Introduction
Informed consent is a crucial aspect of ethical and legal medical practice, ensuring that patients have autonomy over their healthcare decisions. It involves comprehensively understanding the medical intervention or research study at hand. The process of obtaining informed consent should incorporate several critical components, including a clear explanation of the procedure's nature, a discussion of its potential risks and benefits, the presentation of reasonable alternatives, and an exploration of the risks and benefits associated with those alternatives.
However, recent studies have highlighted that informed consent documents are often written in complex language, making it difficult for patients to understand. This article explores the importance of clear communication in the informed consent process and the challenges it faces. It also discusses the purpose and scope of research or procedures, the description of procedures and risks, potential benefits and alternatives, confidentiality and data protection, voluntariness and the right to withdraw, compensation and medical treatment for injury, contact information for questions and concerns, additional costs and consequences of withdrawal, disclosure of new findings and commercial use, special considerations for vulnerable populations, optimizing the informed consent process, clear and accessible information, the use of visual aids, the assessment of participant comprehension, documentation and revision of consent forms, participant and witness signatures, version control and date, storage and accessibility, and informed consent in urgent or emergency situations.
By understanding and addressing these aspects, healthcare professionals and researchers can ensure that the informed consent process is effective and respects the principle of patient autonomy.
Basic Elements of Informed Consent
Informed consent is a cornerstone of both ethical and legal medical practice, ensuring that patients maintain autonomy over their healthcare decisions. At its core, informed consent involves comprehensively understanding the medical intervention or research study at hand. A proper informed consent process should always incorporate several critical components: firstly, a clear explanation of the procedure's nature; secondly, a candid discussion of the procedure's potential risks and benefits; thirdly, the provision of reasonable alternatives to the proposed intervention; and fourthly, an exploration of the risks and benefits associated with these alternatives. It is essential that patients are not only presented with this information but also have their comprehension of these details assessed to confirm that they are making informed decisions.
Recent studies have highlighted that informed consent documents are often written in complex language that may be difficult for patients to understand, potentially hindering their ability to make well-informed decisions. In an analysis of over 100 procedure consent forms from high-volume hospitals, researchers found a significant variation in the disclosure of risks and the explanation of the likelihood of success, suggesting a need for clearer communication in the consent process.
Moreover, the ethical importance of informed consent is underscored by its inclusion in major ethical guidelines, such as the Declaration of Helsinki, which is often considered the 'cornerstone' document pertaining to medical research ethics. Recent guidance on informed consent emphasizes the necessity of presenting key information in a manner that facilitates patient understanding. This includes outlining the research's purpose, the potential risks and benefits of the study, and the study's duration and procedures in a clear and concise way.
Despite these guidelines, the practice of obtaining informed consent has faced challenges. For instance, it is common for consent to be implied for many standard-of-care hospital procedures, presuming that patients desire any tests or interventions that may improve their health or aid in diagnosis unless they explicitly decline. Yet, for more sensitive tests, such as HIV or genetic testing, explicit consent is required due to the potential revelation of sensitive information.
The conversation around informed consent has also evolved with the advent of new technologies. Conversational artificial intelligence using large language models has been suggested as a potential tool to improve the informed consent process, helping to address issues such as the delegation of consent-taking to less informed members of the clinical team.
Lastly, it is imperative to acknowledge recent regulatory changes and societal discussions that have brought informed consent into the spotlight. Notably, news of amendments to the 21st Century Cures Act, which introduced exceptions to the requirement of obtaining informed consent for certain minimal-risk clinical investigations, has sparked debate over the balance between patient autonomy and the progression of medical research.
In conclusion, informed consent is a dynamic concept that must constantly adapt to ethical considerations, regulatory changes, and technological advancements to truly honor the principle of patient autonomy.
- Purpose and Scope of the Research or Procedure
When communicating the essence of a study, it's crucial to articulate the research's aim, methodology, and anticipated results in a manner that resonates with the participants' experiences. This entails not only detailing the technical aspects but also connecting with the lived experiences of individuals involved, whether they are program implementers or recipients. By focusing on the lived experience, we honor and leverage the knowledge and insight that participants bring to the table, ensuring their contributions shape the course of the research.
For instance, adopting a community-centric approach, similar to the 'We are Water' program, which utilizes bilingual newsletters, social media outreach, and a comprehensive website, can effectively engage participants. This program shares water stories that highlight the historical and cultural significance of water, thereby fostering a deeper connection with the community and inviting them to contribute their perspectives through various mediums.
Furthermore, it's essential to present information in plain language to facilitate understanding. Rather than using technical jargon that may obscure the message, simplifying complex terms—like replacing 'positive association' with 'more likely to develop'—ensures clarity. This approach is not only beneficial for participant comprehension but also aids in accurate media representation of the research findings.
Additionally, statistics reveal that healthcare providers sometimes struggle with interpreting complex data presentations in drug promotions. Therefore, incorporating straightforward data displays and offering plain language explanations can significantly enhance understanding among both professionals and participants. Ultimately, by centering the participant experience and committing to clear, transparent communication, we lay the groundwork for ethical and effective research.
