Introduction
Informed consent is a critical ethical and legal principle in healthcare and research, ensuring the protection of individual autonomy, patient well-being, and the integrity of the medical process. It empowers individuals to make informed decisions about their healthcare and research participation by providing them with comprehensive knowledge about the nature of the procedure, associated risks and benefits, reasonable alternatives, and the risks and benefits of those alternatives. The informed consent process aims to foster patient autonomy, shield patients from harm, deter deceptive practices, and encourage sound decision-making.
However, recent challenges have emerged in obtaining informed consent, including time constraints and complexity of consent documents. This has led to the exploration of conversational artificial intelligence and large language models to enhance patient understanding. Additionally, there is a need to simplify and clarify consent documents, which have become cumbersome and difficult for patients to comprehend.
The article explores the importance of informed consent in light of technological advancements, regulatory changes, and the need for continuous attention to ensure it remains a meaningful tool for patient empowerment and participation in healthcare and research.
The Principle of Autonomy and Informed Consent
Informed consent is not merely a formality, but a fundamental ethical and legal cornerstone in healthcare and research, ensuring the protection of individual autonomy, patient well-being, and the integrity of the medical process. At its core, informed consent is about empowering individuals with the requisite knowledge to make enlightened decisions regarding their healthcare and research participation. This involves a comprehensive understanding of the procedure's nature, the associated risks and benefits, reasonable alternatives, and the risks and benefits of those alternatives.
A key objective of the informed consent process is to foster patient autonomy, yet it also serves to shield patients from harm, deter deceptive practices, and encourage sound decision-making. Though consent is often presumed for standard care procedures with minimal risk, like venipuncture or routine imaging, it is obligatory and detailed for interventions such as HIV or genetic testing due to their sensitive nature.
Recent challenges have surfaced in the practical application of informed consent, particularly when the responsibility of obtaining consent is assigned to clinical team members who may lack the time or expertise to fully inform patients. This has sparked interest in leveraging conversational artificial intelligence and large language models to enhance patient understanding and autonomy.
Furthermore, informed consent documentation has grown in complexity over the years, leading to documents that are cumbersome and challenging for patients to understand. This has resulted in barriers to clinical trial enrollment, especially among minority populations, and dissatisfaction among stakeholders. The shift towards more legalistic and lengthy documents, now often surpassing twenty pages, has not been met with efforts to simplify and clarify these critical documents. This is despite evidence that the majority of U.S. adults find current consent documents difficult to comprehend.
Amidst these developments, recent news has highlighted the potential for technological advancements, such as Neuralink's brain-computer interface, to revolutionize patient care and research, underscoring the importance of informed consent in the adoption of novel medical technologies. Simultaneously, regulatory changes, like the amendment to the 21st Century Cures Act, have raised concerns about the preservation of informed consent, especially in situations where research poses minimal risk to participants.
In summary, informed consent is a dynamic concept that requires continuous attention to ensure that it remains a meaningful tool for patient empowerment, protection, and participation in healthcare and research.
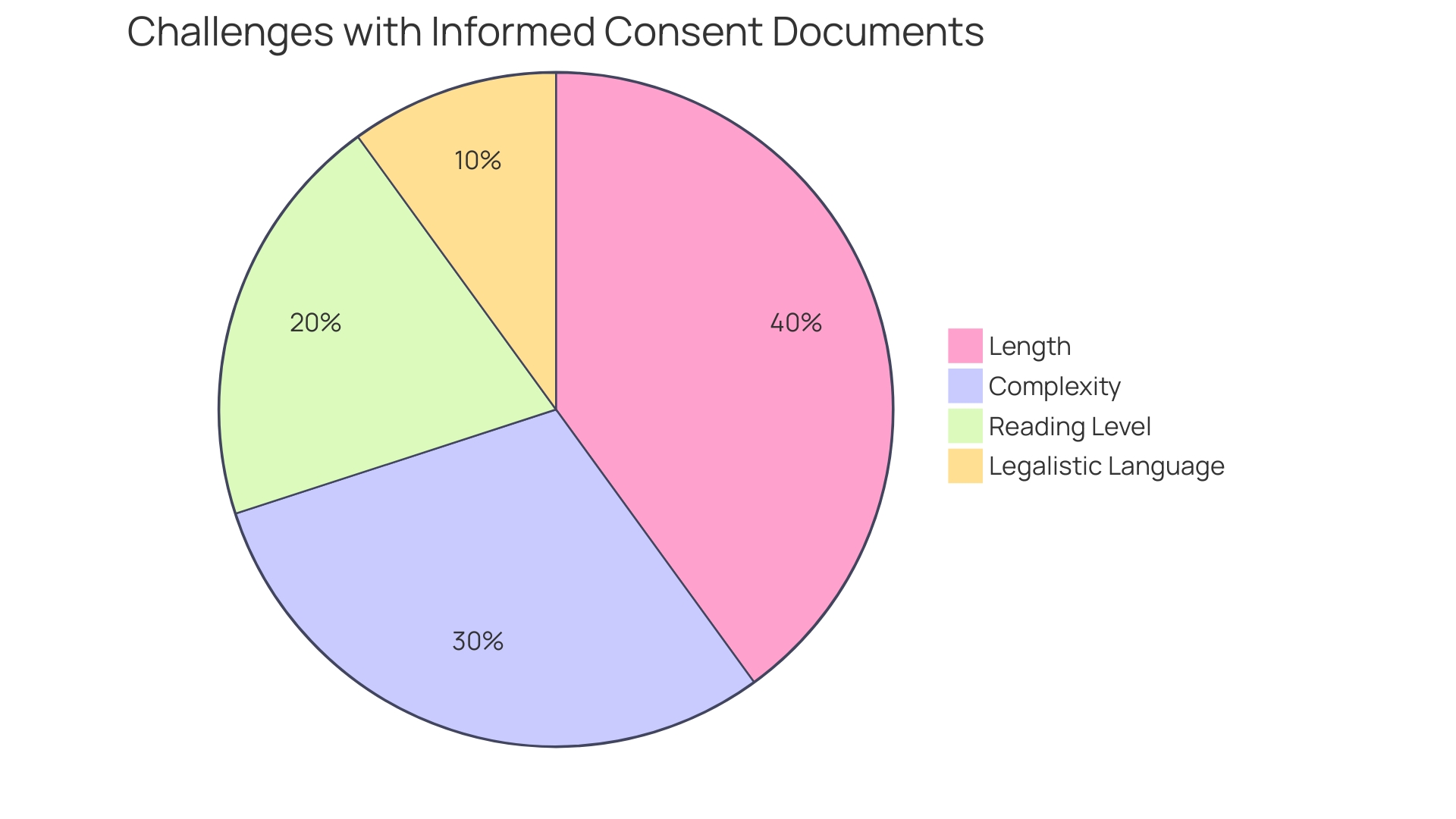
Basic Elements of Informed Consent
Informed consent is a foundational component of both ethical and legal frameworks in healthcare and research, serving to safeguard patient autonomy and ensure well-informed decision-making. At its core, informed consent must transparently outline the nature of the procedure, including any risks and benefits, as well as present reasonable alternatives and their respective risks and benefits. A comprehensive understanding of these elements by the patient is essential.
Recent analyses of informed consent forms from major hospitals have revealed a trend towards complexity, with many documents written at a challenging reading level. This presents a significant barrier, particularly for underserved minority populations, potentially impacting clinical trial enrollment and patient comprehension. In fact, a study has shown that the majority of U.S. adults find consent documents difficult to understand, indicating a pressing need for simplification and clarity.
