Introduction
The U.S. Food and Drug Administration (FDA) plays a crucial role in safeguarding public health by regulating various products, including medical devices, pharmaceuticals, food, cosmetics, dietary supplements, radiation-emitting products, and tobacco. In this article, we will explore the FDA's comprehensive responsibilities, including its rigorous assessment of vaccines and the 510(k) clearance pathway for medical devices. We will also discuss the importance of understanding FDA classifications and the distinctions between FDA approval and clearance.
Additionally, we will delve into the process of obtaining FDA approval or clearance for medical devices and highlight the consequences of misusing FDA terminology. Navigating the FDA's regulatory landscape is essential for companies in the healthcare industry to ensure that their products reach the market legally, safely, and effectively.
What Does the FDA Regulate?
The U.S. Food and Drug Administration (FDA), as part of the Department of Health and Human Services, plays a crucial role in safeguarding public health. Its mandate extends beyond the oversight of medical devices and pharmaceuticals to include the regulation of food, cosmetics, dietary supplements, products that emit radiation, and tobacco. The FDA's comprehensive responsibilities encompass assuring the safety, efficacy, and security of human and veterinary drugs, vaccines, and other biological products.
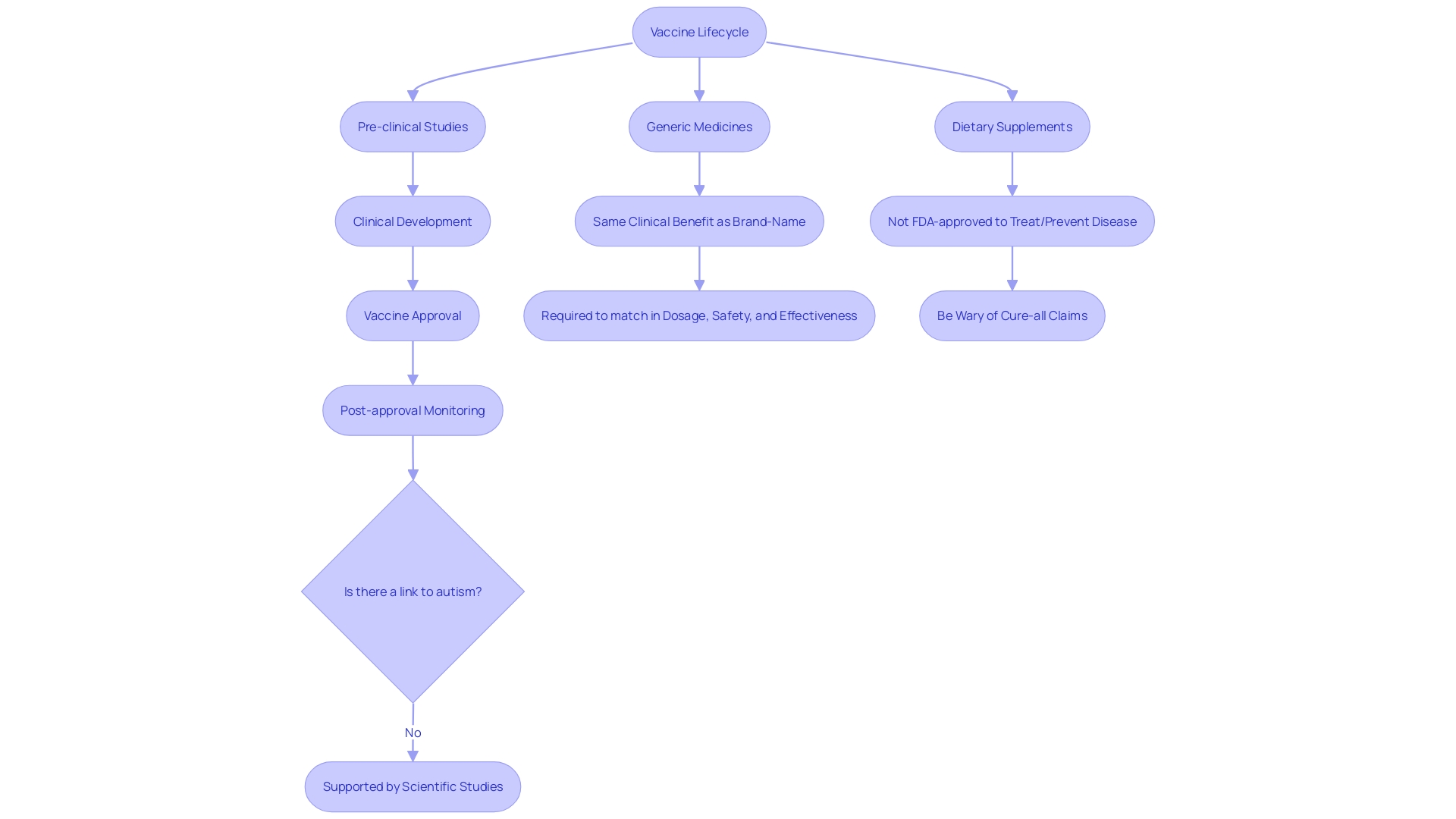
The agency's rigorous assessment of vaccines, for example, spans the full spectrum of a vaccine's lifecycle, from pre-clinical studies to post-market surveillance, to ensure continuous safety and effectiveness.
One notable aspect of the FDA's regulatory process is the 510(k) clearance pathway for medical devices. This mechanism allows for the expedited review of devices that are deemed substantially equivalent to a product already on the market, known as a predicate. However, this process has come under scrutiny, as highlighted by the 2018 documentary 'The Bleeding Edge', which revealed that clinical trials are not always required for device approval under this pathway.
This has led to instances where devices cleared through the 510(k) process caused severe adverse effects, including bleeding, organ puncture, and cobalt poisoning. The film's exposure of these issues contributed to some devices being removed from the market.
The Electronic Code of Federal Regulations (eCFR) provides continuously updated online access to regulations, although it is not considered the official legal edition. The eCFR's content reflects the FDA's editorial process and provides insight into the current regulations governing the agency's oversight.
Understanding the scope of FDA-approved products is also essential. For instance, while FDA-approved generic medicines must match their brand-name counterparts in dosage, safety, and effectiveness, dietary supplements do not receive FDA approval to treat or prevent diseases. The FDA emphasizes that claims of quick fixes or cures from supplements are often unsubstantiated.
In the realm of public engagement, the FDA encourages individuals to submit comments on regulatory matters, with the assurance that these submissions will be made part of the public record. It's important for commenters to remember not to include confidential information, as these comments become publicly accessible. The agency provides guidelines for those wishing to submit confidential information to ensure it is handled appropriately.
