Introduction
The regulatory landscape for medical devices can be complex and daunting, especially when it comes to understanding the processes of FDA clearance and approval. These terms, often used interchangeably, actually have distinct meanings and implications. In this article, we will delve into the key differences between FDA clearance and approval, highlighting the importance of using the correct terminology.
We will also explore the consequences of misusing these terms and the impact it can have on both manufacturers and patients. By gaining a clear understanding of these regulatory distinctions, we can ensure the safety and efficacy of medical devices while facilitating their timely availability to those in need. So, let's dive in and demystify the world of FDA clearance and approval.
Understanding FDA Clearance
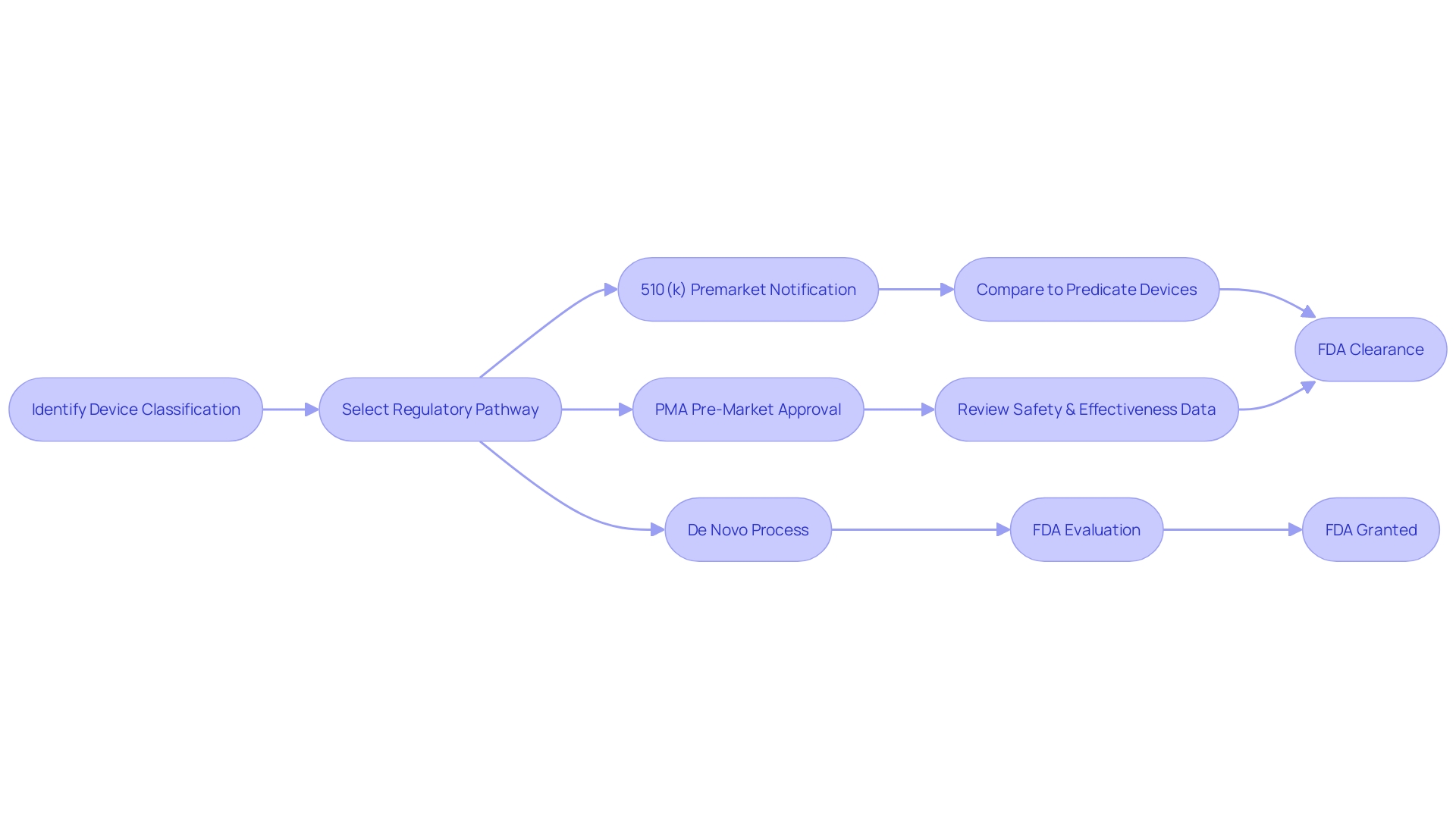
The Premarket Notification 510(k) pathway, often referred to simply as 'FDA clearance,' is a crucial regulatory process for medical devices classified as low to moderate risk. To initiate this process, manufacturers must first determine the correct classification of the device, which can range from Class I to Class III based on the level of risk the device poses to the patient. Class I and II devices, which are considered lower risk, generally follow the 510(k) pathway, where a new device is compared to an already legally marketed device, a predicate, to demonstrate it is 'substantially equivalent' in terms of safety and effectiveness.