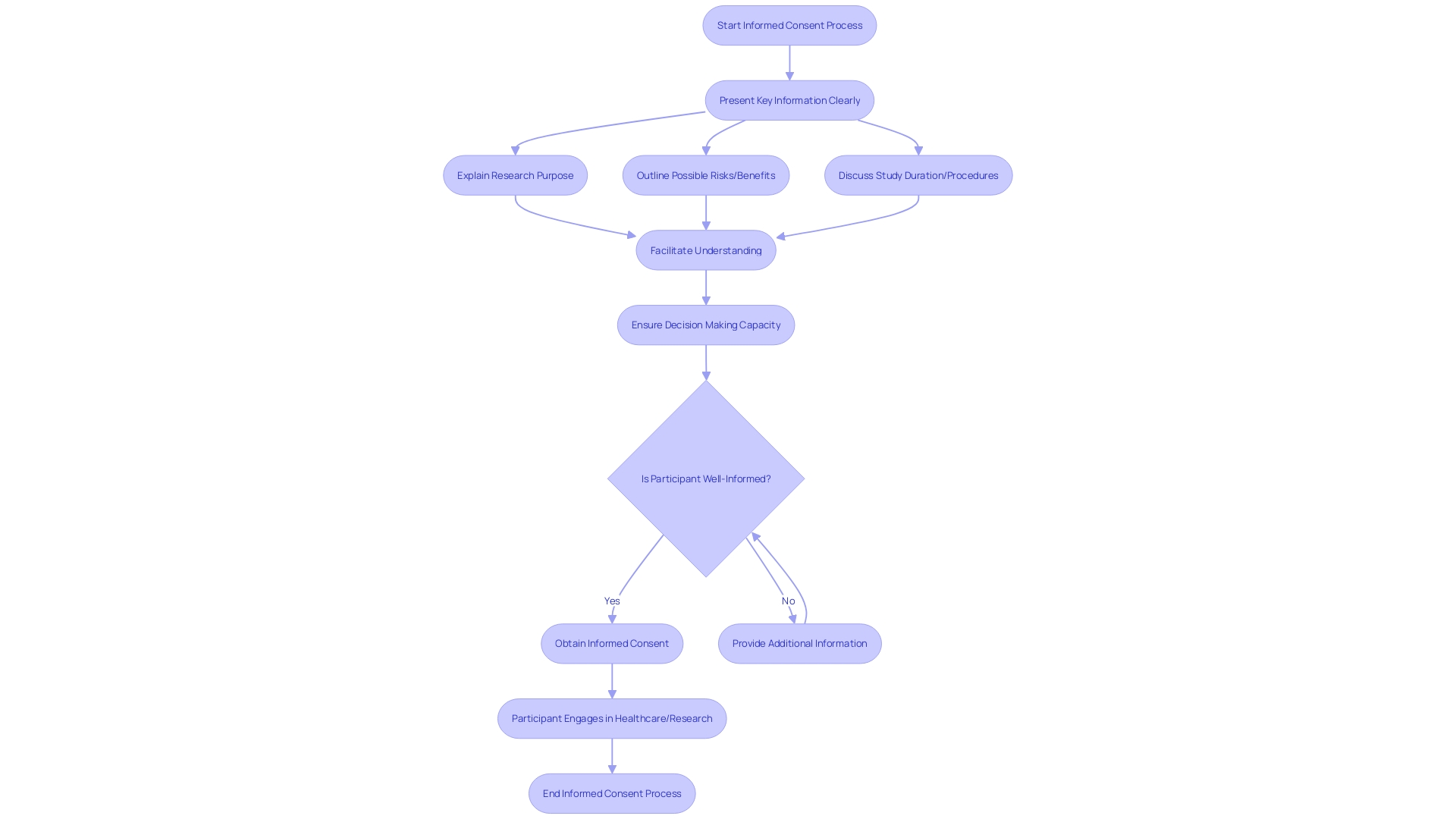
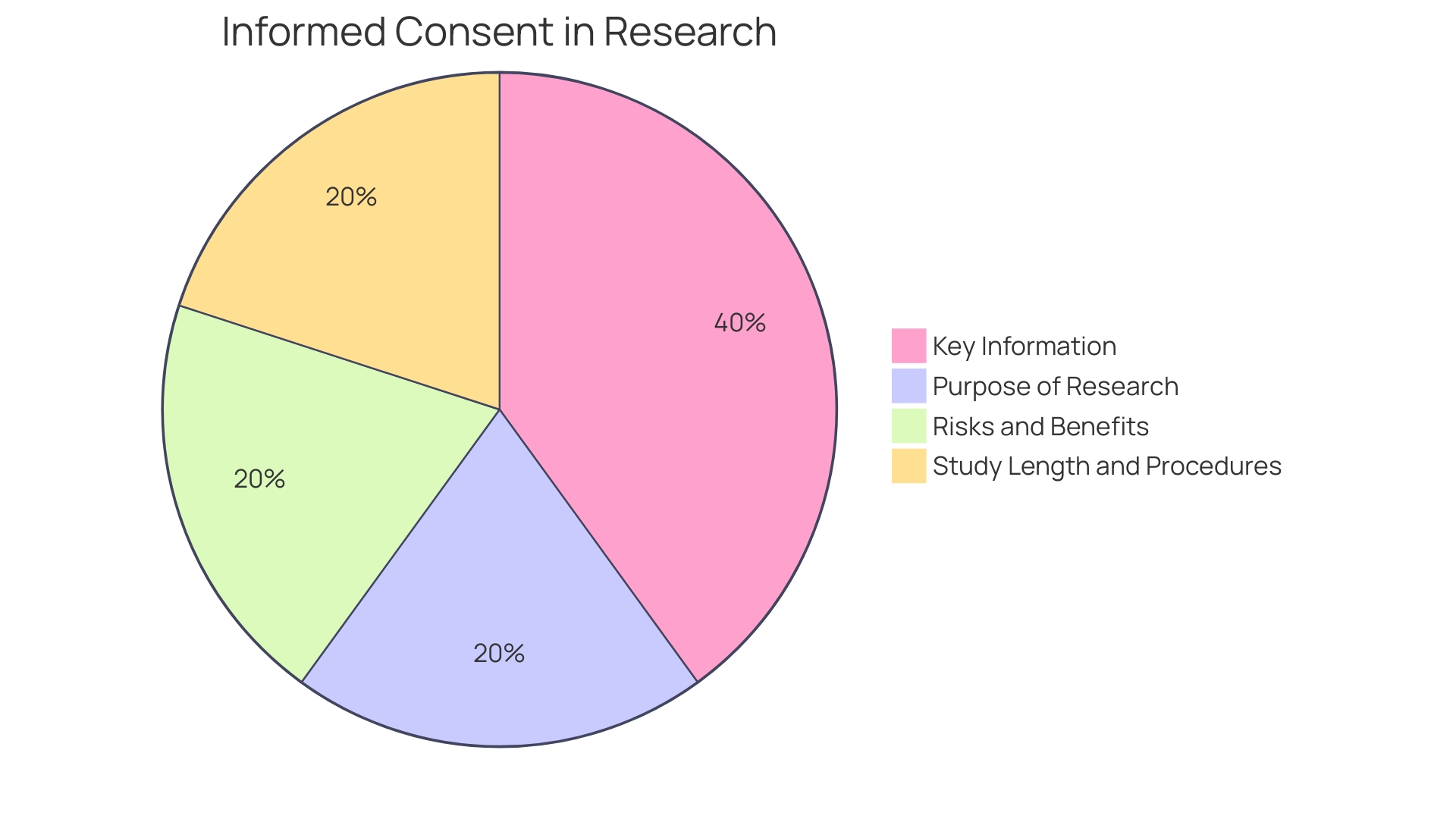
To address these concerns, draft guidance recommends that informed consent should begin with key information presented in a clear and concise manner. This includes the purpose of the research, possible risks and benefits, and the length and procedures of the study. The intention is not merely to list isolated facts but to facilitate a deeper comprehension, aiding prospective subjects in making informed decisions about their participation in research studies.
Despite these recommendations, the reality remains that informed consent documents have, over the last two decades, grown from an average of three to four pages to often over twenty pages in length. The increase in length and legalistic language, partly in response to new legislation such as data privacy laws, has only compounded the issue of readability and accessibility.
In light of these developments, the informed consent process must evolve to better serve its primary purpose: to assist individuals in understanding their choices in healthcare and research settings. This entails creating documents that are both informative and comprehensible, thereby enhancing the consent process and upholding the ethical obligation to respect patient autonomy.
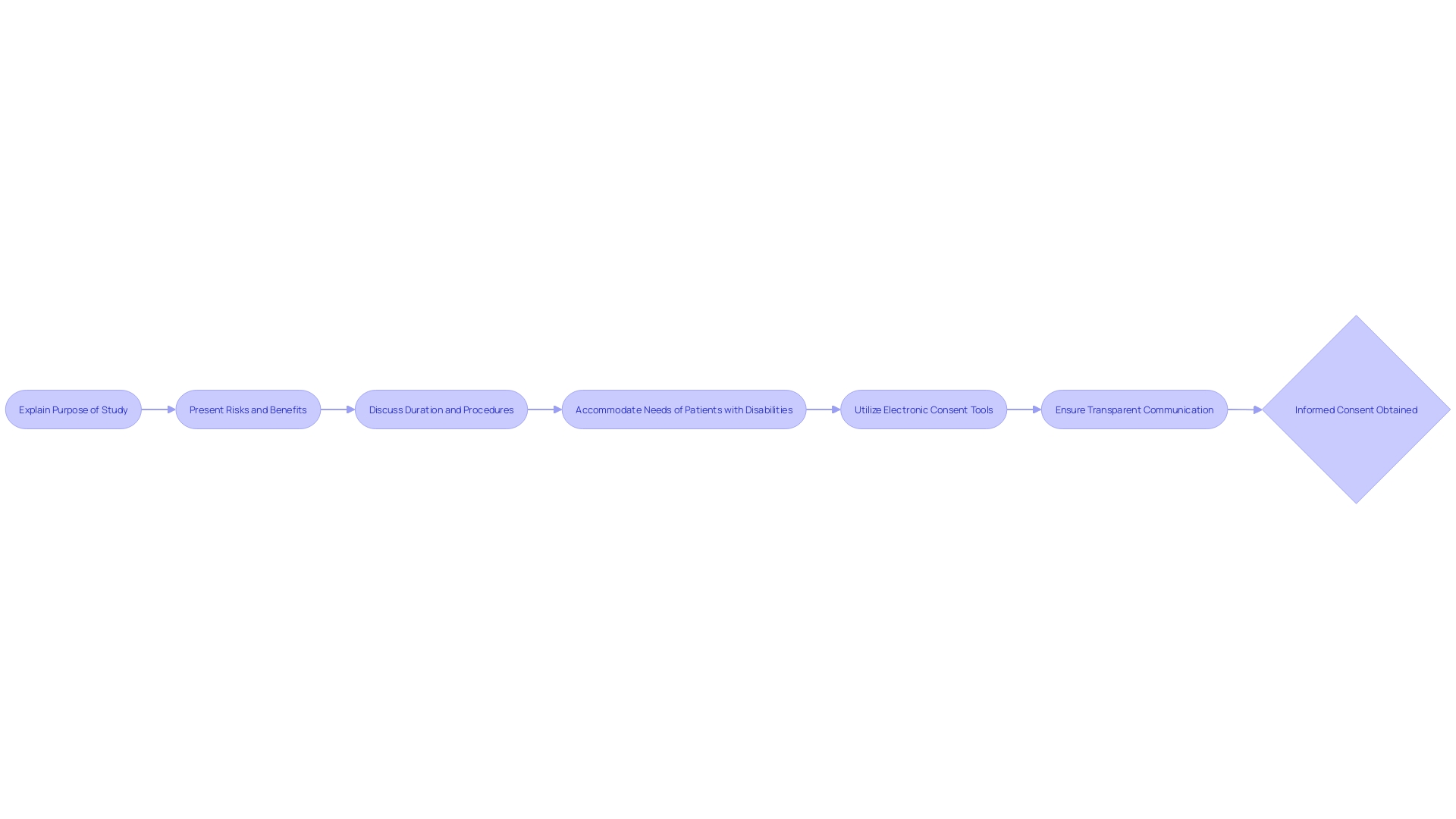
Decision-Making Capacity
Central to informed consent is the concept of decision-making capacity, a clinical judgment about the patient's ability to make specific healthcare decisions. It's crucial to clarify that 'capacity' is distinct from 'competence,' which is a legal term referring to a person's overall decision-making abilities and is adjudicated by the courts. Capacity, however, is evaluated by healthcare professionals and pertains to the patient's ability to understand treatment options, appreciate the consequences of their choices, and communicate their preferences.