As of March 2024, navigating FDA records, such as the 85,791 510(k) application summaries, remains a challenge due to the lack of standardized templates and datasets, highlighting the ongoing need for transparency and accessibility in the FDA's processes.
Understanding FDA Classifications
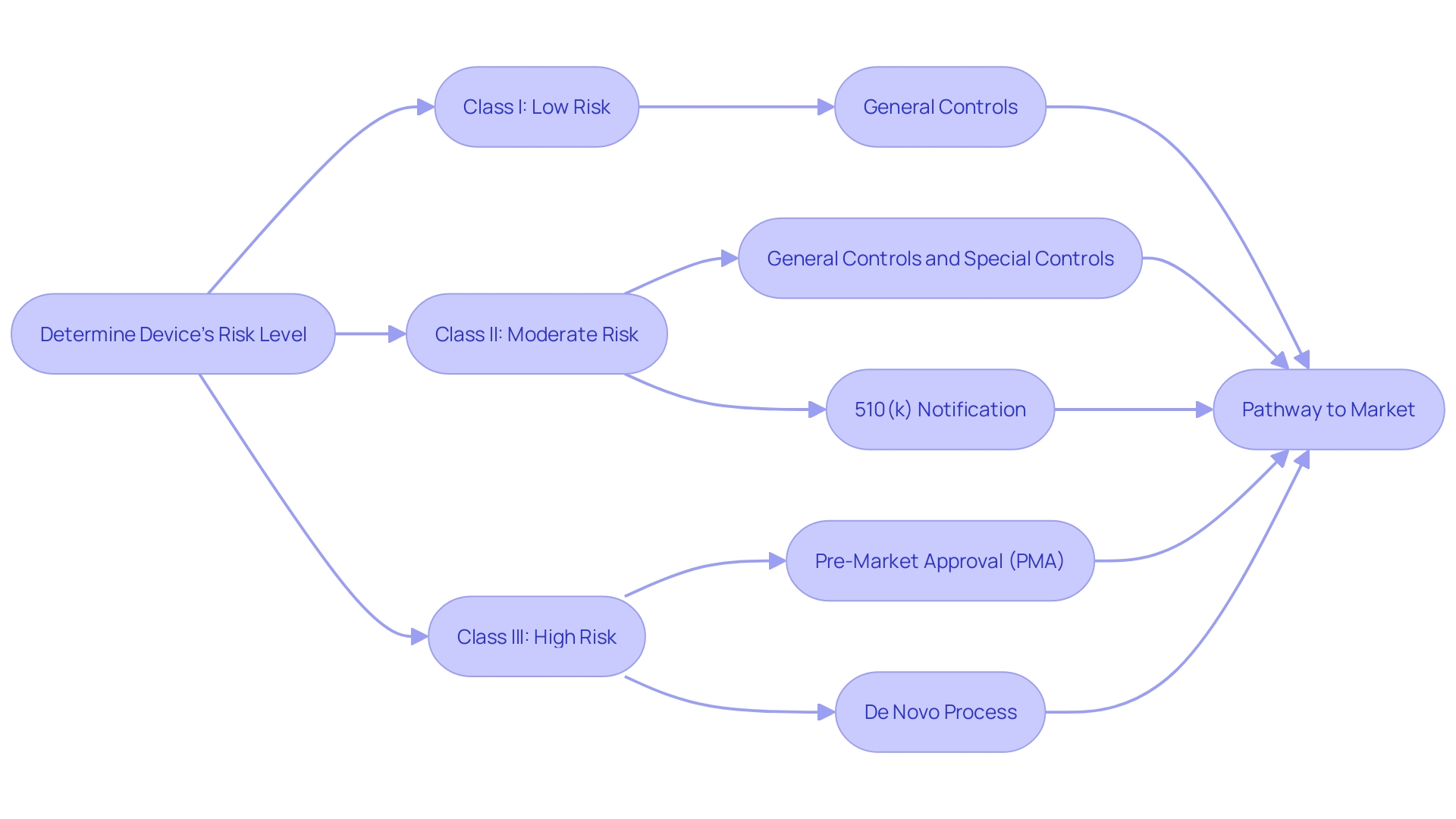
Navigating the regulatory landscape of medical devices, it is crucial to understand the FDA's classification system, which segregates devices into Class I, Class II, and Class III categories, reflecting their associated patient risk levels. Class I devices pose the least risk and typically require the least regulatory control, whereas Class II and Class III devices represent moderate and high risks, respectively, necessitating more stringent controls to assure safety and effectiveness.
The pathway to market for a medical device is contingent upon its classification. Class I devices may only need to comply with general controls, whereas Class II devices frequently necessitate a Premarket Notification, commonly known as a 510(k). Class III devices, which include those that support or sustain human life, are potentially of substantial importance in preventing impairment of human health, or present a potential, unreasonable risk of illness or injury, require Pre-Market Approval (PMA) – the most rigorous type of device marketing application required by the FDA.
Furthermore, the De Novo process offers a route to classify novel devices of low to moderate risk that do not have a valid predicate device. Understanding these distinctions is not just academic; it is a critical component of a medical device manufacturer's strategy to bring new or improved tools to the healthcare industry, an industry that is rapidly evolving with technological advancements and increased consumer demand for digital health solutions.
Recent updates and educational efforts by the FDA, such as webinars and draft guidance on the Breakthrough Devices Program, reflect the agency's commitment to fostering innovation while ensuring safety and efficacy. These initiatives are designed to streamline the process for devices that promise significant advancements in treatments or diagnoses of life-threatening conditions, notably those that can offer substantial benefits over existing alternatives.
In light of the dynamic nature of medical device regulations, the Regulatory Affairs community remains engaged through events like the MedTech Regulatory Intelligence Summit, where professionals can share best practices and gain insights into navigating these complex pathways. The classification and subsequent approval, clearance, or granting process is more than just regulatory compliance—it is a foundational element that supports the FDA's mission to protect public health.
What is FDA Approval?