- Description of Procedures and Risks
In the realm of healthcare and research, informed consent is not merely a procedural formality; it is a fundamental component that safeguards the ethical and legal rights of participants. It is imperative that participants are provided with a comprehensive account of the procedures they will undergo in the study or intervention. This includes a detailed description of each step, along with transparent communication about the associated risks and potential complications. For instance, in a case where the research involves analysis of clinical databases, as was the case in studies exploring the impact of long-term statin use on cancer risk, it's not enough to merely state the hypothesis. It's also crucial to delineate the study design and analysis in a way that participants can understand the magnitude of the risks involved, even if the risk is as nuanced as a 1.01 times greater risk of cancer with long-term statin use versus no use.
Amidst the rapid advances in biomedical research and the subsequent increase in data generation, the integrity and transparency of the research process have never been more important. Researchers must avoid the pitfalls of questionable research practices, which range from the inadvertent to the intentionally detrimental, and can tarnish the trustworthiness of scientific outcomes. These practices can occur at any stage of research and have serious consequences, as highlighted by Martinson and colleagues' findings on the behavior of NIH-funded scientists.
Furthermore, the integrity of the grant application process must be maintained to ensure that the peer review system functions effectively, as exemplified by NIH's handling of plagiarism in grant applications. This underscores the necessity for clear and honest communication about research methods and findings, not only in grant applications but also when conveying results to the public. The use of plain language is recommended to enhance understanding among non-specialist audiences, as exemplified by recent guidance on discussing research findings on topics like the association between air pollution and dementia.
In line with the need for responsible data science, researchers are reminded to be well-versed with the relevant rules, regulations, policies, and laws that govern their work. Ensuring privacy, security, and accountability in data handling is not only a regulatory requirement but also a moral imperative that aligns with the values and standards of the research enterprise. As we navigate the complexities of modern research, these principles serve as beacons, guiding researchers to uphold the highest standards of integrity and respect for human subjects.
- Potential Benefits and Alternatives
Ensuring participants are thoroughly informed about a study is critical, not just for ethical reasons, but also for the integrity of the research. Prospective participants need to know the potential benefits they could gain from the intervention. For instance, a study published in Scientific Reports emphasizes that understanding the effects of aspartame on cognitive abilities is crucial, thus highlighting the importance of participants being informed about the potential personal health knowledge they could gain.
Furthermore, it's just as important to inform them of existing alternatives. This transparency helps participants make well-informed decisions. For example, when new digital technologies are proposed within a healthcare system, a thorough assessment is conducted to ensure that the technology is necessary and that there aren't existing alternatives that are already being used, as noted in internal processes within the NHS.
Additionally, according to Ms. Melissa McGowan and Ms. Dawn Corbett from the National Institutes of Health, clear communication is essential to ensure that participants from diverse backgrounds understand the full scope of the research, including its potential risks and benefits.
Finally, the quality of evidence gathered from research is highly dependent on the informed consent process. The GRADE framework suggests that the overall quality of research evidence is a combined rating based on systematic and transparent criteria, which includes the informed consent process. Informed participants are more likely to engage fully and provide high-quality data, contributing to the reliability and validity of the research outcomes.
- Confidentiality and Data Protection
In the era of Big Data and advanced analytics, the confidentiality and protection of participant data have become paramount. With the vast amounts of data collected from a plethora of sources, including medical, financial, and personal devices, the risk of re-identifying individuals from seemingly anonymized datasets is significant. This concern is especially pertinent in the context of biomedical research, where sensitive personal health information is often involved. The principles of data minimization, de-identification, and anonymization are key strategies employed to mitigate these risks. Data minimization involves collecting only the necessary data elements, while de-identification and anonymization involve altering datasets to prevent the re-association of data with individual subjects.
The recent explosion of data in biomedical research requires that we not only share and reuse data for scientific progress but also protect the individuals behind this data. The study conducted by the University of Lausanne and published in LabAnimal highlights the critical role metadata play in facilitating data sharing while ensuring the privacy of subjects. Metadata, which includes descriptive and administrative information, is essential in maintaining the integrity of the data and the anonymity of the subjects involved.
To address these challenges, data security policies and encryption practices have become fundamental components of data management. A robust data security policy provides a clear framework for handling data, thereby reducing the likelihood of accidental breaches and ensuring regulatory compliance. Encryption adds an additional layer of security, transforming sensitive information into an unreadable format that can only be accessed with the correct decryption key.
The concept of differential privacy further demonstrates the industry's commitment to protecting individual privacy. This approach guarantees that the output from datasets, which differ only by a single data point, will remain similar, ensuring the confidentiality of the data subjects. As Machine Learning and AI continue to permeate various sectors, including healthcare, the importance of safeguarding participant data cannot be overstated. The collective efforts of researchers, policymakers, and technology experts are crucial in maintaining the delicate balance between data sharing for scientific advancement and the protection of individual privacy.
- Voluntariness and Right to Withdraw
It is imperative for participants to be aware that their involvement in any study is entirely voluntary, with the freedom to disengage at any point without repercussions. This principle is rooted in the ethical framework set forth by the Declaration of Helsinki, which emphasizes the autonomy and rights of research subjects. A study by Bohns and Sommers highlighted the complexity of consent, demonstrating that individuals often comply with requests without fully understanding their rights. This underscores the necessity for clear communication about the voluntary nature of participation and the right to withdraw consent. The ethical perspective has evolved to prioritize fairness and respect for participants' contributions. For instance, clinical trials must now account for the costs borne by participants, acknowledging that, like any public service, individuals involved in research are entitled to compensation for their time and effort. Recent news also reveals the need for transparency and accountability in research, with findings showing that a significant number of clinical trials fail to report results, leaving participants uninformed. This aligns with the growing consensus that informed consent is a dynamic process, one that requires ongoing dialogue and clear, concise information about the study's purpose, risks, benefits, and procedures. The aim is to ensure that research not only advances scientific knowledge but also respects and upholds the dignity and rights of every individual who contributes to it.