Recent advancements in healthcare emphasize the importance of shared decision-making (SDM), where patients and clinicians collaborate, taking into account the best available evidence and the patient's values. This process is supported by tools such as Patient Decision Aids (PtDAs), which facilitate understanding of options in clear language, helping patients articulate their treatment preferences.
Reflecting on the evolving healthcare landscape, we see an integration of these concepts into the broader system. For instance, the partnership between Health Services Laboratories and Automata Technologies aims to deliver diagnostic results more swiftly, empowering patients with timely information essential for informed decision-making. Similarly, the establishment of Health Research Centers (HRCs) in the UK is poised to foster innovation in medical technology, with the potential to enhance the patient's involvement in healthcare decisions.
The Cochrane Database of Systematic Reviews underscores the efficacy of decision aids, pointing to improved patient knowledge, more accurate risk perceptions, and decisions more aligned with patient values. The latest update in 2024 incorporates 209 studies with 107,698 participants, reinforcing the role of decision aids in patient-centered care and decision-making processes.
In summary, informed consent is not merely a formality but a patient's right and a cornerstone of modern medical ethics. It requires a nuanced understanding of capacity, the availability of evidence-based tools like Peas, and the support of research and technological advances that prioritize patient empowerment in healthcare decisions.
Explanation of Facts, Benefits, and Risks
A pivotal component of informed consent is the elucidation of facts, benefits, and risks related to a healthcare intervention or research trial. This necessitates articulating the intent behind the procedure or study, the potential advantages for the participant, as well as the foreseeable risks and discomforts that may arise. The conveyance of this information must be accessible and considerate of the recipient's educational and cultural background, fostering an environment where they can make an educated decision about their involvement.
Recent trends show that informed consent documents have grown from a mere three or four pages to a staggering twenty pages or more over the past two decades. This expansion is attributed to an increase in legalistic language intended to meet legislative demands, such as data privacy laws, and to serve as critical documents in legal proceedings. Unfortunately, this complexity has led to a majority of U.S. adults finding these documents difficult to understand, contributing to a barrier to clinical trial enrollment, particularly among underserved minority groups.
To address this, new FDA draft guidance suggests that key information should be succinctly presented at the beginning of informed consent documents. This summary should include essential elements such as the study's purpose, potential risks and benefits, duration, and procedures, all aimed to aid comprehension. By emphasizing clarity and conciseness, this approach aims to remedy the current state of unwieldy consent documentation that has been a source of dissatisfaction among stakeholders—including IRBs, physicians, clinical trial sponsors, research participants, and regulators—for its excessive length and complexity. These recommendations serve as a call to action for collaborative efforts to significantly improve the informed consent process, reinforcing its foundational role in upholding ethical research practices.

Facilitation of Understanding
Informed consent is a process that relies on clear communication, ensuring participants fully understand the research and what it entails. It is essential to convey information in a straightforward manner, utilizing unambiguous language while avoiding technical terms that may be confusing. Charts and diagrams can prove instrumental in elucidating complex concepts. Opportunities for individuals to ask questions and seek further explanation are paramount to ensure a comprehensive understanding of the study they are considering joining.
The ethical foundations of informed consent are deep-rooted in significant historical documents such as the Declaration of Helsinki and the Belmont Report, which advocate for respect, autonomy, and informed, ongoing consent. These principles are echoed in recent guidelines, advocating for the presentation of key information at the forefront of consent documents to aid participant understanding of the study's purpose, duration, procedures, and potential risks and benefits. Such transparency not only honors ethical standards but also supports participants in making informed decisions about research involvement.
Real-world experiences underscore the value of informed consent, with participants like Barbara sharing how enrollment in a study can lead to unexpected health benefits. The use of platforms like The New Normal to recruit participants shows the evolving landscape of research engagement and the importance of accessible information. Meanwhile, controversies in studies outside of clinical research, such as the Twitter job market study, highlight the universal relevance of informed consent principles and the need for their stringent application across all research domains.
Translation quality in research is critical, moving beyond mere word-for-word translation to ensuring the intended meaning is preserved across languages. Translators must navigate document-specific needs, sometimes requiring strict adherence to source text, other times allowing for adaptative strategies to meet the translation's purpose, all while working collaboratively with researchers and community representatives.
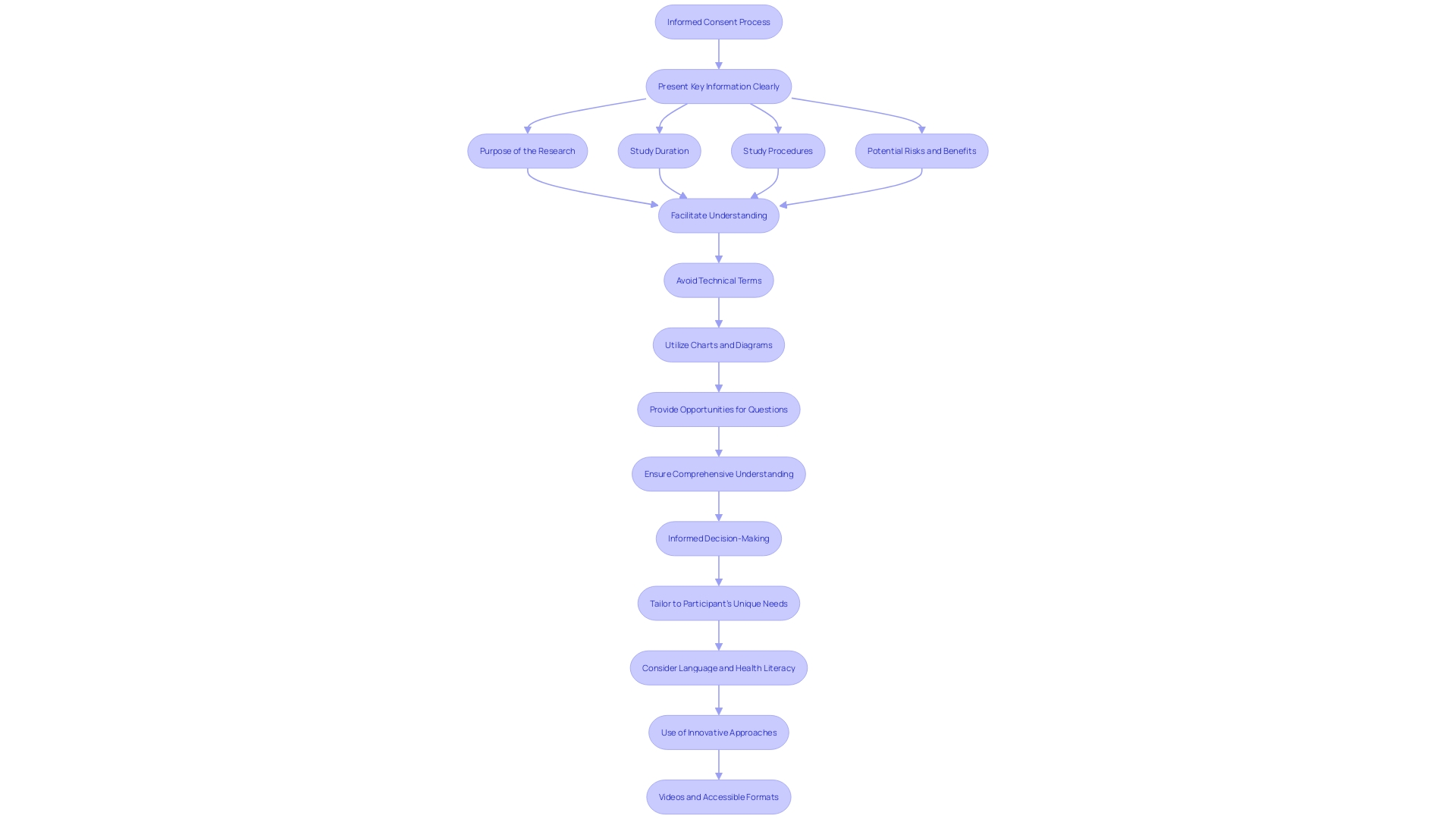
Voluntary Decision
Voluntariness is a cornerstone of informed consent, which mandates that individuals must have the autonomy to decide on their participation in clinical research without being subjected to coercion or undue influence. This fundamental principle ensures that decisions are made based on personal convictions, understanding of the information provided, and without fear of retribution or negative repercussions. In the context of increasingly complex and lengthy informed consent documents, there is a growing need to simplify and clarify these vital materials to avoid deterring participation, particularly among minority populations who are already underrepresented in clinical trials. Studies have revealed that many adults in the U.S. face challenges in understanding these documents, indicating the necessity for stakeholders in drug development to streamline the informed consent process. Recognizing the essence of informed consent, it is crucial to respect individuals' rights to make informed healthcare decisions and uphold their autonomy in the face of any health-related emergency. The World Medical Association's Declaration of Helsinki, which has been a guiding document for medical research ethics, is undergoing revisions that could address current issues and enhance the ethical conduct of research. Simplification efforts are paramount, as consent documents have ballooned from a mere three to four pages to over twenty pages in the last two decades, often written at levels too advanced for the general population to comprehend. The drive to improve these documents should be a coordinated effort across all stakeholders, from Institutional Review Boards (IRBs) to clinical trial sponsors and regulators, to ensure that the process of informed consent remains both meaningful and manageable.