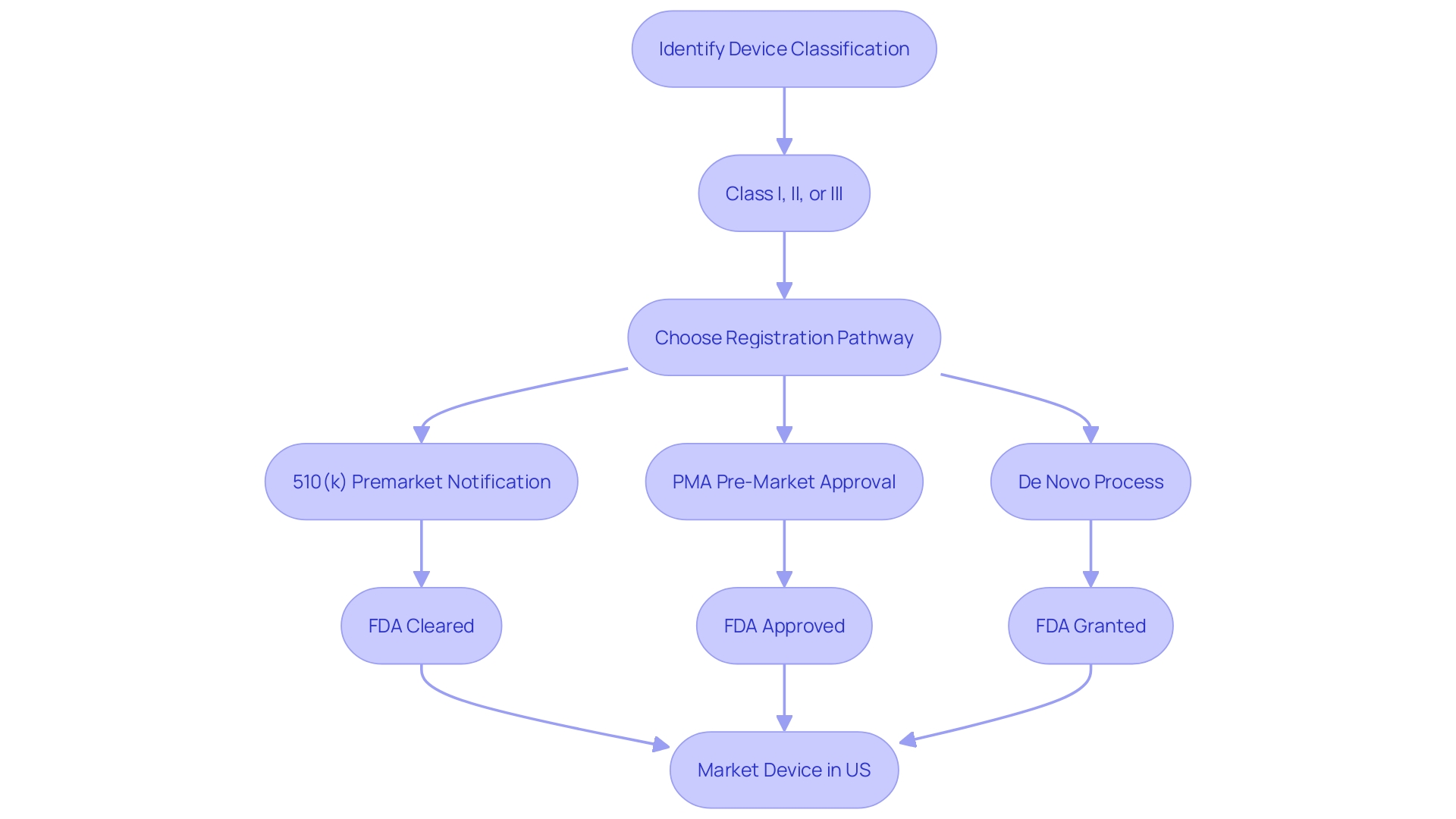
Understanding the distinctions between FDA approval, clearance, and compliance is fundamental in the realm of medical devices and pharmaceuticals. The FDA classifies medical devices into three categories based on patient risk, with Class III encompassing devices that sustain or support life, such as pacemakers, and therefore considered high risk. These devices, alongside new drugs, must undergo the extensive FDA approval process, which includes a rigorous review of scientific evidence, clinical data, and manufacturing information to confirm safety and efficacy.
To legally market a medical device in the U.S., it must be FDA cleared, approved, or granted through the De Novo process. Clearing a device typically involves the 510(k) pathway, where the device is compared to a previously cleared predicate device. This is a less stringent process than the Pre-Market Approval (PMA), which is necessary for Class III devices and demands comprehensive clinical trial data.
Devices that do not have a precedential product are eligible for the De Novo process, which offers a route to classify novel devices of low to moderate risk. Regulatory professionals often navigate complex terms like Registered, Cleared, Approved, and Granted, each signifying different levels of FDA oversight and endorsement.
The FDA's responsibility extends beyond medical devices to ensuring the safety, effectiveness, and security of various products, including human and veterinary drugs. The Center for Drug Evaluation and Research (CDER) oversees the drug approval process, which can span 12 to 15 years, from research to market introduction. This process is marked by meticulous scrutiny by experts to ascertain the therapeutic value and safety of drugs.
Market pathways for devices depend on their classification, and companies must annually register their establishments with the FDA. This registration necessitates listing the devices and activities conducted on them, a preliminary step before choosing the correct regulatory pathway.
In recent times, there have been calls for more efficient regulatory pathways, a movement accelerated by the COVID-19 pandemic, aiming to hasten approvals for devices that meet pressing medical needs. The documentary 'The Bleeding Edge' highlighted issues with the 510(k) clearance process, revealing that not all devices require clinical trials, which has sometimes resulted in patient harm.
The intricacies of FDA regulatory compliance in the pharmaceutical industry are vast, affecting the overall cost of drug development and the potential repercussions of non-compliance. The FDA's recent final rule on direct-to-consumer prescription drug advertisements underscores its commitment to transparency and consumer comprehension in the communication of drug risks.
In summary, navigating FDA regulations is a critical and complex aspect for companies in the healthcare sector. Understanding the nuances between approval, clearance, and compliance is essential for ensuring that medical devices and drugs reach the market legally, safely, and effectively.
What is FDA Clearance?
FDA clearance, also known as the 510(k) clearance, is a regulatory pathway for medical devices that pose moderate to low risk to patients. To achieve clearance, a device must be demonstrated to be substantially equivalent to a predicate device, meaning a similar product already legally on the market. This involves a thorough comparison of the new device's intended use and technological characteristics with those of the predicate device.
Equivalence in safety and effectiveness, or substantial equivalence, is key to securing FDA clearance. The process does not always necessitate clinical trials, as highlighted by the 2018 documentary 'The Bleeding Edge', which revealed instances where fast-tracked devices led to patient injuries. It's crucial for manufacturers to first precisely classify their medical devices since the FDA assigns devices to one of three classes based on the level of risk they present to patients.