This comparison is vital as it determines whether the new device can be cleared for market without requiring the more rigorous Pre-Market Approval (PMA) process reserved for Class III devices, which are higher risk and often life-sustaining. The 510(k) process has been under scrutiny, as highlighted by the 2018 documentary 'The Bleeding Edge,' which revealed that clinical trials might not be mandatory for many devices cleared under this pathway, a fact that has led to some devices causing patient injuries.
Recognizing the evolving landscape of medical technology, the FDA has been updating its guidance to better address the challenges of new device innovation, such as those presented by digital health and personalized medicine. Notably, there has been a concerted effort to streamline regulatory pathways to expedite the approval process for devices that fulfill unmet medical needs, especially pertinent in the wake of the COVID-19 pandemic.
A clear understanding of these regulatory distinctions and processes is an essential component of medical device development and commercialization. As the industry continues to advance with innovations like medical 3D printing, which is projected to generate $4 billion by 2026, staying abreast of the latest FDA regulatory changes and requirements remains a priority for ensuring patient safety and bringing new medical solutions to market.
Understanding FDA Approval
The FDA's approval process for high-risk medical devices, such as Class III devices that are crucial in sustaining or supporting life, is indeed a more comprehensive evaluation compared to the clearance process for lower-risk devices. Class III devices, which include advanced technologies like the HeartMate 3 Left Ventricular Assist System, are subject to rigorous scrutiny due to their significant role in patient care. These devices must provide clinical data to demonstrate their safety and efficacy before they can be approved.
This is to ensure that they safely fulfill their intended purpose, especially since they are not substantially equivalent to any other legally marketed device.
Moreover, the FDA's role in safeguarding public health extends beyond approval, as evidenced by cases where post-market surveillance identifies potential risks not previously recognized. For instance, the Philips Respironics' recall of sleep apnea devices, due to concerns about carcinogenic particles from industrial foam, demonstrates how the FDA continues to monitor and act on safety issues even after initial market entry. This vigilance is crucial in maintaining trust in medical devices that millions of patients rely on.
The FDA's regulatory framework is designed to balance the need for innovation with the imperative of patient safety. Recent efforts to streamline regulatory pathways and improve collaboration between the FDA and industry stakeholders, especially during the COVID-19 pandemic, have aimed to expedite the approval process for devices that address unmet medical needs. Nonetheless, the agency maintains stringent standards, as exemplified by the recent guidelines for direct-to-consumer prescription drug advertisements, ensuring that major statements about drug side effects are presented in a clear, conspicuous, and neutral manner.
In summary, the FDA approval process for high-risk medical devices is a critical step in ensuring that these devices provide their intended benefits without posing undue risks to patients. The process involves thorough evaluation of clinical data and ongoing post-market oversight to protect public health.
Key Differences between FDA Clearance and Approval
Navigating the regulatory landscape of medical device approval by the FDA involves a keen understanding of the classification system which stratifies devices according to their associated patient risk levels. Low to moderate-risk devices typically follow the Premarket Notification, or 510(k), pathway, which allows a new device to be marketed by demonstrating that it is substantially equivalent to a legally marketed device that is not subject to Premarket Approval (PMA). On the other hand, high-risk devices must undergo the more rigorous PMA process, which demands comprehensive clinical data to support claims of safety and effectiveness.