- Compensation and Medical Treatment for Injury
The ethical underpinnings of clinical research underscore the importance of informed consent, which encompasses the need for clear communication regarding potential compensation or medical care in case of an injury or adverse event during a study. Ethical guidelines, such as those in the Declaration of Helsinki, assert that participants must be fully aware of their rights and the recompense they are entitled to should any harm occur as a result of their participation. This is not only a matter of fairness but also ensures data integrity and the overall quality of research outcomes.
Fair compensation for research participants is a topic of significant importance. The Fairwork Cloudwork Report 2022 highlighted the lack of adequate pay and clear contracts across numerous research platforms, underscoring the need for equitable treatment of contributors. Aligning with these ethical standards, participants should be informed about what they may receive, which could include financial compensation, healthcare services, or additional medical treatment relevant to their participation.
Moreover, the practicality of such disclosures is evident in the broader context of healthcare research. For instance, Efrain Torres, PhD, received a grant to develop a low-cost MRI, aiming to bring medical advancements to underprivileged communities. This type of research, which strives to translate scientific discoveries to real-world applications, also emphasizes the importance of ethical considerations in participant treatment.
In the realm of data reliability, the U.S. Bureau of Labor Statistics provides a framework for measuring the precision of an estimate, ensuring its appropriateness for the intended purpose. Similarly, in clinical research, the precision of informed consent documents must be scrutinized to guarantee that participants are adequately informed about potential compensatory measures. The March 2023 estimates from the Bureau indicate a systematic approach to benefits provision, which can serve as a model for the structured delivery of information to research participants.
In light of these considerations, it is essential that participants are concisely informed about any compensation or medical treatment they may receive, which must be in accordance with the ethical principles governing the responsible conduct of research.
- Contact Information for Questions and Concerns
Ensuring that participants have access to contact information for questions and concerns is a fundamental component of informed consent. This practice not only empowers participants by providing them a reliable point of contact but also addresses potential issues that may surface during the study or procedure. For instance, Barbara's experience, highlighted by Mehta's research team, underscores the importance of participant follow-up. After enrolling through The New Normal, an online platform designed to enhance health research awareness, Barbara discovered a previously undetected heart condition. Her case exemplifies how unexpected findings can emerge, necessitating prompt attention and further medical action.
Moreover, the BISON-PRO Quality of Life Study, led by Dr. Major and Dr. Buckner, illustrates a structured approach to engaging with participants through online surveys and various communication channels, ensuring ongoing contact and support throughout the research process. This approach aligns with the prescribed pipeline for conducting online surveys, which emphasizes adherence to data protection guidelines, such as GDPR in Europe, and requires ethical board approval for study designs and any changes thereto.
In the realm of research communication, it is increasingly recognized that using clear, plain language when discussing study details is crucial, as evidenced by the advice given to researchers for crafting press releases and public statements. This clarity extends to the communication strategy with participants, ensuring they fully understand the study's implications and their own health discoveries.
The insights drawn from these case studies, expert advice, and statistical data reflect a comprehensive view of the multifaceted relationship between researchers and participants. They highlight the critical role of transparent communication and the ethical imperative to maintain accessible, responsive channels for participant inquiries and concerns.