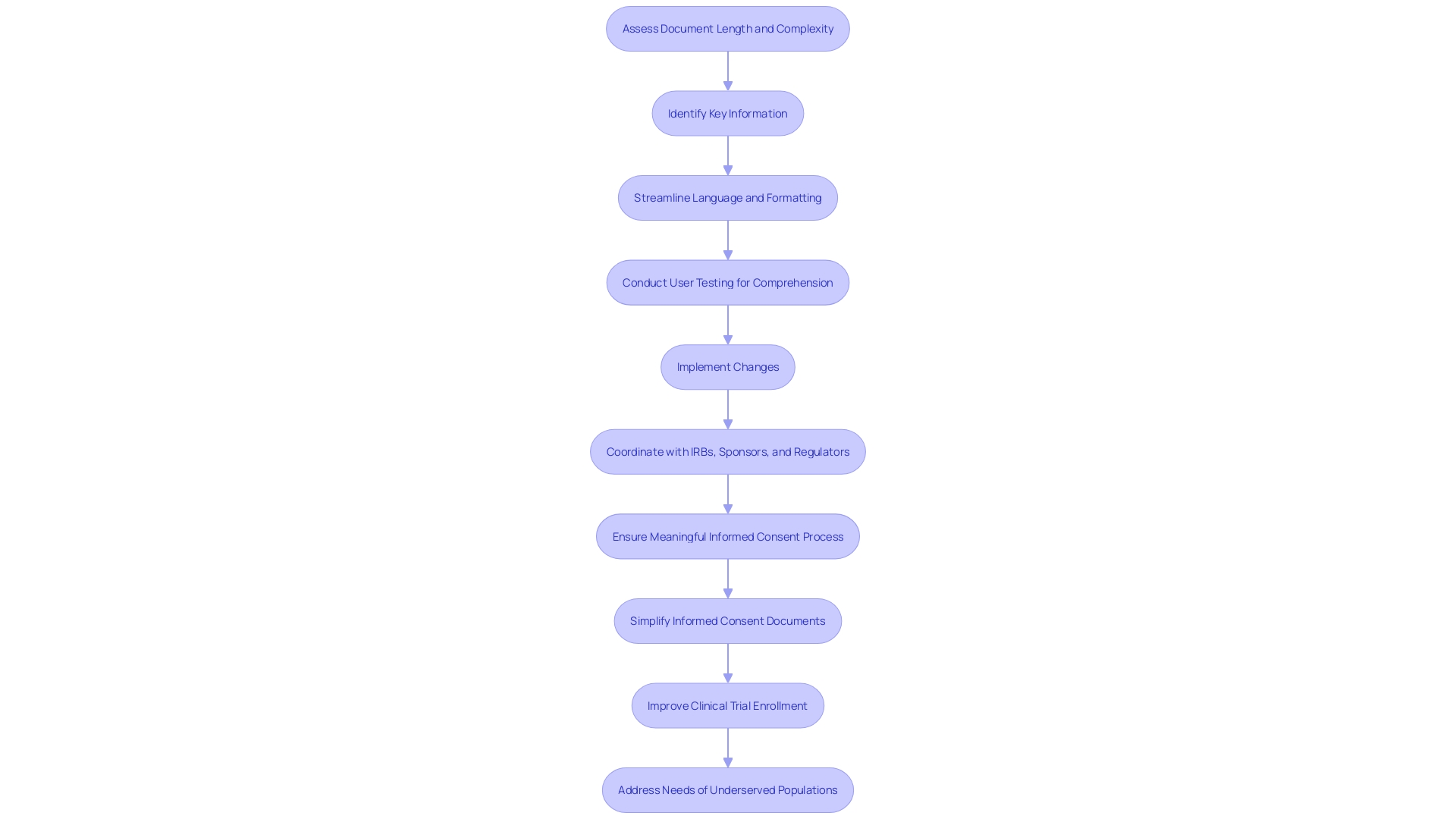
Detailed Components of Informed Consent
The informed consent process is an essential component of patient autonomy and ethical clinical research. It is a dynamic conversation between the healthcare provider or researcher and the patient or participant, aimed at ensuring that individuals are fully informed about their medical condition, treatment options, and the potential risks and benefits associated with those treatments. Over the years, informed consent documents have evolved to become quite complex, often posing a challenge for individuals to comprehend and make informed decisions, particularly among underserved minority populations. According to statistics, these documents have ballooned from an average of three to four pages to over twenty pages in the past two decades, becoming increasingly legalistic and written at a high reading level. This trend reflects a growing disconnect between the original intent of facilitating comprehension and the current state, where the sheer length and complexity can deter clinical trial enrollment and diminish the quality of the informed consent process.
The importance of informed consent is highlighted by experts who emphasize that it requires not just the provision of comprehensive and accurate information but also the freedom for individuals to make decisions without coercion or pressure. Key information should be presented clearly and concisely at the outset to help potential participants understand the study's purpose, duration, procedures, and the risks and benefits involved. This approach supports the core principle that informed consent is not merely about providing a list of isolated facts but about facilitating an understanding that empowers individuals to make well-informed decisions about their healthcare.

a. Purpose and Duration of the Procedure or Research
In the realm of healthcare and research, informed consent is not merely a formality but a fundamental component that respects the autonomy and decision-making capacity of participants. It is crucial for individuals to receive a transparent explanation of the research study's objectives as well as the healthcare procedure's purpose. Additionally, they should be clearly informed about the anticipated duration of their involvement. This clarity not only aids participants in understanding the reasons behind the research or procedure but also sets realistic expectations regarding the time investment required. The significance of conveying this information cannot be overstated, as it directly impacts the participant's ability to make an informed decision about their participation.
Recent investigations by ProPublica into vascular treatments for peripheral artery disease—a condition affecting over 6.5 million Americans—underscore the importance of informed consent. Findings revealed that some doctors may prematurely recommend invasive procedures, potentially exposing patients to unnecessary risks. This highlights the ethical imperative for clear communication about healthcare interventions.
Furthermore, expert problem solvers and researchers agree that understanding the problem is paramount before seeking solutions. This approach is mirrored in the formulation of a research question, which should be focused and answerable, guiding the methodology of the study. In instances where the human subjects' research component cannot be fully defined at the application stage, a 'delayed onset' designation is used, necessitating initial research results to finalize the human subjects study plans later.
These insights, combined with data revealing over 1.5 billion surgeries performed in 2022 and the distinct considerations for inpatient and outpatient procedures, emphasize the critical nature of informed consent. It ensures that participants are well-informed about the purpose, process, and time commitment of healthcare procedures and research studies, enabling them to make decisions that are in their best interest.
b. Description of Procedures and Identification of Experimental Procedures
Informed consent is essential, encompassing more than just a signature on a document; it is about ensuring individuals are fully aware of what they are agreeing to when participating in a healthcare intervention or research study. The process must begin with a presentation of the key information in a clear, concise, and understandable manner. Prospective participants should be made aware of the study's purpose, the potential risks and benefits, the duration, and the procedures involved. Central to the process is the need for information to be conveyed in a way that is easily comprehensible, helping participants make informed decisions about their involvement.
For consent to be genuinely informed, it must not merely be a list of facts but rather a facilitation of understanding. Over recent years, however, informed consent documents have become increasingly complex, lengthy, and more challenging to understand. This trend has been observed industry-wide, with documents expanding from an average of three to four pages to over twenty pages in the last two decades. The language used has also become more technical and legalistic, posing a significant obstacle, particularly for underserved minority populations, and affecting clinical trial enrollment. It is reported that the majority of U.S. adults find these documents difficult to comprehend, yet there has been no significant collective effort to improve their accessibility and understandability.
One innovative approach to addressing the complexities of informed consent is the potential use of conversational artificial intelligence (AI) with large language models to assist in the process. This AI could provide a means to offer clear, tailored explanations and support the consent process, promoting patient autonomy and informed decision-making. As such, the consent process must evolve to be more inclusive and patient-centered, ensuring that participants can make truly informed choices without the added burden of navigating through overly complex documentation.

c. Risks and Discomforts
A fundamental component of informed consent is the thorough delineation of potential risks and discomforts that may arise from a healthcare procedure or research study. Patients must be apprised not only of the known risks but also of any foreseeable complications, with clear communication regarding the probability and severity of such risks. Case studies, such as the experiences of individuals with Ehlers-Danlos Syndrome (EDS), highlight the importance of this principle. EDS patients often endure exacerbated symptoms due to invasive treatments, as illustrated by the story of Talia, who suffered unexpected consequences post-surgery. This underscores the critical need for patients to be fully cognizant of the possible adverse effects prior to consenting.
The discussion of risks is further complicated by the economic dynamics within healthcare, as demonstrated by the history of spinal epidural steroid injections. Initially a highly profitable procedure, changes in insurance reimbursements led to an increase in their frequency to maintain revenue, illustrating how financial incentives can influence medical practices and potentially impact the informed consent process.
Recent news also sheds light on the interconnectedness of conditions such as Parkinson's disease with autoimmune disorders, suggesting that patients must be informed of broader health implications when considering treatment options. Statistics reveal a striking figure: Americans undergo an average of 9.2 surgical procedures over a lifetime, with a peak operation rate at age 75. This prevalence amplifies the necessity for meticulous informed consent protocols to ensure patients are equipped with comprehensive information about the interventions they may encounter.
Informed consent is not merely a formality but a cornerstone of ethical medical practice, as enshrined in the Declaration of Helsinki. It is incumbent upon healthcare providers to ensure that consent is obtained without coercion, presenting all relevant information in a manner that is easily understood. This includes not only the research or procedure's purpose but also its duration, methods, and the balance of risks and benefits. Key information should be conveyed at the outset, serving as a guide for the consent discussion between the investigator and potential participants, and ensuring that patients are empowered to make truly informed decisions about their healthcare.
d. Benefits to the Subject or Others
Informed consent is a cornerstone of ethical healthcare and research, ensuring that individuals are thoroughly aware of what they are agreeing to when they undergo a medical procedure or participate in a study. This process includes explaining the potential benefits, which may range from personal health improvements to broader contributions to medical science. For example, patients participating in novel operational models such as those inspired by Formula One pit stops might see expedited surgical care with reduced waiting times, as seen in the streamlined processes where downtime in operating theatres is eliminated. Similarly, individuals involved in diabetes research contribute to tackling a global health crisis that not only impacts millions of lives but also imposes a significant economic burden.