Understanding and navigating the distinctions between FDA regulatory terms like Registered, Cleared, Approved, and Granted is essential for compliance and to ensure the safe introduction of new medical devices into the healthcare market.
Key Differences Between FDA Approval and Clearance
Navigating the regulatory landscape of the FDA for medical devices requires a clear understanding of the specific pathways and terminology associated with bringing a device to market. Devices are categorized into three classes by the FDA, based on the level of risk they pose to patients. Class I devices are deemed low-risk, Class II devices are intermediate-risk, and Class III devices are high-risk and often critical to sustaining or supporting life.
The route to market, whether via Premarket Notification (510(k)), Pre-Market Approval (PMA), or De Novo classification, hinges on the device's classification.
A 510(k) submission is often employed for Class I and II devices, and it hinges on demonstrating that a new device is substantially equivalent to a predicate device already legally on the market. Conversely, the PMA process, which is more stringent, is typically reserved for Class III devices. These devices undergo rigorous scrutiny, with the FDA evaluating extensive data on safety and efficacy.
Lastly, the De Novo pathway offers a route for low to moderate risk devices that lack a predicate, allowing them to be classified as Class I or II rather than automatically being considered Class III, which would necessitate a PMA.
The distinctions between FDA 'clearance,' 'approval,' and 'granting' are pivotal for regulatory professionals to grasp. While 'clearance' through the 510(k) route implies that a device is safe and effective by comparison to existing similar devices, 'approval' denotes a more comprehensive vetting process, addressing the safety and efficacy of novel or high-risk devices. The term 'granted,' used in the context of the De Novo process, indicates authorization to market a device following its reclassification into a lower risk category than initially presumed.
The FDA's mission to ensure the public's health safety encompasses not only medical devices but also human and veterinary drugs, biological products, and more. It's imperative for companies to align with the FDA's regulatory framework to avoid the financial and reputational repercussions of non-compliance, which can also lead to significant delays in bringing new treatments to market. Such delays can have profound implications, as highlighted by the critical breakthroughs in Alzheimer's treatment, which were contingent upon navigating these regulatory complexities.
The Process of Obtaining FDA Approval
Securing FDA approval is a meticulous process requiring manufacturers to demonstrate the safety and effectiveness of their products. The journey begins with rigorous preclinical testing, often involving laboratory research and animal studies to collect initial safety and performance data. This is followed by comprehensive clinical trials on human participants, which are crucial to further assess the product's safety profile and therapeutic efficacy.
Once these studies are satisfactorily completed, the manufacturer compiles a thorough application, which must include all pertinent data and documentation. It is then submitted to the FDA for review. The FDA, tasked with ensuring public health by regulating a wide array of products from drugs to medical devices, may then request further information or clarifications.
This step is essential to ensure that the medical products meet the high standards for safety, effectiveness, and security set by the Agency.
The FDA's commitment to public health is exemplified by its recent clearance of innovative therapeutic options for chronic diseases, such as diabetes, which affects more than 11% of the American population. This clearance offers new technologies that automate tasks, potentially easing the daily management of the disease for patients.
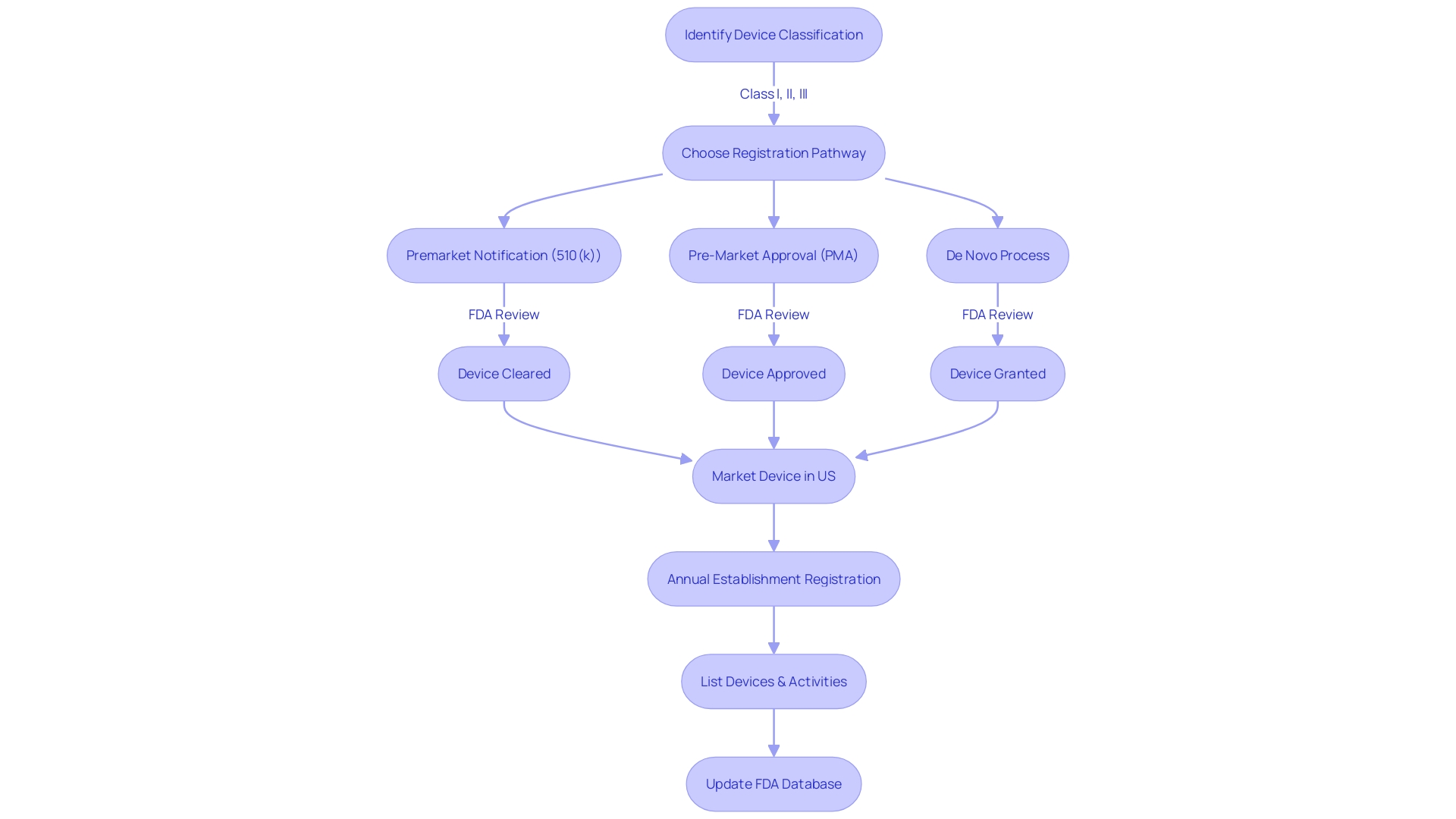
It's important to recognize that medical devices are categorized into three classes based on the risk they pose to patients. The classification determines the regulatory pathway for approval, be it Premarket Notification (510(k)), Premarket Approval (PMA), or the De Novo process. Class I and II devices, which are considered lower risk, may be cleared through the 510(k) process, which involves comparing the new device to one already on the market.