The 510(k) clearance process, while less stringent, ensures that a device performs similarly to its predicate without necessitating extensive clinical studies. This pathway often expedites the availability of innovative technologies to patients, as observed with the inclusion of heart monitoring functions in a widely-used wearable technology in 2018. In contrast, the PMA process involves a detailed review, including a thorough evaluation of clinical trial data to assess the high-risk device's safety and efficacy, addressing the potential for significant patient injuries.
Moreover, the FDA's adaptability in accommodating new technologies within established regulatory frameworks facilitates market entry and subsequent real-world application, enhancing the healthcare landscape. For example, the FDA's firm-based approach to the regulation of novel medical devices, as demonstrated in the clearance of consumer-focused wearable technology, reflects a balance between rigorous oversight and the encouragement of innovation.
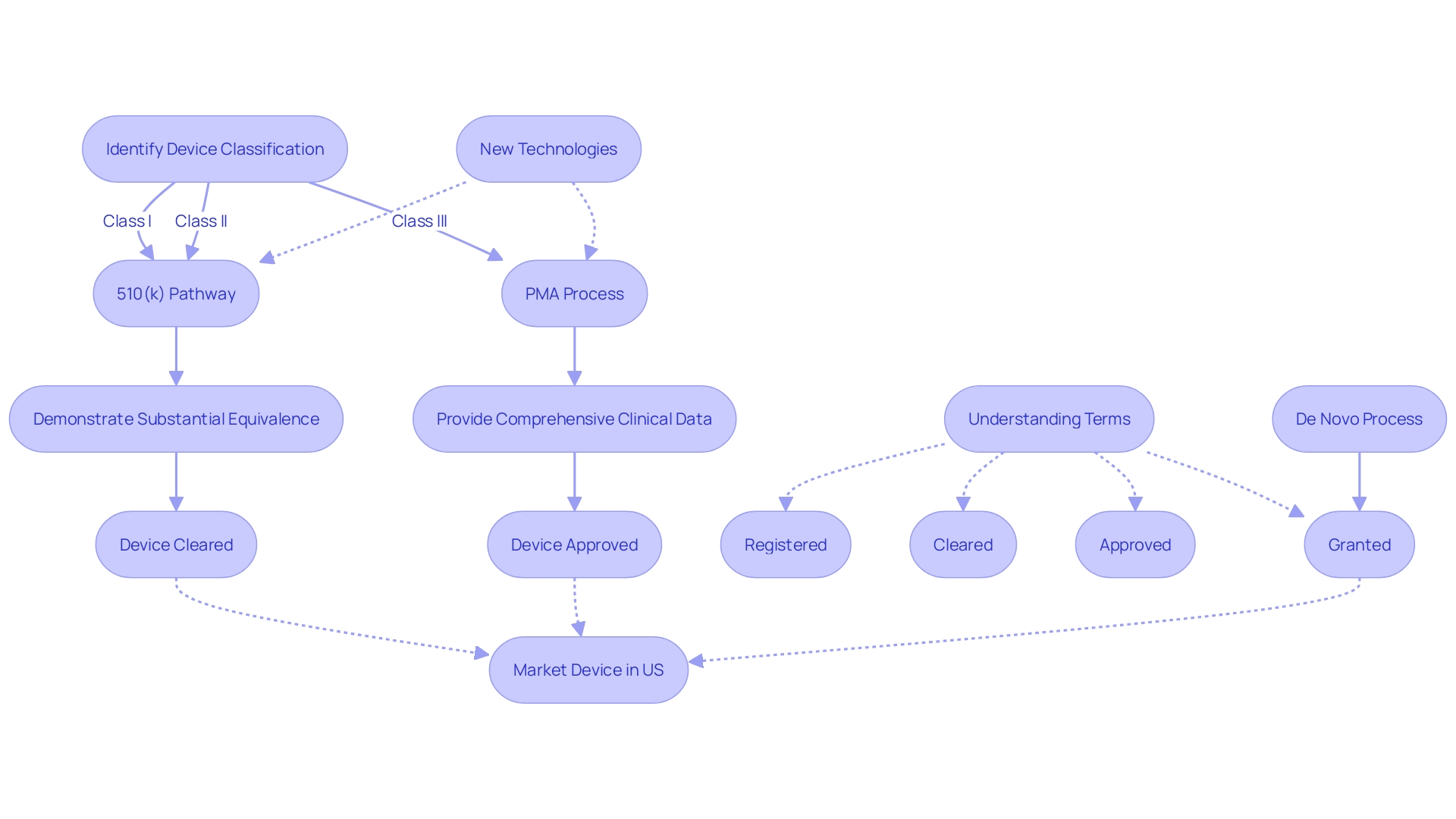
It's important for regulatory professionals to discern the nuances between terms such as 'Registered', 'Cleared', 'Approved', and 'Granted,' as each signifies a different level of FDA evaluation and oversight. This distinction is crucial in the context of medical device regulation, where the safety and efficacy of products must be assured before they reach consumers.