The potential benefits relayed to participants must be framed within realistic expectations. Advanced therapies like stem cell treatments hold the promise of regenerating damaged tissues and testing individual responses to drugs, as described by leading scientists in the field. However, it is crucial to communicate the current stage of research and the realistic possibilities of clinical application. This honest dialogue supports the decision-making process of potential participants, who may be motivated by the chance to access cutting-edge treatments for conditions such as macular degeneration or to aid in the discovery of new therapies that could revolutionize the management of chronic diseases.
Moreover, the informed consent process must be clear and understandable. Analysis of procedure consent forms from high-volume hospitals reveals that complex language often obfuscates the risks and benefits, hindering patient comprehension. To address this, strategies such as presenting key information at the outset of the consent document—covering the purpose, risks, benefits, duration, and procedures of the study—are recommended to facilitate understanding.
Ultimately, informed consent is not just a legal formality but an educational resource empowering patients to make informed health decisions. By presenting potential benefits in a comprehensible and realistic manner, healthcare providers and researchers respect the autonomy of individuals and foster an environment of trust and transparency.
e. Alternative Procedures or Courses of Treatment
When determining the best course of action in healthcare or research, it is crucial for individuals to be fully informed about all available alternatives. For example, the case of Charlotte*, a 68-year-old diagnosed with diabetes, illustrates the impact of a patient-centric approach. Her treatment at the Royal Melbourne Hospital involved open dialogue about her values and preferences, leading to a personalized treatment plan that was both accessible and aligned with her goals.
Similarly, the digital-assurance process employed by certain healthcare trusts ensures that new technologies meet rigorous standards of security and compliance before adoption. This process includes a thorough initial assessment to verify whether the requested technology is already in use or available within the healthcare system, highlighting the importance of informed decision-making based on comprehensive information.
In the realm of surgical innovation, the recent acquisition of the da Vinci 5 multiport surgical system by Hackensack Meridian Hackensack University Medical Center demonstrates the significance of informing patients about cutting-edge treatment options. The da Vinci 5 system offers unprecedented precision and control, potentially improving surgical outcomes and patient comfort.
The informed consent process must, therefore, encompass a clear explanation of potential benefits, risks, and any uncertainties associated with each alternative. This aligns with the views expressed by experts who advocate for the development of lay-friendly summaries of clinical studies and the publication of rationales behind drug approval decisions, ensuring that patients and stakeholders have access to crucial information that can guide their choices effectively.
f. Confidentiality of Records
The safeguarding of personal health information and research data is not merely a procedural step; it is a fundamental human right deeply rooted in the principles of privacy and autonomy. When individuals participate in healthcare services or research, they entrust their most sensitive information with the expectation that it will be protected. Healthcare providers and researchers bear the responsibility of detailing the security measures in place to preserve confidentiality, which includes robust protections against unauthorized access and data breaches.
Recent case studies have underscored the vulnerabilities in data sharing practices. For instance, a report by Privacy International shed light on data being shared through Facebook's Software Development Kit without user consent, raising significant privacy concerns. This led to crucial changes in data-sharing practices, emphasizing the paramount importance of informed consent. Similarly, another report examining menstruation-tracking apps resulted in overhauled terms of use and privacy policies, demonstrating the impact of informed consent on human rights.
Furthermore, the significance of privacy in health is magnified as technological advances, such as telehealth services, become more prevalent, especially during unprecedented times like the COVID-19 pandemic. While these advances offer greater autonomy in managing health, they also increase the risk of personal health information being misused for commercial or political purposes. Privacy, confidentiality, and security are distinct yet intertwined concepts, each vital to maintaining the integrity of the patient-physician relationship and the trust of individuals in healthcare systems.
The National Health Service (NHS) illustrates the delicate balance between using data for patient care, improving service effectiveness, and advancing medical research. As reported by The Times, the NHS's commitment to data sharing for these purposes is predicated on the assurance of data security and privacy for legitimate use, which aligns with the expectations of individuals who contribute their data to the system.
Healthcare providers and researchers must be transparent regarding their authority to collect, use, or share data, the purposes behind their data-focused activities, and the measures in place to protect against emerging threats to data privacy. This comprehensive approach to informed consent and data protection not only complies with privacy laws and principles but also fosters trust and respect between individuals and the institutions responsible for their care and the advancement of medical knowledge.
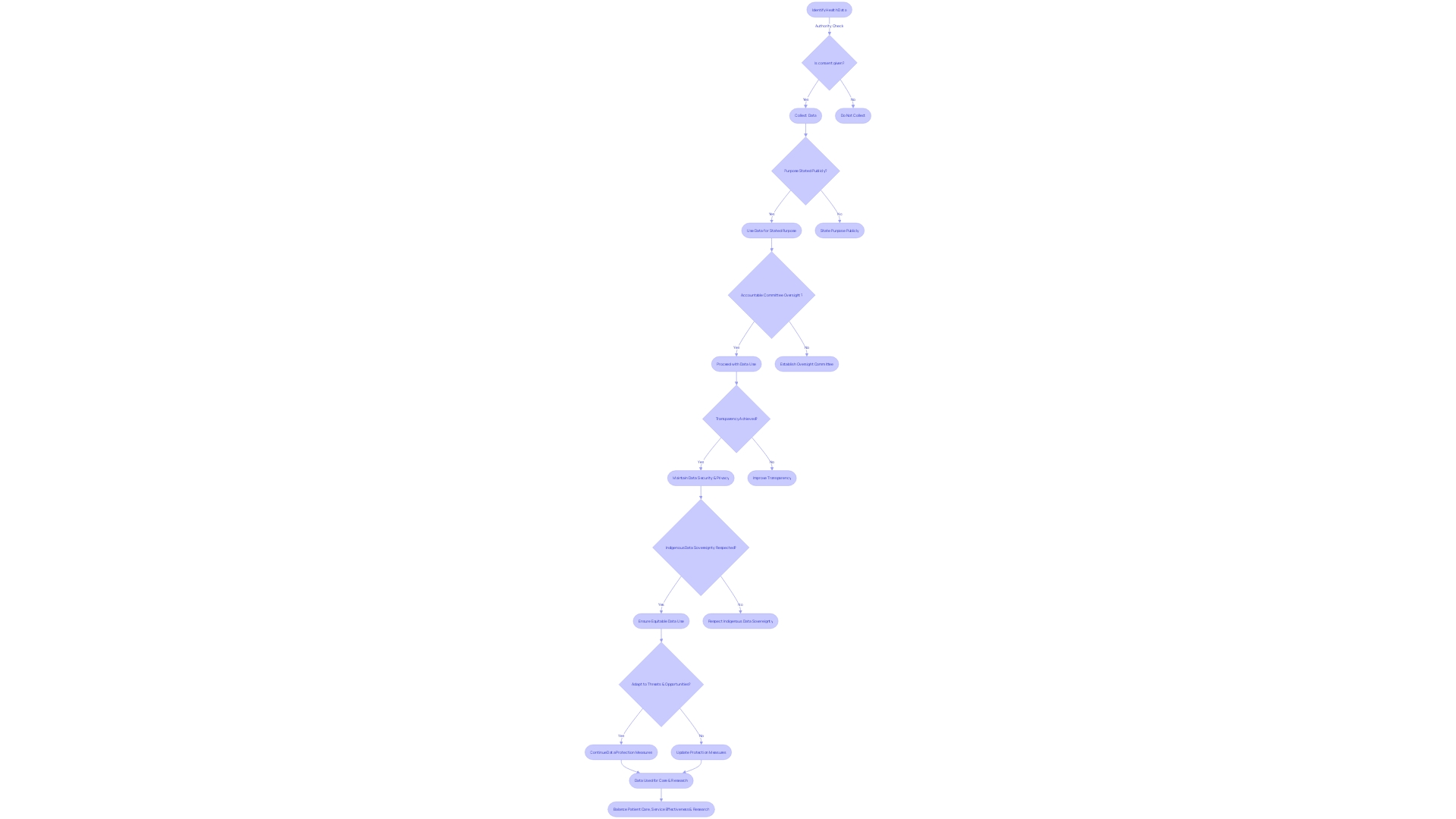
g. Compensation and Medical Treatments for Injury
When healthcare procedures or research studies carry a risk of injury or harm, it is crucial to inform participants about the compensation and medical treatments available in case of an adverse outcome. This communication should encompass the specifics of any insurance coverage, including what it entails and any constraints that might affect its applicability. Additionally, it's important to discuss compensation programs that may provide financial relief and detail the medical support services that can be accessed. Such transparency is not only a matter of ethical responsibility but also aligns with the International Statistical Classification of Diseases and Related Health Problems (ICD) framework, which standardizes data collection and supports large-scale research by providing consistent health recording and statistics on disease and mortality.
Recent case studies highlight the importance of this practice. For instance, frontline healthcare workers, after contracting SARS-CoV-2 on the job and suffering long-term effects such as long COVID, have engaged law firms specializing in negligence to seek legal accountability and compensation for their injuries and financial losses. Likewise, MGMA compensation data serves as a benchmark for contract negotiations, ensuring that medical professionals have access to fair compensation in alignment with industry standards.
In the context of ICD-11, which became effective in January 2022, the classification and terminology allow for the systematic recording and comparison of health data from various regions and times. It underscores the necessity for legally mandated health standards as outlined by the World Health Organization. These standards ensure that in events of harm due to occupational exposure or medical interventions, there is a clear framework to address the consequences and provide appropriate compensation and medical care.