- Additional Costs and Consequences of Withdrawal
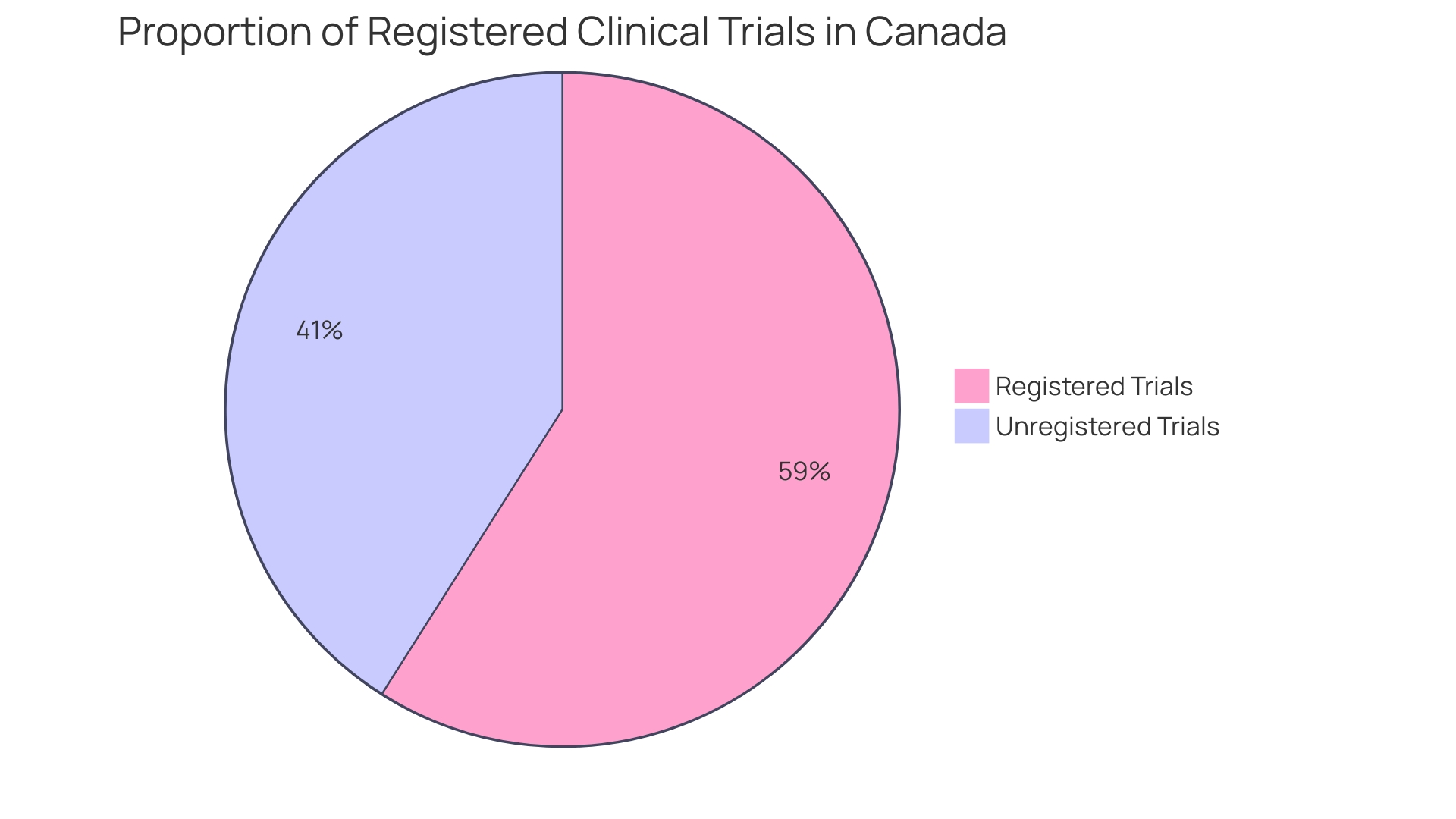
When it comes to informed consent in healthcare and research, participants must be fully apprised of not just the procedures and benefits, but also of any additional expenses they may face by partaking in the study. This transparency extends to comprehensively communicating the ramifications should they choose to withdraw, ensuring that participants make well-informed decisions. Two crucial aspects, delayed start and delayed onset, epitomize the intricacies of planning research involving human subjects. A delayed start means the human subjects research is planned but will commence later in the funding period; all details are provided upfront. Conversely, a delayed onset study cannot be fully defined at the application stage and requires initial research results to inform the human subjects study's planning. Misunderstanding these terms could lead to applications lacking critical information, potentially jeopardizing the study's integrity and funding opportunities. The importance of integrity in research is underscored by cases such as the retracted study by Studnicki et al., initially published in Health Services Research and Managerial Epidemiology, which aimed to describe emergency room visit incidences after abortion procedures. This study was retracted due to methodological concerns, highlighting the imperative of adhering to the core values of honesty, accuracy, efficiency, and objectivity in research. Moreover, a study published in Facets revealed that out of 6,720 clinical trials in Canada between 2009 and 2019, 32 percent failed to report results or publish findings. This lack of transparency affects around 612,000 Canadian patients, underscoring the risk that unreliable data poses to public health and the validity of clinical research. Ensuring that research findings are methodologically sound and transparently reported is paramount for upholding the integrity of the scientific literature that informs policy decisions and public health initiatives.
- Disclosure of New Findings and Commercial Use
Research participants have the right to be informed about any new discoveries that might occur during a study, including the potential commercial application of their data or the results. This transparency is not only ethical but also aligns with the increasing demands for openness in research. For instance, in the biomedical sciences, the conceptualization of a journal transparency tool (JTT) reflects the need for stakeholders to understand a journal's transparency practices, which directly impacts the credibility of the research they publish or consume.
When we look at clinical trials, the commitments to transparency can vary significantly. It is essential that trial protocols, statistical analysis plans, and raw data be made publicly available for research to be truly transparent and reproducible. Studies published in major journals often register in databases like ClinicalTrials.gov, but this registration may not provide enough information for comprehensive transparency. Full disclosure of all research components is required, yet industry-sponsored trials often keep raw data internal, analyzed only by their statisticians, with limited sharing even among trialists.
Furthermore, recent studies highlight the importance of transparency in research. For example, an observational study found a potential link between routine metabolic screening results for newborns and the risk of sudden infant death syndrome (SIDS). With over 2 million infants screened in the US, researchers identified patterns in the metabolites of those who died from SIDS, underscoring the significance of accessible and detailed research data. This level of detail in research findings is crucial for advancing medical knowledge and improving patient outcomes.
As such, participants must be made aware of how their contributions to research might be used, including any potential commercialization. This is not only a matter of respect for the individuals involved but also a foundational aspect of advancing science in a way that remains credible and beneficial to society as a whole.