In contrast, Class III devices, which are high-risk and often life-sustaining, undergo a more stringent approval process due to the critical role they play in patient care.
The distinctions between FDA terms such as Cleared, Approved, and Granted are significant, carrying implications for the level of evidence required and the degree of regulatory scrutiny. Understanding these differences is crucial for regulatory professionals navigating the complex landscape of medical device approval.
Recent initiatives to streamline regulatory pathways, particularly in response to urgent medical needs highlighted by the COVID-19 pandemic, underscore the FDA's role in fostering innovation while maintaining rigorous standards for public health and safety. As the Agency continues to adapt and refine its processes, it remains a cornerstone in the advancement of medical treatments and devices, ensuring they deliver on their promise of patient care without compromising on safety.
The Process of Obtaining FDA Clearance
Securing FDA clearance for a medical device is a critical step in ensuring it can be legally marketed in the United States. The first order of business is to accurately classify the device according to the FDA's three-tiered system, which assesses the level of risk to patients. Class I devices pose the least risk, while Class III devices, such as life-sustaining pacemakers, are subject to the most stringent controls due to their high-risk nature.
Once the classification is established, the manufacturer must select the appropriate regulatory pathway: a 510(k) Premarket Notification, a Pre-Market Approval (PMA), or the De Novo process.
For many Class I and II devices, a 510(k) submission is the gateway to demonstrating substantial equivalence to an existing, legally marketed predicate device. This involves a meticulous comparison of intended use, technological characteristics, and safety and effectiveness data. While some may perceive the 510(k) process as a fast track to clearance, it remains a thorough review, ensuring that new devices meet rigorous standards without necessarily requiring new clinical trials.
The FDA's commitment to public health is unequivocal, as evidenced by its recent rules requiring clear and conspicuous presentation of major side effects in direct-to-consumer drug advertisements. Such measures reflect the agency's dedication to transparency and consumer understanding, which parallels its approach to medical device regulation.
Regulatory professionals and manufacturers alike must navigate the complexities of FDA terminology and processes. Despite potential confusion surrounding terms like 'Registered,' 'Cleared,' 'Approved,' and 'Granted,' the distinctions hold significant implications for the pathway a medical device must follow. The FDA's classification system and regulatory pathways are designed not just to facilitate market entry, but more importantly, to uphold the safety and efficacy of medical devices for American consumers.
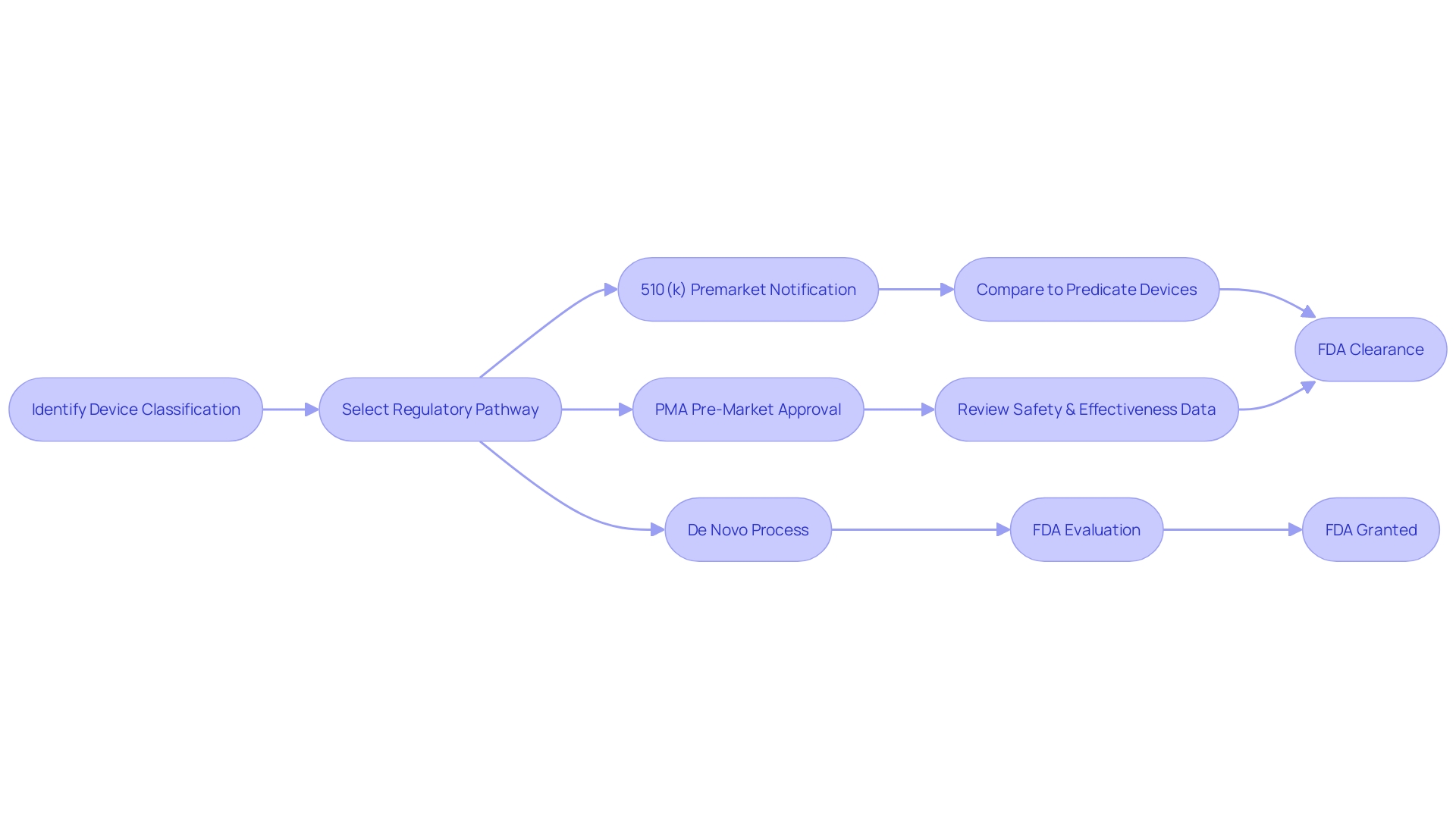
Examples of FDA-Approved and FDA-Cleared Devices
Navigating the regulatory requirements of the FDA is a critical step for medical device manufacturers. Understanding the distinction between FDA approval and clearance can be illustrative of the regulatory process. For instance, a Class III medical device, which is considered a high-risk product such as an implantable cardiac pacemaker, would necessitate FDA approval due to its potential impact on patient health and safety.