Moreover, expert opinions, such as those from Bijan Elahi who has extensive experience in safety risk management for medical devices, emphasize the importance of clear and precise definitions when managing risks, which is similarly applicable in defining the scope of compensation and treatment options for research participants or patients. His insights suggest that a comprehensive understanding of risk management can lead to more informed and confident decision-making in healthcare settings.
Given the complexity and potential for far-reaching impact on individuals' lives, it's imperative that all involved parties are fully informed of their rights and the protections in place. This not only supports the ethical conduct of medical research and healthcare delivery but also reinforces the trust and transparency that should be the foundation of any patient-caregiver or participant-researcher relationship.
h. Contact Information for Questions and Injuries
In the realm of healthcare and research, informed consent is a cornerstone of ethical practice. It is not merely about delivering a list of facts; it is about ensuring individuals can make educated decisions regarding their participation in studies or medical procedures. Central to this process is providing clear and concise information upfront, especially about the purpose of the research, the potential risks and benefits, and the procedures involved, including their duration. Moreover, it is crucial to present this information in a manner that facilitates understanding, catering to the diverse comprehension levels of potential participants.
One vital aspect of facilitating understanding is the provision of accessible contact information. Participants must have a direct line to the healthcare provider or researcher who can promptly address inquiries or concerns that may emerge throughout the study or medical intervention. This open channel of communication is indispensable for ongoing support and reassurance, allowing individuals to feel secure in their healthcare or research journey.
Despite the importance of simplicity and clarity in informed consent documents, they have grown in length and complexity over the years. The evolution has been such that a document that was typically three to four pages long two decades ago now often exceeds twenty pages. These documents have become increasingly challenging to create, review, and comprehend, not only for participants but also for Institutional Review Boards (IRBs), physicians, and clinical trial sponsors. The language used often skews towards the legalistic, potentially as a response to legislative requirements and previous legal disputes. Unfortunately, this shift has resulted in documents written at a reading level beyond the average adult's comprehension, creating barriers to enrollment in clinical trials, particularly for underserved minority populations.
The sheer length and complexity of these documents, with mandatory items running to more than 270 words, has led to dissatisfaction across all stakeholders involved in drug development. This sentiment is backed by findings that most U.S. adults struggle to understand current consent documents. Yet, despite this recognition, there has been no coordinated industry-wide effort to substantially improve the situation, leaving a critical need for more straightforward and participant-friendly informed consent documentation.

i. Voluntary Participation and Withdrawal
Informed consent is a cornerstone of ethical healthcare and research, underlining the necessity for participants to make decisions free from coercion, fully aware of all pertinent information regarding medical procedures, research studies, and their voluntary nature. It's paramount that individuals understand they can withdraw consent at any time without repercussions, and the process for doing so should be straightforward and transparent.
Biomedical research must be grounded in solid scientific principles, incorporating thorough literature reviews and prior laboratory and animal experimentation. Every research protocol involving human subjects should be meticulously outlined and reviewed. This is not just about adherence to ethical codes but about respecting the individuals who place their trust in the medical research process.
Recent studies, like the one conducted by the University of Ottawa, highlight the need for transparency and accountability in clinical trials. They found that a significant percentage of trials failed to report results, leaving participants uninformed about the outcomes that directly affect them. This lack of reporting can erode trust and underscores the importance of informed consent as a dynamic and ongoing process, not a one-time formality.
The ethical considerations extend to the fair treatment of participants, who should not be expected to shoulder financial burdens for their contributions to public health advancements. Just as first responders receive compensation for their public service, so too should clinical trial participants be reimbursed for expenses, time, and the risks they undertake.
Ultimately, informed consent is about respecting fundamental human rights. It demands that healthcare providers honor and support individuals' decisions regarding their care and participation in research, providing all necessary information without any form of pressure or threat. As we strive for progress in healthcare and research, we must always prioritize the autonomy and well-being of each participant, ensuring that their rights remain inviolable even in times of declared emergency.
Additional Elements of Informed Consent
In specific healthcare or research contexts, informed consent documents can include additional elements tailored to address the unique challenges and ethical considerations present. For example, in a campaign to combat domestic violence, technology was used to animate photos of victims to create a more visceral impact and raise awareness. Such sensitive applications underscore the importance of ethical usage and informed consent in deploying novel technologies.
Moreover, in emergency medical research, where obtaining proactive consent is challenging due to the acuity of patient conditions, alternative consent models are being explored. Research presented at the American College of Emergency Physicians illustrated the use of post-study consent under certain conditions, recognizing that traditional methods may not be representative of the diverse patient populations.
The importance of informed consent is further emphasized by the evolution of documents over time. They have transitioned from simple, concise outlines to complex, lengthy documents, often exceeding twenty pages. This complexity can hinder patient understanding and participation, particularly among underserved populations. A study found that the majority of U.S. adults struggle with the comprehension of consent forms, signaling a need for simplification and clarity.
Regulatory frameworks, such as the GDPR in Europe and the revised Common Rule in the U.S., are in place to govern the use of health data, reflecting the layered conditions of use from ethical, legal, and institutional perspectives. These instruments aim to protect data subjects' rights and foster public trust, ensuring the continued availability of valuable data for research.
Consent documents are not merely lists of isolated facts; they are meant to clearly present information and facilitate comprehension. Stakeholders, including IRBs, physicians, clinical trial sponsors, regulators, and participants, have expressed dissatisfaction with the current state of informed consent documents, calling for a coordinated effort to improve their accessibility and effectiveness.
a. Unforeseeable Risks
In healthcare procedures and clinical research, informed consent is a cornerstone of ethical practice, ensuring that individuals are fully informed of potential risks, including those that may arise unexpectedly. The reality of unforeseen risks in medical interventions is starkly highlighted by case studies such as Mia's, where unexpected complications led to tragic outcomes. The handling of such events is crucial, as seen in the case of atherectomy procedures, where extensive public Medicare data demonstrates the magnitude of operations conducted by a small group of specialists, earning substantial reimbursements. This data underscores the importance of transparent communication regarding risks and their management, especially when high volumes of complex procedures are involved.
In the realm of clinical research, the distinction between delayed start and delayed onset terms is pivotal. A delayed start allows a fully defined human subjects study to commence later in the funding period, while delayed onset requires initial research results to finalize plans for the human subjects study. This differentiation is vital for understanding the timeline and expectation for addressing risks in clinical research.
Moreover, the need for clarity and confidence in risk management is echoed by experts such as Bijan Elahi, who emphasizes the importance of defining risk acceptance criteria and knowing when risk reduction efforts should cease. His insights, derived from decades of experience, are invaluable for those navigating the complexities of risk in medical research and procedures.
News reports such as those covering the repercussions of using unapproved products in spine surgeries at a reputable Manhattan hospital further illustrate the critical nature of informed consent. The FDA's warning about potential contamination underscores the potential dangers patients face when not properly informed.
Statistics on forecasting pandemic risks from laboratory accidents reveal the need for a robust understanding of 'risky research units'—a concept that can be extrapolated to the healthcare setting, where informed consent must account for the unpredictable nature of medical treatments and research studies. With this understanding, clinical researchers and healthcare providers can better manage the unpredictable, ensuring that consent forms reflect the real possibility of unforeseen risks and the strategies in place to address them should they arise.
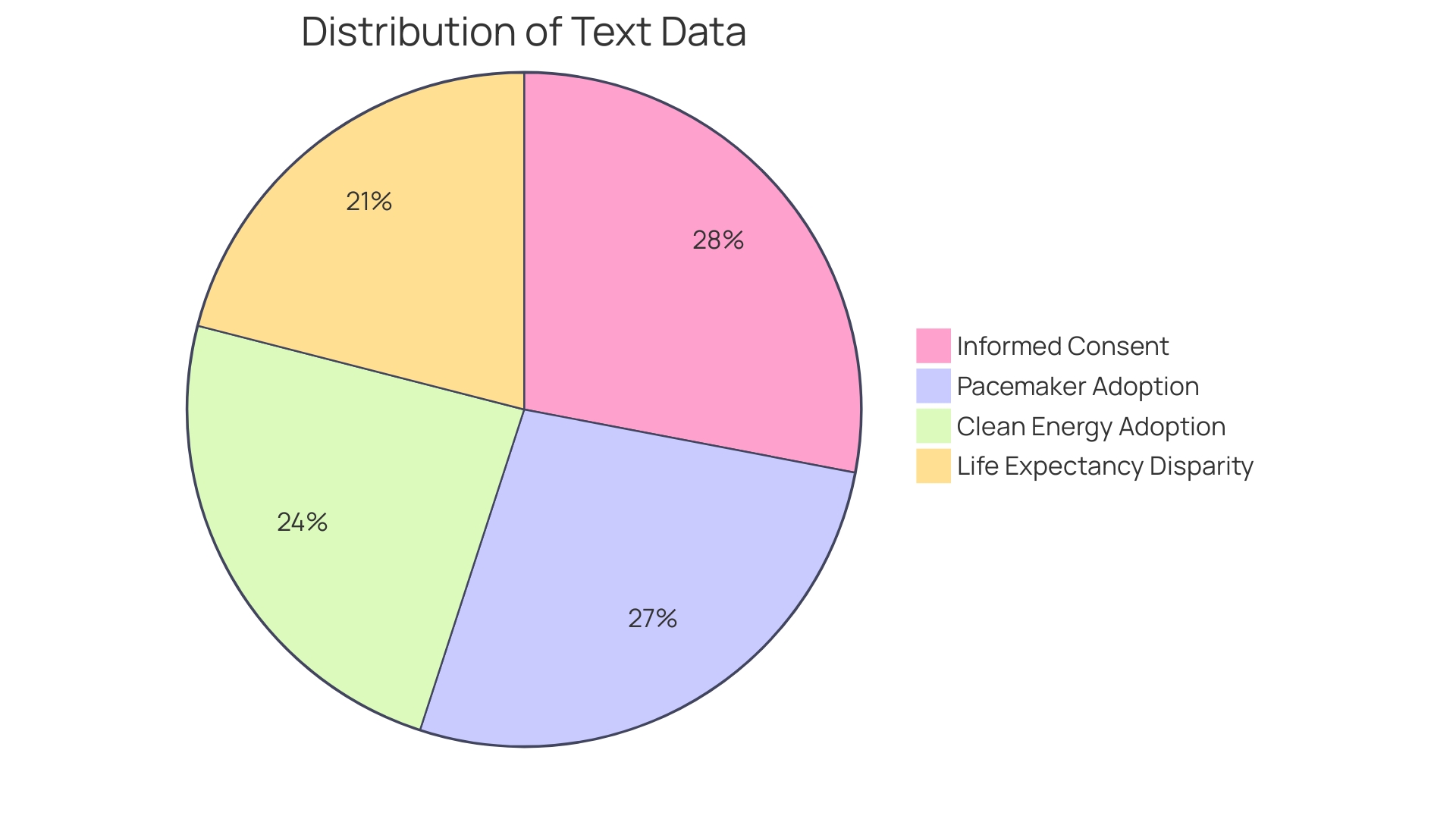
b. Termination of Participation by the Investigator
In the context of clinical research, informed consent is not just a formality—it's a cornerstone of participant rights and ethical standards. Participants must be aware of the possibility that their involvement can be discontinued and under what circumstances. For instance, an individual's participation may need to be terminated for reasons like non-adherence to the protocol or if new information arises that affects their eligibility or safety. This was exemplified by a participant who discovered an unexpected health condition during a study, which required immediate medical attention, emphasizing that research can uncover critical health information beneficial to participants.