Importance of Correct Terminology
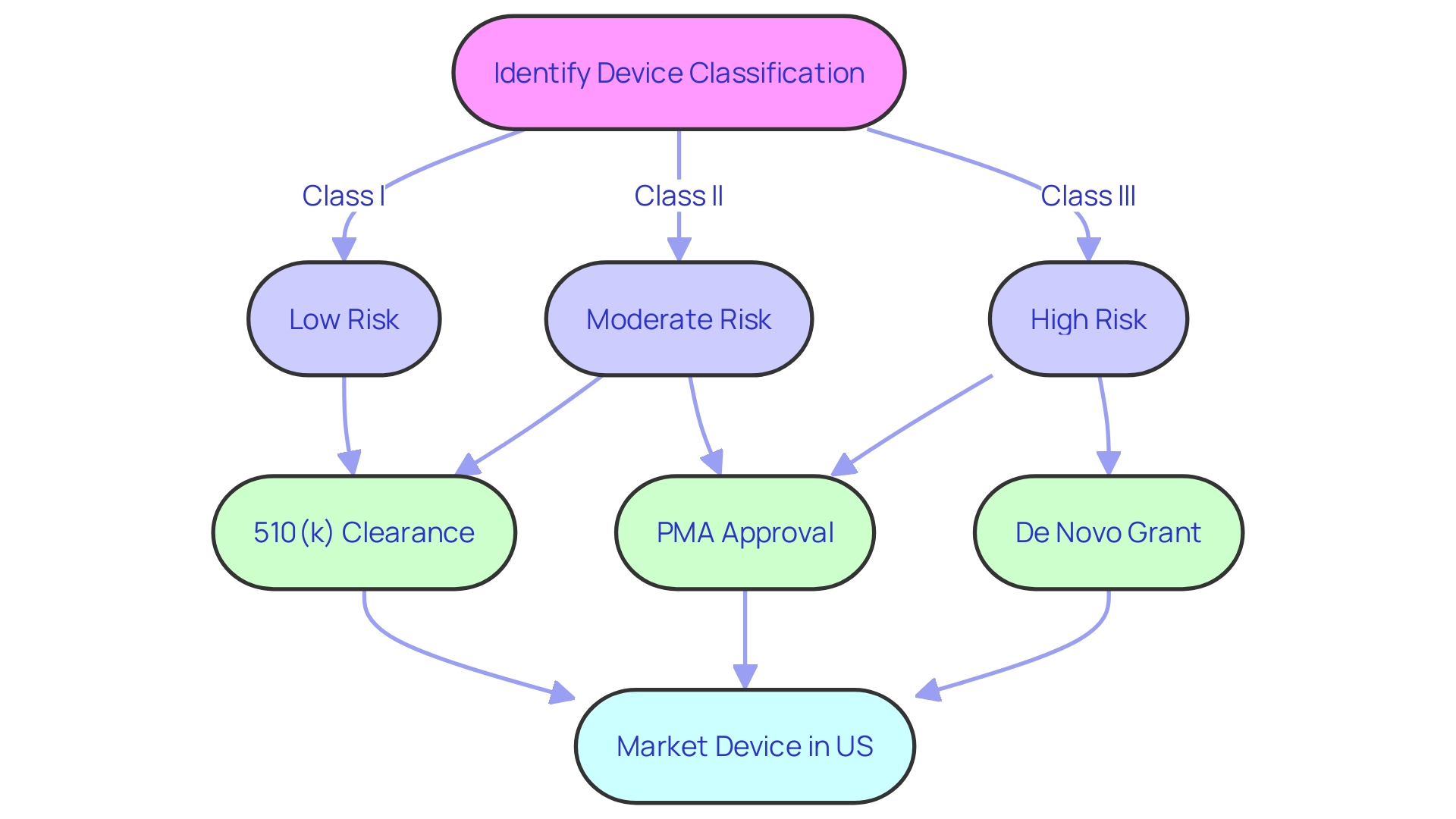
The precise use of 'FDA cleared,' 'FDA approved,' and other regulatory terms is a cornerstone of medical device marketing, as it guides healthcare professionals and patients in their understanding of a product's safety and efficacy. The FDA classifies medical devices into three categories, each reflecting a different level of risk to patients. Determining a device's classification is the initial step in selecting the appropriate FDA registration pathway—be it Premarket Notification (510(k)), Pre-Market Approval (PMA), or the De Novo process.