- Special Considerations for Vulnerable Populations
Special attention is required in the informed consent process when dealing with vulnerable populations, such as minors and individuals with cognitive impairments or limited decision-making capacity. It is imperative to tailor the communication of key information in a manner that is clear, concise, and understandable to accommodate their unique needs and circumstances. This includes conveying the purpose of the research, potential risks and benefits, study length, and procedures in a way that enhances comprehension. Using innovative approaches, like videos and illustrations, as endorsed by organizations like the National Organization for Rare Disorders (NORD) and the Pharmaceutical Research and Manufacturers of America (PhRMA), can greatly aid in this process. These methods can address challenges like language barriers, sensory impairments, and varying levels of health literacy, ensuring that all participants, regardless of their abilities, can fully grasp the implications of participating in clinical trials and make informed decisions.
Optimizing the Informed Consent Process
To optimize the informed consent process, it is fundamental to address the complexity and accessibility of informed consent documents. These documents have expanded significantly, from a more manageable three to four pages, to a daunting twenty pages or more over the past two decades. The content often exceeds a reading level comprehensible to the average U.S. adult, with a focus on legalistic language that can act as a barrier to enrollment in clinical trials—particularly for underserved minority populations.
Efforts to streamline informed consent involve presenting crucial information effectively—not merely as isolated facts but in a manner that enhances understanding. This includes outlining the purpose of the research, potential risks and benefits, procedures, and the anticipated duration of the study at the onset of the document. One approach to support comprehension is incorporating multimedia resources, such as explanatory videos, which can offer a more accessible format for diverse participants with varying literacy levels and language proficiencies.
Furthermore, leveraging the vast amounts of health data available can aid in creating more inclusive clinical trials. Pharmaceutical companies are encouraged to utilize this data judiciously in participant recruitment and site selection to meet the latest FDA diversity requirements. These strategies, together with a concerted effort by all stakeholders, aim to reverse the trend of lengthening and increasingly complex informed consent documents, making the process more transparent and participant-friendly.

- Clear and Accessible Information
Effective communication in healthcare is paramount, especially when discussing informed consent with patients. It's crucial to avoid technical medical terminology that may confuse the patient. Instead, we should employ simple, understandable language. For example, use 'high blood pressure' instead of 'hypertension' and make sure to explain its significance and implications clearly.
Infographics offer a powerful tool to convey complex medical information in a more digestible format. These visual aids combine simple wording with graphic elements and symbols to enhance understanding. Research indicates that infographics can significantly improve a patient's ability to focus on, comprehend, and remember medical information, which is particularly beneficial when discussing treatment options and potential side effects.
In practice, initiatives like the Plain Numbers Approach have demonstrated the effectiveness of simplifying complex information. This approach, which applies insights from psychology and behavioral science, has been shown to double the number of people who can correctly comprehend medical information.
Moreover, it's essential to regularly check for understanding by asking patients to repeat information back in their own words. This technique ensures that the patient has truly grasped the concepts discussed. Such strategies not only foster better patient-clinician communication but also empower patients to make informed decisions about their healthcare.
In summary, using plain language, visual aids like infographics, and interactive communication techniques can significantly improve patients' understanding, which is a cornerstone of informed consent. These methods are instrumental in ensuring patients are fully informed and can actively participate in their healthcare decisions.
- Adequate Time for Decision-Making
The process of informed consent is a cornerstone of ethical conduct in both healthcare and research. It requires that participants be fully informed about the study's purpose, potential risks and benefits, procedures, and duration, enabling them to make a voluntary and educated decision on whether to participate. Regulatory bodies and experts emphasize the importance of delivering this information in a clear and concise manner, with draft guidance suggesting that key information be placed prominently at the beginning of consent documents. This is not merely a formality but a fundamental part of respecting participants' autonomy and ensuring that consent is truly informed.
As we learn from initiatives like Australia's affirmative consent laws and the Make No Doubt campaign, clarity and understanding in consent are crucial. These efforts highlight the need for explicit communication and mutual understanding, principles that are directly applicable to the context of informed consent in research. Providing participants with ample time to digest the information, without feeling rushed, supports a transparent consent process where questions can be addressed, fostering a trustful environment.
In the words of experts, "Consent means someone gives someone else permission to do something. We explain what we are trying to understand in our research, and we ask people to agree to participate." This statement underscores the ethical imperative for researchers to communicate effectively, allowing every individual, including those with intellectual disabilities, to exercise their right to informed consent.
In light of recent critiques on decision-making models, it's acknowledged that individuals often make decisions under time constraints, which can influence the quality of their consent. Thus, it's critical for the informed consent process to accommodate the cognitive and temporal aspects of decision-making, ensuring that participants are not just informed, but fully equipped to make decisions that align with their values and interests.
- Verbal and Written Communication
To ensure a comprehensive understanding of informed consent among diverse patient populations, it's critical to combine clear verbal explanations with written materials. Verbal discussions of consent allow for interactive clarification and immediate answers to any questions. Accompanying these conversations with written consent forms or information sheets provides patients with a reference they can review at their own pace, aiding in the retention of information. This dual approach is especially vital in regions like Southern Nevada, where a significant portion of the population may speak a language other than English at home. Tailoring consent materials to the demographic's linguistic needs not only fosters inclusivity but also aligns with regulations mandating accessibility.
