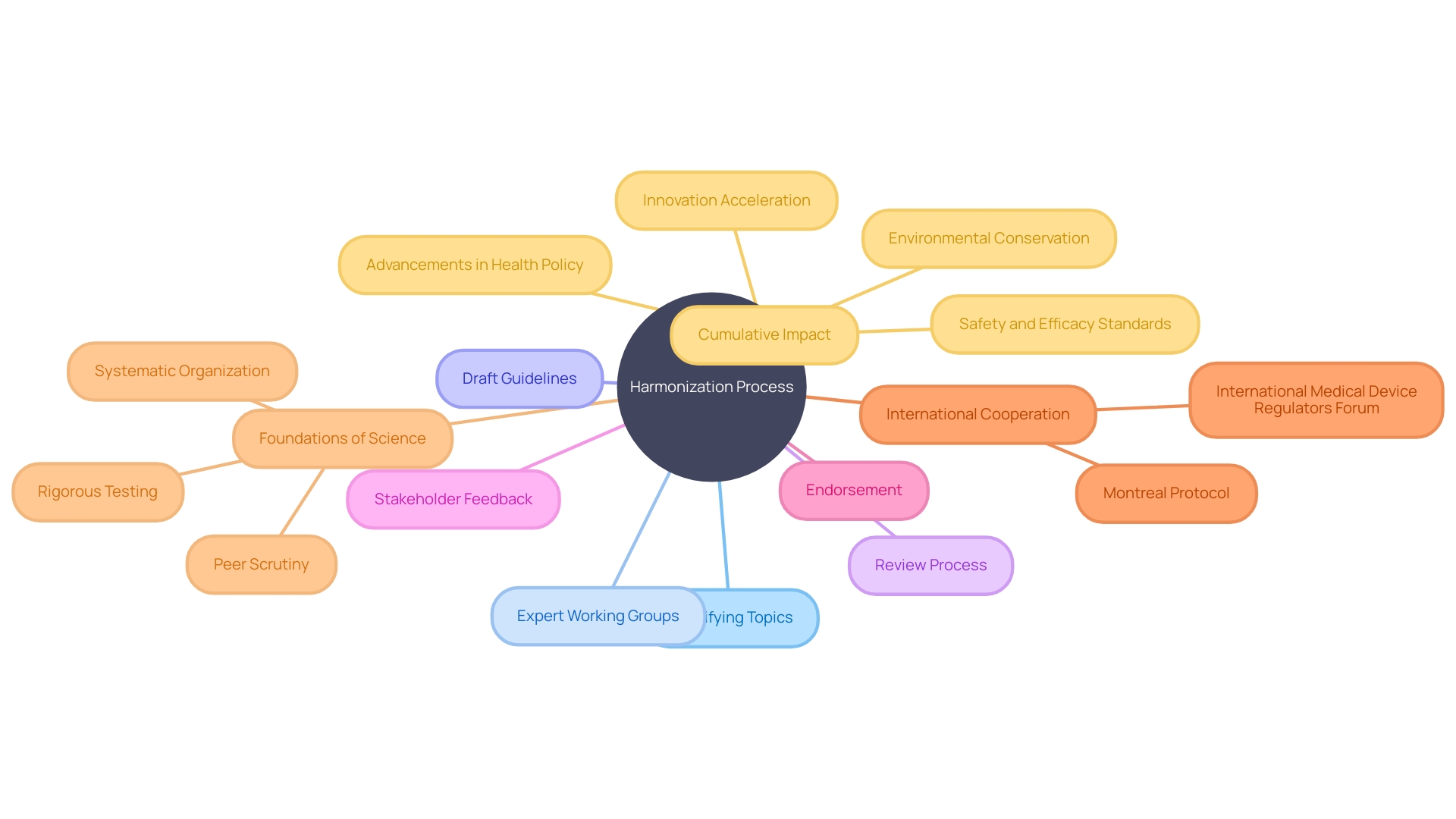
The International Council for Harmonization (ICH) adopts a synergistic approach to the harmonization of requirements, ensuring that safe, effective, and high-quality medicines are developed and registered in the most resource-efficient manner. The harmonization process initiates with pinpointing topics that necessitate alignment across regions. To achieve this goal, Expert Working Groups, which include authority members and pharmaceutical industry experts, are assigned to devise draft guidelines. These drafts are meticulously scrutinized through a multi-tiered review process, which encompasses public consultation phases to solicit widespread input from relevant stakeholders.