This rigorous approval process is designed to ensure the highest level of scrutiny for devices that can significantly affect patient outcomes.
Conversely, a Class II device, such as a non-invasive blood pressure monitor that shares substantial equivalence with a device already on the market, would generally be subject to FDA clearance. This clearance confirms that the device is safe and effective for use, and that it complies with the necessary regulatory standards without necessarily suggesting that it is superior or more effective than other similar devices.
The FDA's classification system categorizes medical devices into three classes based on the level of control necessary to assure the safety and effectiveness of the device. Once a device's classification is determined, the appropriate regulatory pathway, whether it's Premarket Notification (510(k)), Pre-Market Approval (PMA), or the De Novo process, must be selected prior to legal marketing in the United States.
It is important to recognize that the terms 'FDA Registered', 'FDA Cleared', 'FDA Approved', and 'FDA Granted' are not interchangeable but signify different levels of regulatory compliance and oversight. For example, the HeartMate 3 Left Ventricular Assist System Implant Kit, which assists in pumping blood for patients with severe left ventricular heart failure, underwent a correction recall—a process that does not entail product removal but rather addresses issues to meet FDA standards.
These distinctions underscore the FDA's commitment to ensuring that medical devices are evaluated thoroughly, taking into account the innovation and complexity of each product, as well as the potential risks and benefits to patients. The goal is to protect public health while also facilitating access to the latest advancements in medical technology.
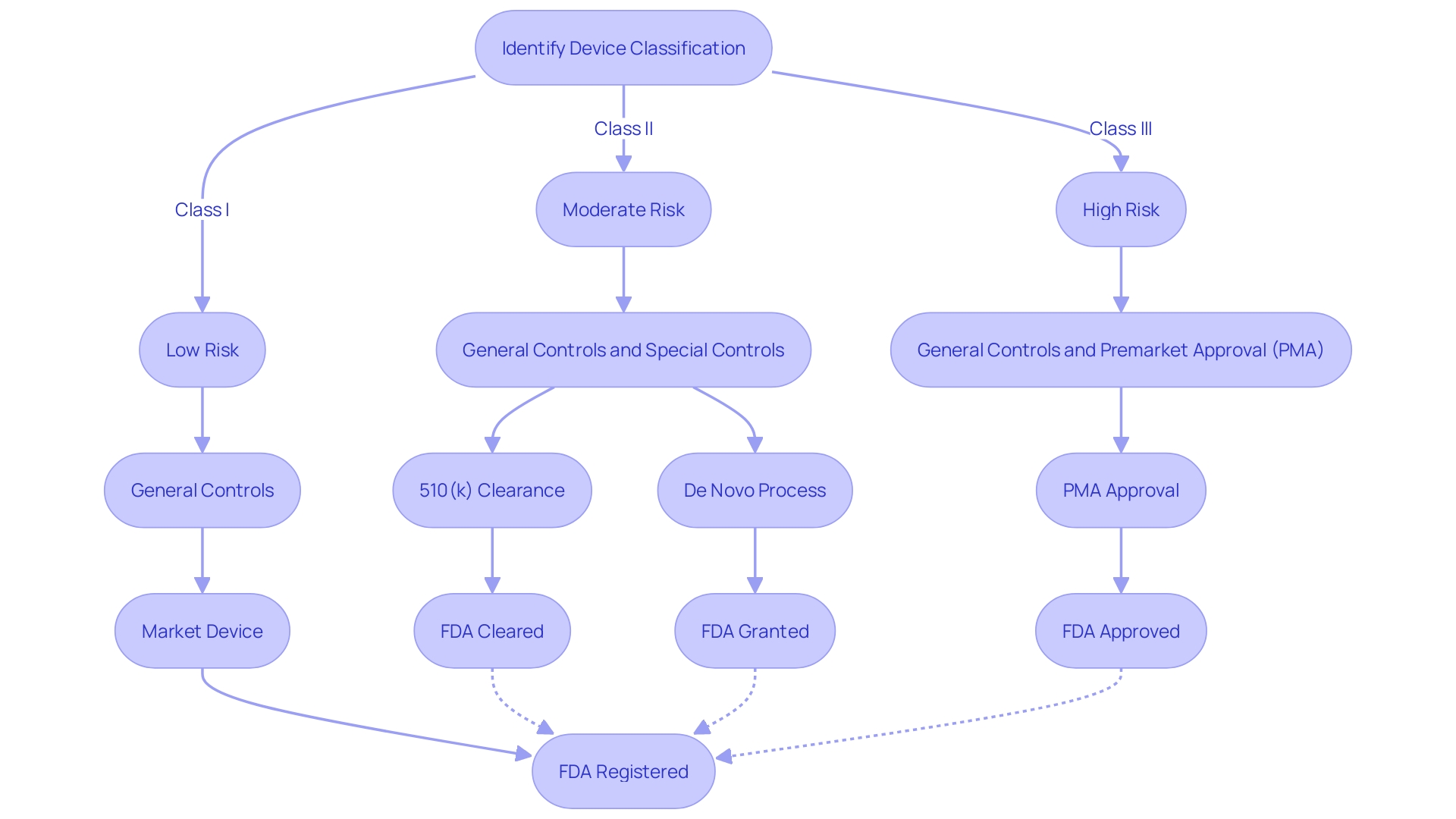
Importance of Correct Terminology
Understanding the intricacies of FDA regulations is essential for ensuring that medical devices meet the required safety and efficacy standards before they reach the market. The FDA categorizes medical devices into three classes based on the level of risk they pose to patients. Class I devices pose the lowest risk and often do not require premarket submission, while Class II and Class III devices, which carry higher risks, typically necessitate a more rigorous review process.