Furthermore, personalized communication is paramount in medical contexts. For instance, Summer Health's practice of supplementing text message consultations with detailed medical visit notes respects the importance of comprehensive patient communication post-consultation. This documentation ensures continuity of care and empowers parents through a clear understanding of the discussed care plans and follow-up actions. Informed consent materials that are easy to understand and cater to the specific needs of the audience, as advocated by experts, are more likely to resonate and be accepted by patients.
Moreover, recent guidelines suggest that informed consent documents should begin with key information presented simply and succinctly to facilitate understanding. This approach can be particularly beneficial to health literacy and could be considered a form of returning value to participants in research studies. Health research summaries, for example, provide a concise and informative overview of the study's findings, contributing to the participants' understanding and potentially increasing their engagement.
Statistics have shown that readability is a crucial factor in patient comprehension, with many consent forms being too complex for the general public. Employing readability assessment tools can ensure that written materials meet the necessary standards for patient comprehension. This is essential for informed consent, where understanding risks, benefits, and treatment options is critical for patient autonomy and ethical medical practice. Overall, the goal is to create informed consent materials that are not only legally compliant but also genuinely informative and respectful of patients' needs and backgrounds.
- Use of Visual Aids
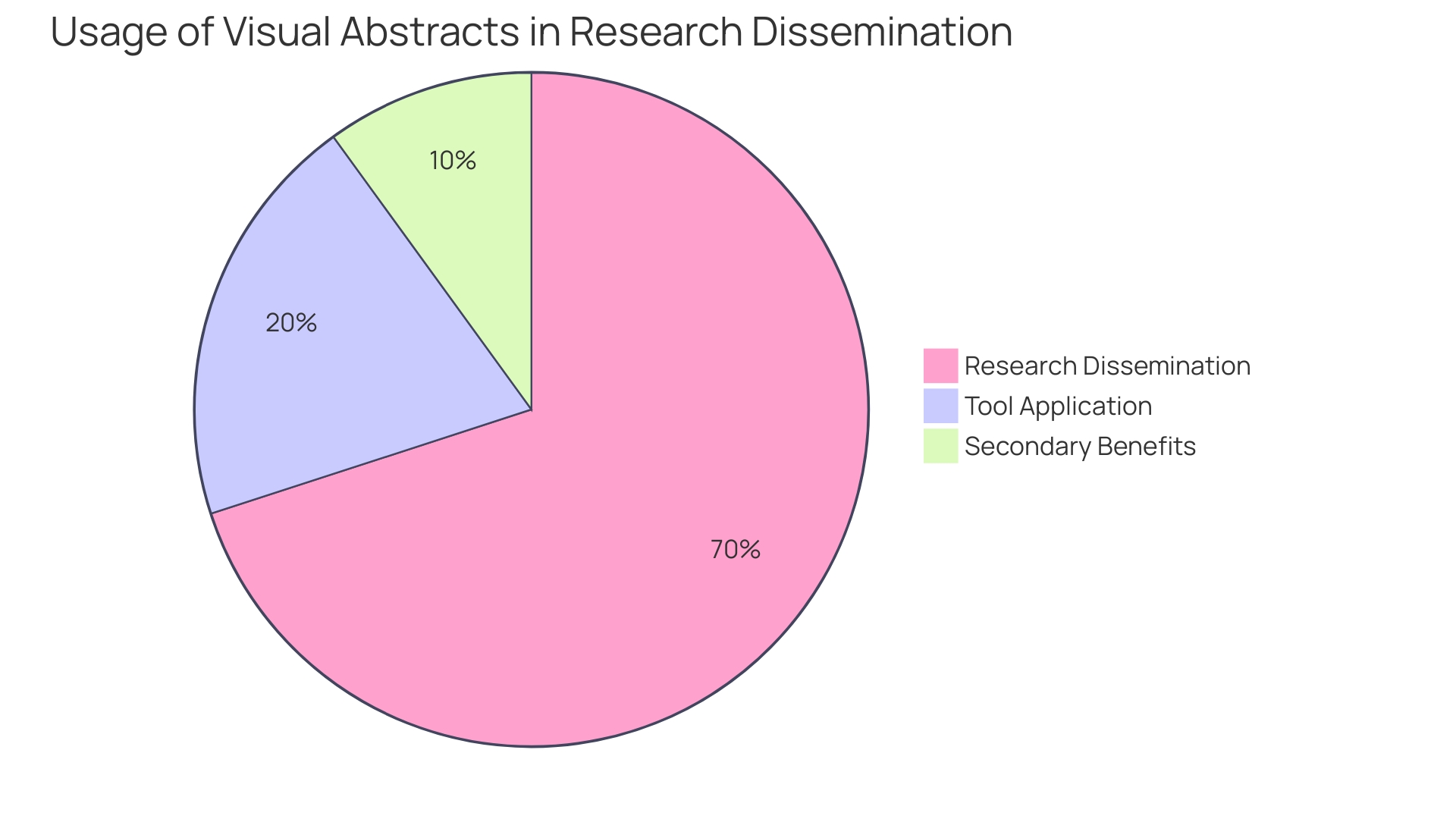
The absorption and retention of complex medical information can be significantly enhanced through the use of visual aids. For instance, the introduction of visual abstracts has revolutionized the way research findings are communicated, allowing for a succinct summary of an article's content with an emphasis on the research question, methodology, and primary outcomes. This practice has not only been adopted by over 100 journals and institutions but has also demonstrated through randomized trials to increase research dissemination. When the first visual abstract was published in 2016, it detailed the effects of establishing a pan-regional trauma system in London and subsequently garnered over 30,000 views on social media within two weeks. This marked a threefold increase in article visits compared to traditional sharing methods, underscoring the visual abstract's potential to capture attention and disseminate knowledge effectively.