Only after receiving FDA Clearance, Approval, or a Grant through the De Novo process can a device be lawfully marketed in the United States. Each term—Registered, Cleared, Approved, and Granted—carries a distinct meaning, and even seasoned regulatory professionals sometimes struggle with their nuances. Nevertheless, understanding and accurately conveying these differences is imperative for maintaining regulatory compliance and upholding public safety, as underscored by the FDA's mission to ensure the efficacy and security of medical devices.
Consequences of Misusing FDA Clearance and Approval Terms
The distinction between FDA approval, clearance, and compliance is not just semantic but carries significant legal and regulatory weight. Medical device manufacturers must navigate the FDA's classification system, which categorizes devices based on the risk they pose to patients. Devices are classified into three levels, with each level dictating the type of premarket submission required.
For lower-risk devices, a 510(k) premarket notification might suffice, allowing a device to be "cleared" if it is substantially equivalent to a legally marketed device. Higher-risk devices may require a rigorous Pre-Market Approval (PMA) process, resulting in "approval" after demonstrating safety and effectiveness. The De Novo pathway, on the other hand, provides a route for novel, low to moderate risk devices without a valid predicate to be "granted" marketing authorization.
The implications of misrepresenting a device's status are serious. Manufacturers incorrectly claiming FDA approval instead of clearance or grant may face stringent regulatory actions, including financial penalties. Such missteps can also tarnish a company's reputation, potentially causing long-term damage.
A recent draft guidance from the FDA amidst the Covid-19 pandemic highlights the agency's efforts to combat misinformation about medical products. This emphasizes the importance of accurate representation of a device's regulatory status.
To prevent such costly errors, it is crucial for companies to ensure that submissions, electronic or paper, do not inadvertently disclose confidential information. The FDA has detailed procedures for submissions that contain confidential data, underscoring the responsibility of the applicant to protect sensitive information.
Understanding and appropriately communicating the nuances of FDA regulatory terms is vital. It not only ensures compliance with federal regulations but also safeguards the integrity of the medical device industry and protects public health. Regulatory professionals and manufacturers alike must be diligent in their representations to uphold these standards.
Conclusion
In conclusion, understanding the distinctions between FDA clearance and approval is crucial for navigating the complex regulatory landscape of medical devices. FDA clearance, through the 510(k) pathway, is used for low to moderate-risk devices, while FDA approval is required for high-risk devices.
The FDA's regulatory framework balances innovation with patient safety, streamlining pathways and expediting approval for devices addressing unmet medical needs. However, strict standards and post-market surveillance ensure ongoing safety.
Using the correct terminology, such as "FDA cleared" or "FDA approved," accurately conveys a device's regulatory status and ensures compliance. Misusing these terms can result in legal and regulatory consequences, including penalties and reputational damage.
Understanding FDA clearance and approval processes is vital for device development and commercialization, ensuring safety and efficacy while facilitating timely availability. Regulatory professionals and manufacturers must be diligent in their representations to uphold standards and maintain the integrity of the medical device industry.