Manufacturers must navigate through distinct pathways such as Premarket Notification (510(k)), Premarket Approval (PMA), or De Novo classification to obtain the necessary FDA clearance, approval, or grant for their devices.
For a device to be marketed legally in the United States, it must be FDA Cleared, FDA Approved, or FDA Granted in the case of the De Novo process. The nuances among these terms are substantial. FDA clearance, typically via the 510(k) pathway, indicates that a device is substantially equivalent to another legally marketed device.
FDA approval, generally through the PMA process, is granted for high-risk devices and involves a more comprehensive review, including clinical data to support efficacy and safety. Meanwhile, the De Novo process is a route for novel devices of low to moderate risk that have no existing equivalent.
The FDA's mission to safeguard public health extends beyond medical devices to food, cosmetics, tobacco products, and more. However, the documentary 'The Bleeding Edge' exposed some of the pitfalls within the 510(k) clearance process, revealing that certain devices were fast-tracked without clinical trials, leading to adverse events including organ puncture and cobalt poisoning. While some devices were subsequently removed from the market, the documentary highlights the importance of transparency and vigilance in the regulatory process to prevent harm and maintain public trust.
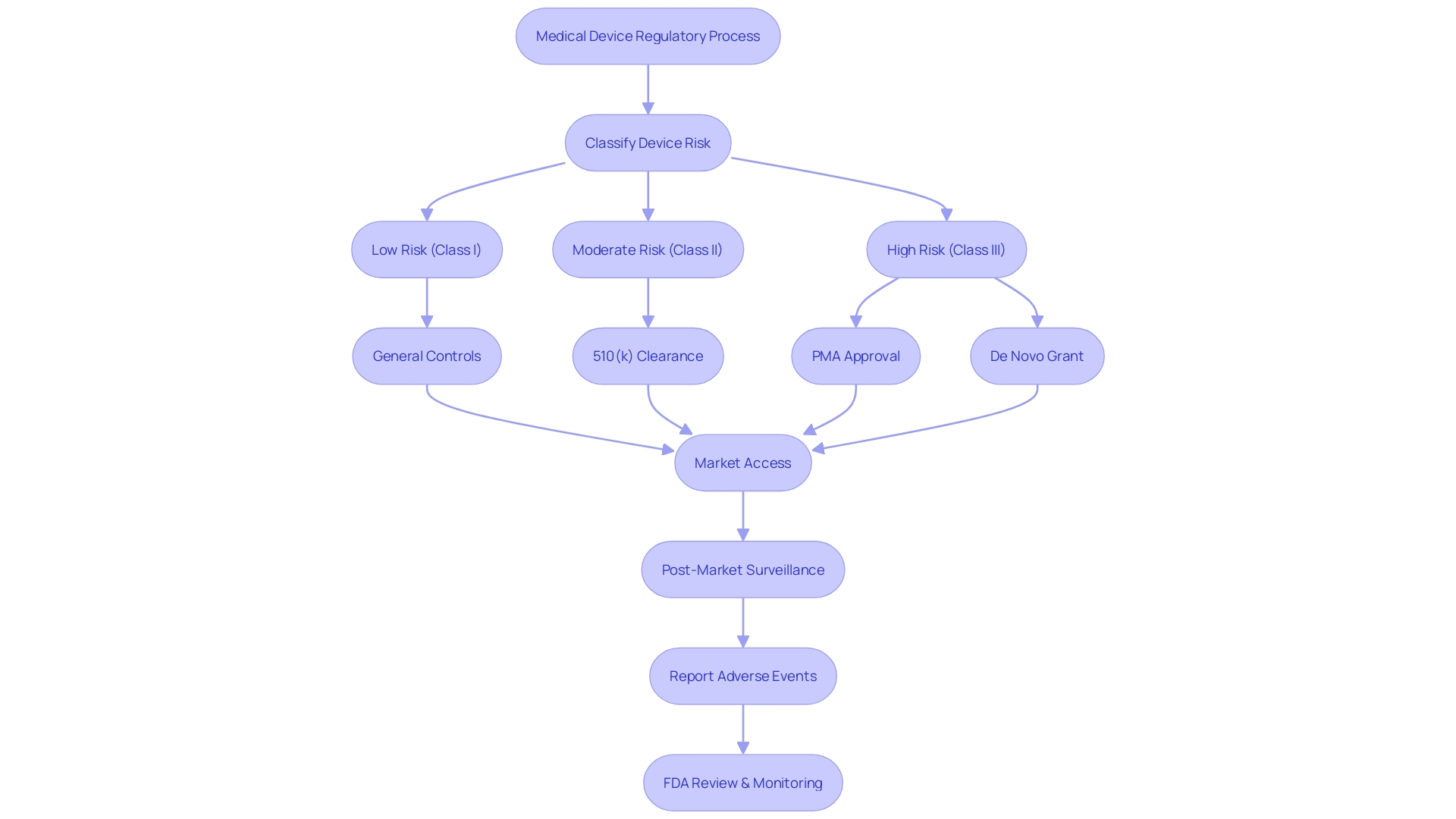
Consequences of Misusing FDA Terminology
The nuanced differences between FDA approval, clearance, and compliance are critical for healthcare professionals, patients, and the general public to understand. Misusing these terms can lead to significant misinterpretations of a medical device's safety and efficacy. For instance, a medical device that has been 'cleared' by the FDA has met certain regulatory requirements for sale, but this does not equate to the rigorous process of 'approval,' which involves a comprehensive evaluation of safety and effectiveness for the device's intended use.
One notable case that highlights the consequences of miscommunication regarding FDA terminology involves Philips Respironics. The company had to recall its sleep apnea devices due to safety concerns, but later claimed the devices were not expected to cause long-term health consequences based on new tests. Such a reversal can cause confusion and potentially undermine public trust.
Furthermore, the FDA's regulatory landscape is complex, as demonstrated by the challenges associated with classifying products such as foods and dietary supplements compared to drugs and vaccines. The case of probiotics and their potential to prevent necrotizing enterocolitis (NEC) illustrates the tension between providing accurate health information and adhering to regulatory classifications.
The FDA's role extends beyond the initial approval or clearance of products. As highlighted by the agency's continuous assessment of vaccines, including addressing myths like the disproven link between vaccines and autism, the FDA's regulatory vigilance is ongoing.
In the realm of medical devices, a lack of clarity can lead to significant implications. Servicing and remanufacturing of devices, for example, require precise guidelines to avoid unintentional modifications that could lead to non-compliance with federal law. The FDA's guidance on these matters is crucial for ensuring the safety and intended use of medical devices throughout their lifecycle.