Moreover, interactive data visualizations have been shown to help readers process numerical information more systematically, making it more vivid and easier to understand complex social issues. An NSF-funded study published in Digital Journalism supports this, highlighting the advantages of interactive visuals over plain text or static representations.
In clinical research, the integrity of shared images is paramount. Proofig AI's founder, Dr. Dror Kolodkin-Gal, emphasizes that accurate visual documentation is critical for the validation of research findings. This is particularly significant in light of instances where the validity of crucial studies, such as a major Alzheimer's disease investigation in 2006, was called into question.
Educational visuals in the academic setting, such as diagrams illustrating the pelvic anatomy and its correlation with OB/GYN surgical procedures, can bridge the gap in medical education by enhancing the application of anatomical knowledge in clinical practice. Similarly, the creation of academic posters serves dual purposes: to convey research succinctly for broad and serendipitous insights, and to facilitate networking among peers with similar research interests.
In conclusion, visual aids are an indispensable tool in healthcare and research communication, offering a multifaceted approach to presenting information that is both engaging and accessible. They not only simplify complex concepts but also play a vital role in improving participant understanding, ensuring that research findings are effectively communicated and comprehended.
- Assessment of Participant Comprehension
To truly grasp the essence and implications of informed consent, healthcare practitioners and researchers must step beyond the mere delivery of information and engage in meaningful dialogue with participants. This dialogue should employ open-ended questions, fostering a two-way exchange that ensures individuals fully comprehend the nature of their involvement in research or clinical care. By doing so, we uphold the integrity of the consent process, ensuring that participants are not only informed but also actively engaged in the decision-making process.
Research underscores the value of using straightforward, jargon-free language when conveying complex medical information, thereby enhancing participants' understanding and retention of the material. This approach not only facilitates informed consent but also serves as an educational resource, empowering participants to make informed decisions about their health and the research in which they partake.
Moreover, summaries that resonate with the readers' values, such as contributing to meaningful research or improving health equity, can significantly bolster engagement and trust. As demonstrated by the All of Us Research Program, which engaged over 300,000 participants through tailored email summaries, such strategies can lead to higher levels of participant engagement, particularly when the content is relevant to their interests and health priorities.
These insights align with sentiments expressed by experts in the field, who emphasize the importance of clear, focused communication in research. As Dawn Corbett, NIH's Inclusion Policy Officer, notes, ensuring comprehensive understanding throughout the study is crucial for the full inclusion of diverse individuals in research. This commitment to effective communication is echoed by Melissa McGowan, Deputy Director of the Office of Clinical Research at the National Institute on Aging, who stresses the significance of language access in reaching a broad spectrum of people with varying health needs.
In conclusion, by embracing these principles and prioritizing participant comprehension through thoughtful, accessible communication, healthcare professionals and researchers can foster a more inclusive, informed, and engaged participant base, thereby advancing the quality and impact of clinical care and research.
Documentation and Revision of Consent Forms
Ensuring that the informed consent process is meticulously documented is not only a legal necessity but also an ethical imperative. The consent form must be crafted with precision, undergo thorough reviews, and be updated as required to reflect any changes. The significance of comprehensive documentation lies in its ability to serve two main purposes: firstly, to aid potential participants in making informed decisions about their involvement in a study by presenting necessary information in an understandable format, rather than merely listing disjointed facts; and secondly, to enhance their comprehension of the research they may partake in.
Despite these goals, creating, revising, and understanding informed consent documents have become increasingly challenging. These documents, essential for legal and ethical research conduct, have evolved into lengthy and complex texts that can impede clinical trial enrollment, particularly among minority populations who are already underserved. Stakeholders, ranging from Institutional Review Boards (IRBs) and physicians to clinical trial sponsors, participants, and regulators, have expressed dissatisfaction with the current state of informed consent forms. The growing length of these documents, sometimes exceeding twenty pages with over 270 mandatory items, paired with their often high-level language and legalistic tone, reflects an effort to adhere to legislative requirements, but at the cost of accessibility and clarity.
The crux of informed consent is the fair and voluntary decision-making process by participants, which is why the understanding of 'competence' and 'capacity' is crucial. Competence, a legal concept, speaks to a person's overall ability to make decisions across various domains such as finance and property. It is determined within the legal system, distinct from 'capacity,' which is a medical term assessed by physicians to gauge a patient's capability to make informed decisions specifically about their medical care.
To address these challenges, guidance has been proposed to streamline informed consent by highlighting key information at the outset of the document. This approach aims to clarify the purpose of the research, delineate potential risks and benefits, and outline the study's duration and procedures in a concise and intelligible manner. Such guidance not only serves as a tool for investigators to facilitate discussions with potential participants but also acts as a resource for current study participants to better understand the research they are involved in.
Healthcare providers must honor and respect the decisions of individuals, ensuring that informed consent is an expression of their right to make educated choices regarding their healthcare without coercion or undue influence. It is imperative that all information provided is complete, accurate, and varied, enabling individuals to grasp the full scope of medical and non-medical procedures and treatments.