The termination process and its implications for the individual must be transparently communicated, ensuring that participants are not left in the dark regarding the potential outcomes of their involvement. As the landscape of participant recruitment evolves with technology, offering easier access to studies and streamlining communications, the ethical obligation to provide clear, understandable consent remains unchanged. Recent data emphasizes the necessity of this transparency, revealing that out of thousands of clinical trials, a substantial portion failed to report results, underscoring the importance of accountability and proper closure in research participation. Prompt and comprehensive communication, as well as the responsible handling of consent and study termination, are not merely regulatory requirements but also affirm the trust and reliability that participants place in research institutions.
c. Additional Costs to the Subject
Understanding the financial implications of healthcare procedures and research studies is paramount for patients and participants. It is not uncommon for additional costs to arise that may not be covered by insurance or other funding sources. These could encompass expenses for diagnostic tests, specific treatments, and even logistical considerations like travel and lodging. For instance, in vitro fertilization (IVF), a complex procedure involving multiple steps and potential cycles, can present varying rates of success and disparate costs between programs. The precise financial burden of IVF depends on numerous factors, including the number of cycles undertaken and the definition of 'success' used by the program, which can lead to a wide range of expenses for the individuals involved. Additionally, the new No Surprises Act is designed to mitigate the shock of unforeseen medical bills, such as those from out-of-network care during emergency surgery. With healthcare costs constituting a significant portion of tax revenues and individual spending, as seen in the Canadian healthcare system, it is essential that individuals are afforded the opportunity to fully understand their financial responsibilities prior to consenting to any procedure or study. This level of transparency not only aligns with ethical standards but also allows individuals to make informed decisions about their health and financial wellbeing.
d. Consequences of Withdrawal
Informed consent is not only a fundamental ethical obligation but also a legal requirement in healthcare and research. It is crucial for participants to fully understand not just the benefits and risks of a procedure or study, but also the implications of opting out. This includes a clear communication of how withdrawing might affect their ongoing medical care, eligibility for future treatments, or the continuity of their participation in research projects. For instance, the American Society of Reproductive Medicine outlines protocols for embryo transfer in IVF treatments, which includes the scenario of not achieving a live birth and the subsequent options for the patient. In a similar vein, the study led by NYU Grossman School of Medicine delves into the complex decisions around life support withdrawal, emphasizing the need for tailored conversations that address disparities in end-of-life care. Such candid discussions ensure that individuals are empowered to make informed choices based on a comprehensive understanding of the potential outcomes of their decisions.

e. Disclosure of New Findings
In the dynamic landscape of clinical research, informed consent is a living document that must adapt to new findings that could influence a participant's willingness to continue. It is essential to outline the method for conveying significant updates to participants, ensuring that they can make choices based on the most current data. This is critical for maintaining the integrity of the study and respecting participant autonomy.
For instance, when discrepancies arise—such as differences between study protocols and subsequent publications or registry entries—researchers must communicate these effectively. This is not only a matter of ethical research practice, but also a cornerstone of maintaining trust with participants who have agreed to partake based on specific information and conditions.
A recent survey of psychology-journal editors highlighted the importance of transparent reporting of deviations from preregistered plans, illustrating how unexpected changes could impact study outcomes. The development of standardized templates for reporting these deviations underscores the commitment to transparency in the research community.
Furthermore, with technological advancements and the increasing volume of data, studies have shown that observational research can reveal patterns and associations that were not initially anticipated. For example, large-scale screenings have identified potential risk factors for conditions like sudden infant death syndrome (SIDS) and the long-term effects of mild brain injuries. Such findings could have immediate implications for ongoing participant involvement and must be communicated promptly and effectively.
It is evident that keeping participants informed with the latest findings is not just a procedural necessity but a fundamental component of ethical research. The method section of any research paper serves as the 'recipe' for the study, detailing the ingredients and the process, allowing for reproducibility and transparency. By adhering to these principles, researchers can ensure that participants remain informed and empowered to make decisions throughout the course of a study.
f. Use of Biospecimens and Commercial Profit
In the realm of healthcare and research, informed consent is more than just a formality—it is a cornerstone of ethical practice. When it comes to the collection of biospecimens, such as blood or tissue samples, transparency is paramount. Participants must be given clear information about how their samples will be used, and this includes any potential for commercialization. The possibility of profit-sharing from the use of these biospecimens should also be openly discussed, allowing individuals to make informed decisions about their contributions to science.
The use of biospecimens can lead to significant scientific breakthroughs. For instance, the study of human cell lines has been pivotal in biomedical research, leading to the development of vaccines and treatments for various diseases. These cell lines, which can divide indefinitely in the lab, often become a staple in research without much thought given to their origin. For example, the HeLa cell line, derived from a cervical carcinoma patient in 1951, has been integral in medical milestones, including the development of the first polio vaccine.
Understanding the implications of biospecimen use is not only a matter of respecting individual autonomy but also of recognizing the long-term impact of today's scientific decisions. As one professional reflected, the decisions we make now may lead us to wonder in 40 years, 'What on earth have we done?' This underscores the importance of ensuring that informed consent is not just procedural but truly informed, with individuals understanding the full scope of how their contributions to science might be used.
Moreover, the need for clear communication in informed consent documents is reinforced by draft guidance from regulatory bodies. These documents should facilitate understanding by presenting key information in a clear and concise manner at the outset, including the purpose of the research, potential risks and benefits, and the procedures involved in the study.
In light of the increasing importance of life and health insurers in managing risks related to illness and injury, the role of informed consent in protecting individual interests is all the more critical. As the industry evolves, and the global demand for insurance products grows, the ethical management of biospecimens remains a vital concern, reflecting the industry's commitment to safeguarding individual rights while advancing medical science.
g. Disclosure of Research Results
The essence of informed consent in research is not only to inform participants about the nature and procedures of the study but also to address how the outcomes will be communicated. This is pivotal to ensure that participants are aware of the impact of their involvement and the potential implications of the results. For instance, in clinical trials, participants may anticipate learning whether their contributions have made a meaningful difference in the advancement of medical knowledge or treatment options.
In recent years, concerns have been raised about the transparency of research studies. A study analyzing clinical trials in Canada highlighted that a considerable proportion of studies failed to report their results or publish findings, leaving participants in the dark about the outcomes of the research they contributed to. This gap in reporting is not only alarming but underscores the ethical imperative to clearly communicate with participants about the fate of study results.
Moreover, the translation of research findings is not a straightforward task. It requires not just accuracy but the conveyance of data in a manner that serves its intended audience. This means that the results section of a study should do more than just present data; it should provide context and make the findings accessible and understandable. As scholars have noted, back translation methods for evaluating translation quality in health research may be outdated and insufficient for modern needs.
In the context of informed consent, it is therefore crucial to articulate to participants if and how they will receive updates about the research results. This commitment to transparency can foster trust and respect between researchers and participants, acknowledging their significant role in the research process. As one clinical and policy researcher noted, for some participants, their involvement in a trial might be linked to the hope that their contributions will benefit future generations, despite not directly benefiting themselves.
In sum, informed consent must include clear communication about the disclosure of research results, ensuring that participants are not left wondering about the impact of their participation, and upholding the integrity of the research process.
Implementing Informed Consent in Practice
To optimize the informed consent process in healthcare and research, the essential approach is to simplify and clarify the presentation of key information. It is critical to distill this information into a format that is not only comprehensive but also accessible to a broad audience, including those from underserved communities. In light of the fact that the complexity of consent documents has escalated over the years—growing from an average of three to four pages to over twenty pages—there is a pressing need to counteract this trend and enhance participant understanding.
Recent guidance recommends that consent documents commence with a clear summary of the most crucial details, such as the research's purpose, the potential risks and benefits, and the procedures involved. This approach aims to assist prospective subjects in making an informed decision by focusing on the essence of the study without overwhelming them with excessive detail.
Research reveals that the majority of U.S. adults find current consent documents challenging to understand, indicating a disconnect between the documents' intended purpose and their actual efficacy. To address this gap, innovative methods such as eConsent are being introduced. EConsent employs digital media, including videos and interactive elements like glossaries and quizzes, to facilitate a better understanding of the study. Such multimedia tools can cater to diverse literacy levels and learning styles, making informed consent more inclusive.
The use of visual aids, too, is gaining traction. Illustrations and videos can effectively convey complex information, making key points more digestible. Organizations such as the National Organization for Rare Disorders (NORD) endorse these innovative approaches, recognizing the importance of tailoring the informed consent process to the unique needs of each participant.