Statistics from a 2018 documentary on the FDA's medical device clearance process reveal that not all devices undergo clinical trials before reaching the market. The 510(k) clearance process allows some devices to be fast-tracked based on similarity to existing products. However, this process has led to adverse events, including bleeding and organ puncture, underscoring the importance of accurate representation of FDA regulatory actions.
In light of ongoing challenges, such as combating misinformation during the Covid-19 pandemic, the FDA has provided draft guidance on how companies should address misinformation about their products. It's crucial for manufacturers to accurately communicate the status of their products in relation to FDA regulations to maintain their reputation and ensure public safety.
Conclusion
In conclusion, the FDA plays a crucial role in safeguarding public health by regulating various products, including medical devices, pharmaceuticals, food, cosmetics, dietary supplements, radiation-emitting products, and tobacco. Their responsibilities encompass ensuring the safety, efficacy, and security of drugs, vaccines, and other biological products.
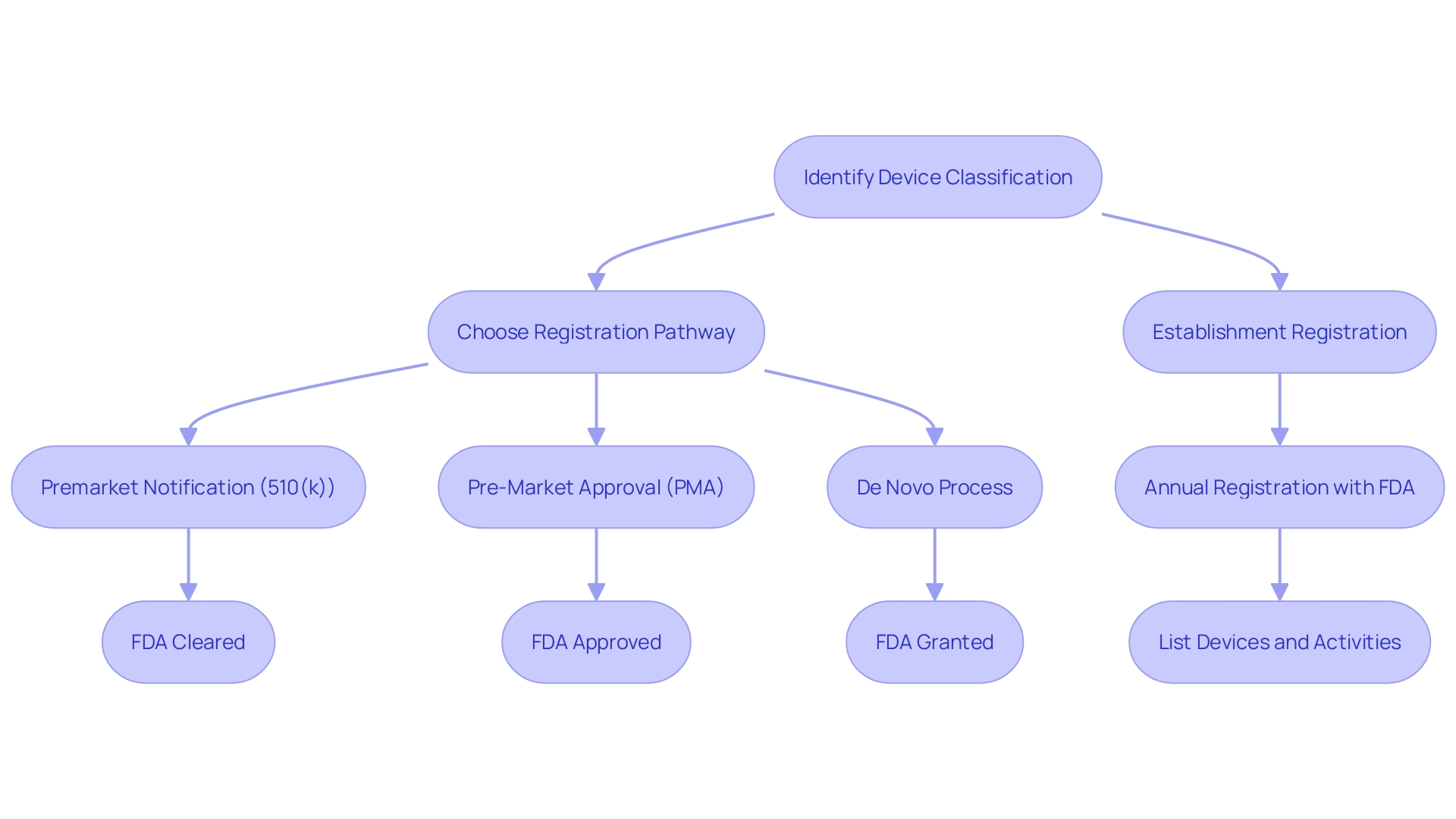
Understanding the FDA's classification system is essential for navigating the regulatory landscape of medical devices. Devices are categorized into Class I, Class II, and Class III based on their associated patient risk levels. The pathway to market for a device depends on its classification, with Class I devices requiring general controls, Class II devices often needing a 510(k) clearance, and Class III devices typically requiring Pre-Market Approval (PMA).
It is important to distinguish between FDA approval and clearance. Approval is for high-risk devices and new drugs, involving a rigorous review of scientific evidence, clinical data, and manufacturing information. Clearance, on the other hand, is a pathway for moderate to low-risk medical devices, where the device must be substantially equivalent to a predicate device already on the market.
Obtaining FDA approval or clearance for medical devices is a meticulous process involving preclinical testing, comprehensive clinical trials, and the submission of thorough applications to the FDA for review. The FDA's commitment to public health is exemplified by its recent clearance of innovative therapeutic options for chronic diseases, offering new technologies that improve patient management.
Using correct FDA terminology is crucial for regulatory compliance and ensuring the safe introduction of medical devices into the healthcare market. Misusing FDA terminology can lead to significant misinterpretations of a device's safety and efficacy. It is crucial to understand the distinctions between terms like "cleared," "approved," and "granted" to accurately communicate the regulatory status of a medical device.
Navigating the FDA's regulatory landscape is complex but essential for companies in the healthcare industry to ensure that their products reach the market legally, safely, and effectively. The FDA's role extends beyond initial approval or clearance, with continuous assessment and vigilance to protect public health. Accurate representation of FDA regulatory actions is crucial to maintaining public trust and ensuring the safety of medical devices.