The integration of stakeholder feedback is a critical component in refining these guidelines to their final form. The endorsement of these guidelines by the regulatory bodies of ICH member regions is pivotal, as it facilitates their implementation across various countries, thereby streamlining drug development and approval processes globally.
This collaborative framework is not unique to the ICH; it reflects a broader trend in international cooperation as seen in other successful initiatives. For instance, the Montreal Protocol's triumph in reducing atmospheric hydrochlorofluorocarbons underscores the efficacy of international partnerships and policy synergy. Similarly, the International Medical Device Regulators Forum (IMDRF) exemplifies engagement with various organizations to advance medical device regulation convergence.
The collective efforts in harmonization are further underpinned by the foundations of science, which is the bedrock of these guidelines. As articulated by a prominent voice in the scientific community, science's systematic organization and adherence to rigorous testing against reality and peer scrutiny are what make it universally comprehensible and applicable across diverse challenges.
Moreover, the harmonization process echoes the principles of science in its pursuit of standardization and interoperability, as evidenced by the International Statistical Classification of Diseases and Related Health Problems (ICD). The implementation of ICD standards allows for consistent data collection and promotes extensive research, which is vital for public well-being monitoring and decision-making.
The cumulative impact of these harmonized guidelines is significant, as reflected in the latest statistics showing advancements in health policy and environmental conservation. The adoption of these guidelines not only accelerates innovation but also ensures that it is done within a framework that respects the highest standards of safety and efficacy.
Conclusion
In conclusion, the International Council for Harmonisation (ICH) streamlines regulatory requirements and technical standards for pharmaceutical development and registration. By harmonizing guidelines, ICH creates a predictable regulatory environment, enabling efficient drug development and faster access to life-saving medications for patients.
ICH's comprehensive approach to assessing drug interactions and ensuring medication safety is crucial for public health. Recent developments, like the ICH M14 initiative and longer clinical trials, highlight its ongoing relevance. These demonstrate ICH's commitment to enhancing post-approval studies and promoting efficient clinical development.
ICH's mission revolves around protecting and promoting public health and safety. Collaborative networks, such as the International Regulatory Cooperation for Herbal Medicines (IRCH), exemplify ICH's focus on improving the regulation of herbal medicines. Harmonized guidelines and standards facilitate international regulatory cooperation and enhance the availability of safe and effective pharmaceuticals worldwide.
ICH's membership and governance structure, including regulatory authorities, the pharmaceutical industry, and observers, ensures the creation of universally accepted guidelines. This collaborative model allows for diverse perspectives, leading to practical and relevant standards that meet market needs. It aligns with human rights principles and contributes to consistent evaluation criteria for health and wellness technologies.
The harmonization process involves expert working groups and a multi-tiered review process that incorporates stakeholder feedback. This collaborative framework, supported by science and international cooperation, facilitates the development and implementation of harmonized guidelines across regions. Adoption of these guidelines accelerates innovation while ensuring the highest standards of safety and efficacy.
In conclusion, the ICH remains a cornerstone in the global effort to enhance the quality, safety, and efficacy of pharmaceuticals. Its mission to protect and promote public health and safety is reflected in its work to harmonize guidelines, address emerging challenges, and facilitate international regulatory cooperation. By fostering a predictable regulatory environment and promoting efficient drug development, the ICH plays a crucial role in ensuring the availability of safe and effective medications worldwide.