The current landscape of informed consent is ripe for transformation, with a collective effort required from all stakeholders—regulatory bodies, research sponsors, healthcare professionals, and IRBs—to streamline the process. By embracing clarity, simplicity, and innovative communication strategies, the informed consent process can be made more efficient and user-friendly, ultimately fostering greater trust and participation in clinical research.
a. Involving Patients in Decision-Making
Active engagement between healthcare providers and patients is a cornerstone of informed decision-making, which dates back to the principles set forth by the Presidential Commission in 1982. The Commission advocated for a collaborative environment where patients are informed about their medical conditions, treatment options, and likely outcomes, allowing them to make choices reflective of their personal values and lifestyle considerations. The Courageous Parents Network (CPN), through its dialogues, underscores the importance of addressing decision-making fatigue and fully comprehending the patient's experience, particularly for those managing complex or chronic conditions. A patient's understanding of their medical situation is crucial for meaningful participation in shared decision-making. For instance, without basic knowledge of chromosomal abnormalities, a patient may find it difficult to engage in discussions about miscarriage prevention. Furthermore, statistics suggest that patient involvement in healthcare decisions not only increases satisfaction but also leads to better treatment outcomes. Despite advancements in medical education that promote patient engagement, there still exists a gap in implementing these principles at the health system management level. It is recognized that engaging patients in their healthcare decisions is as beneficial as a 'blockbuster drug' of the century, yet the healthcare system often resists fully embracing patient participation. Shared decision-making can sometimes lead to frustration, with patients feeling overwhelmed by the expectation of engagement or feeling that their preferences are not adequately considered by healthcare professionals.

b. Documenting the Consent Process
Thorough documentation of the informed consent process is pivotal in healthcare and research settings, where it serves as a record of the information conveyed to patients, their comprehension, decisions made, and any ensuing dialogue or requests for clarification. This level of meticulous documentation is crucial not only for evidencing that informed consent was indeed procured, but also for safeguarding the rights of both parties involved—the patient and the healthcare provider or researcher.
The imperative to capture every facet of the informed consent process is underscored by the challenges faced when consent responsibilities are passed to clinical team members who may not be performing the procedure, such as junior doctors. These individuals often grapple with a deficit of time and requisite clinical knowledge to foster patient independence and informed decision-making. To combat these issues, one innovative solution is the deployment of conversational artificial intelligence, leveraging large language models (LLMs) to enhance the consent process. The integration of such technology can potentially streamline and improve patient comprehension and autonomy.
Moreover, the use of generative AI is being explored as a means to refine medical documentation, as seen with companies like Summer Health. This approach is aimed at alleviating the administrative burden that medical shorthand translation into comprehensive visit summaries places on pediatricians. This shift not only aids in reducing the time dedicated to administrative tasks, which currently consumes over half of care providers' time, but also promises to deliver clearer, more understandable visit notes to parents, thus enhancing patient care and reducing the risk of burnout among healthcare providers.
The relevance of this is further highlighted by the Peterson Health Technology Institute's commitment to the independent assessment of digital health tools, considering equity factors such as cost reduction and accessibility for diverse populations. Such advancements in digital health documentation and AI have the potential to transform the informed consent process, making it more efficient and patient-centered, and thus aligning with the aims of co produced health research. This collaborative approach to research prioritizes shared control and joint design, analysis, dissemination, and evaluation, ensuring research is responsive to stakeholder needs and geared towards real-world application.
In embracing technology and innovative methodologies, the healthcare industry can address the ethical and practical complexities of informed consent, ensuring that patients are fully informed and able to exercise their right to make autonomous decisions about their healthcare. This progression towards enhanced documentation and understanding in the consent process is a testament to the industry's ongoing commitment to ethical standards and patient empowerment.
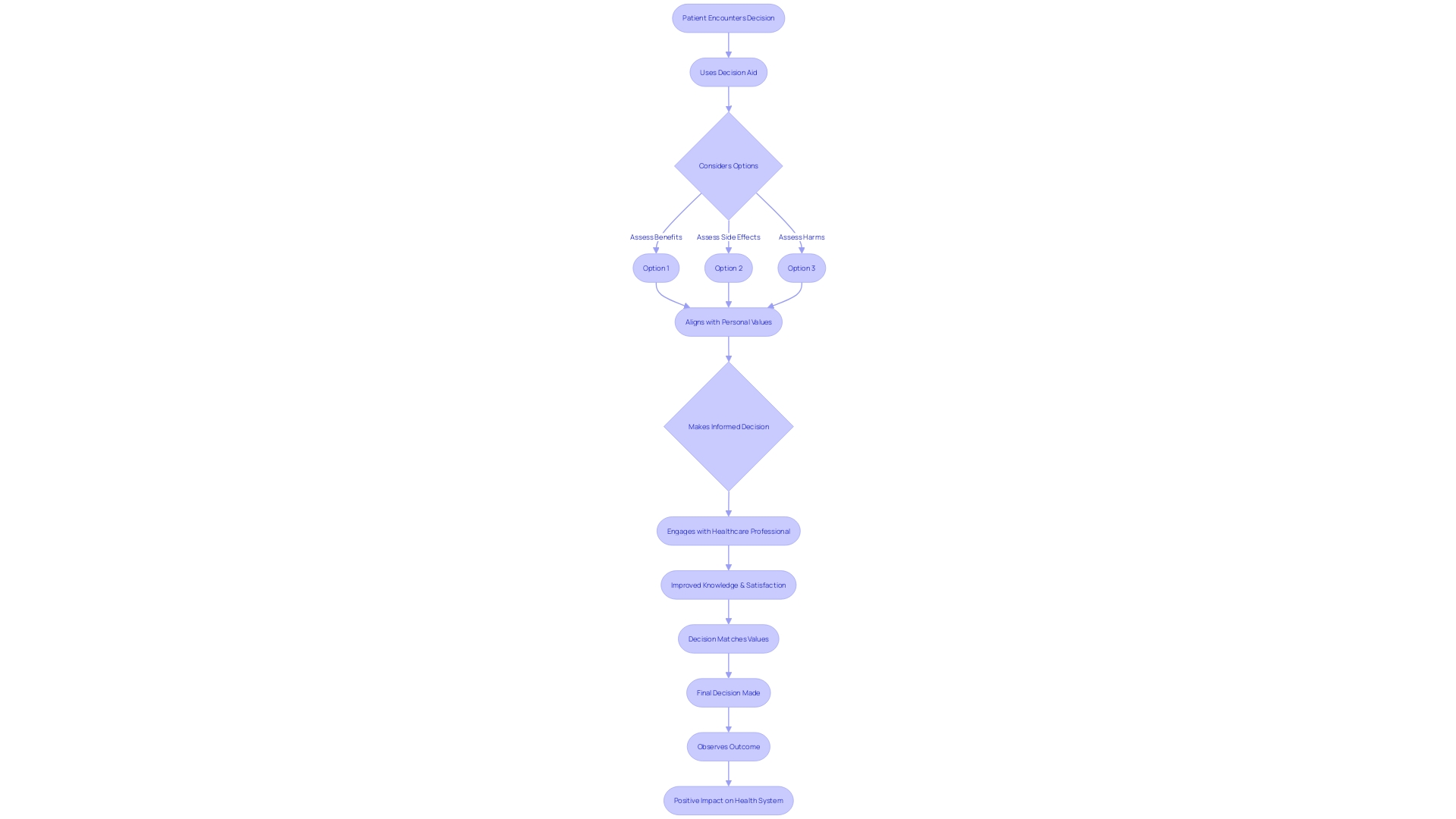
c. Using Decision Aids and Educational Materials
The use of decision aids, such as pamphlets and videos, has proven to significantly improve the informed consent process by empowering individuals with a better understanding of their healthcare options. These tools are designed to clearly outline the decisions to be made, provide detailed information on the various options including their potential benefits and harms, and assist in aligning choices with personal values and preferences. Through the comprehensive work of lead author Dawn Stacey from the University of Ottawa and Ottawa Hospital Research Institute, the Cochrane Review has been meticulously updated every few years since 1999, with the 2024 update marking a substantial expansion of the review since 2017. The latest findings highlight the effectiveness of decision aids in fostering better knowledge among patients, enhancing their perception of risks, and leading to decisions that more accurately reflect their values.
The substantial improvements observed with the deployment of decision aids also include a more active engagement of patients in the decision-making process, in partnership with their healthcare providers. This form of shared decision-making is a cornerstone of person-centered care and is integral to advancing health system improvements. The 2024 Cochrane Review evidences that patients who use decision aids not only feel more informed but are also more satisfied with the decision-making process, without experiencing increased distress or regret about their health decisions. Furthermore, informed consent documents, vital to the consent process and necessary for clinical trial participation, have become lengthy and complex over the years, posing challenges for comprehension, particularly among underserved minority populations. This calls for a coordinated effort among all stakeholders to streamline these documents, ensuring they are both informative and accessible, to support informed decision-making in healthcare.
Conclusion
In conclusion, informed consent is a critical principle in healthcare and research, empowering individuals to make informed decisions about their treatment and participation. Recent challenges, such as time constraints and complex consent documents, have led to the exploration of conversational AI and simplified documentation to enhance patient understanding.
To optimize the process, it is important to simplify and clarify information, using innovative approaches like eConsent and multimedia tools. Thorough documentation is essential to protect the rights of both patients and healthcare providers/researchers.
Shared decision-making and patient involvement are crucial in promoting informed consent. Decision aids and educational materials improve patient knowledge and satisfaction with the decision-making process. Streamlining consent documents is necessary to ensure accessibility and support informed decision-making.
Informed consent is a dynamic concept that requires continuous attention to remain meaningful. By simplifying the process, involving patients, and utilizing educational tools, we can enhance patient understanding and autonomy, upholding the ethical principles of informed consent.