The integrity of scientific research hinges on practices that maintain trust and transparency. Questionable Research Practices (QRPs), which deviate from traditional research values and standards, threaten this trust and can occur at any stage of the research process. There is a recognized need for comprehensive perspectives to understand the prevalence and impact of QRPs.
Moreover, the transparency of clinical trials is paramount, yet the commitment to this transparency is inconsistent across the industry. Full disclosure of trial protocols, statistical analysis plans, and raw data is essential for research to be transparent and reproducible. While initiatives to enhance data sharing have been proposed, their implementation remains suboptimal, highlighting the need for routine access to raw data for a broader range of stakeholders.
In conclusion, informed consent documents should not be seen as mere formalities but as critical tools that empower participants to make knowledgeable and autonomous decisions. By simplifying these documents and focusing on clear communication, we can uphold the ethical standards of research and foster an environment of trust and respect for participants' rights.
- Participant Signatures
Consent in research is a crucial element that embodies respect for individuals and their autonomy, allowing participants to make informed decisions about engagement in healthcare and research projects. It is essential that participants are presented with clear, concise information regarding the nature and purpose of the research, the procedures involved, potential risks and benefits, and any reasonable alternatives to participation. This is not just an ethical imperative but a legal one, ensuring that participants are not subject to deception or coercion and are safeguarded against unnecessary harm.
Particularly in clinical settings, informed consent is a nuanced process. While consent is implied for routine procedures such as venipuncture, more sensitive examinations, like those involving HIV or genetic testing, necessitate explicit consent due to the potentially profound implications for the individual's privacy and wellbeing. In cases where individuals are unable to provide consent, such as those with intellectual disabilities, it is paramount that consent is sought in a manner that respects their right to autonomy, using plain language and adequate support to facilitate understanding.
The evolving landscape of consent in digital realms, as seen in recent developments in blockchain technology, underscores the importance of consent as a dynamic and binding agreement. This technology ensures transparency and control over personal data, mirroring the principles of informed consent in healthcare, where participants must have clarity and control over their involvement.
In research, the inclusion of diverse populations is vital for the generalizability of findings. However, underserved groups may be overlooked, leading to a lack of representative participation and potential biases. This not only affects the quality of research but also perpetuates health disparities. Adequate informed consent processes, tailored to address the needs of various populations, can foster inclusivity and trust, thereby enhancing both the ethical integrity and the scientific validity of research.
In summary, the act of obtaining informed consent is an ongoing commitment to respect, transparency, and inclusivity. It is a foundational aspect of both ethical conduct and legal compliance in healthcare and research, ensuring that all participants are fully informed and have willingly agreed to partake in the procedures or studies proposed.
- Witness Signatures
When dealing with vulnerable populations or higher-risk procedures, the process of obtaining informed consent may necessitate additional safeguards, such as the presence of a witness during the signing of the consent form. This practice underscores the commitment to ensuring that participants comprehend the full scope of the research study, including its purpose, potential risks, benefits, and the expected duration and procedures involved. The FDA's draft guidance on informed consent emphasizes the importance of presenting these key elements at the outset of the consent document as a means to facilitate understanding and support informed decision-making. Furthermore, the Americans with Disabilities Act mandates that healthcare facilities accommodate the needs of patients with disabilities, including the provision of reasonable measures such as telehealth services, isolated waiting areas, and enhanced ventilation to safeguard against easily transmitted diseases. In the realm of clinical care and research, informed consent is a multifaceted process that encompasses permission for procedures, data utilization for care enhancement, and active research participation. It is paramount that consent is given freely and with a comprehensive understanding of the activity in which the participant is engaging. Electronic consent (eConsent) tools, which utilize audio, video, and interactive elements, have emerged as innovative methods to simplify the consent process and improve patient comprehension. These tools offer interactive glossaries and knowledge checks to ensure participants have a clear grasp of the study's implications. Ultimately, the integrity of the informed consent process is maintained through transparent communication and the provision of easily accessible, essential information to participants.

- Version Control and Date
Informed consent forms are vital tools in research, ensuring that participants are fully aware of the study details and their rights. These documents must be meticulously crafted to include comprehensive variable lists with full definitions and spelled-out abbreviations, especially for tabular data and units of measurement. They should also detail any computational methods used, such as scripts or software versions, and provide references to related research articles or data sources, ensuring everything is clearly understood, not assuming universal knowledge of acronyms or scoring keys.
Moreover, the forms should reflect the complexity of health data governance, adhering to ethical and legal standards set by instruments like the GDPR or the Declaration of Taipei, which protect data subjects' rights and foster public trust. They must be kept up-to-date, with version control and dates of signing prominently displayed, to guarantee participants have access to the latest information, a practice underscored by digital preservation experts who emphasize the necessity of maintaining accessible and long-lasting records.
In the dynamic digital age, where 'twenty years is ancient,' as highlighted by digital preservation professionals, the consent process must ensure future accessibility and comprehension of consent documentation. This includes not just the content but also the format, which must be preserved in a way that remains openable and understandable for years to come, as demonstrated by the challenges faced by software engineers in retrieving historical documents.
The consent form should serve as a testament to the integrity of the research, embodying principles of honesty, accountability, and stewardship, and should be structured to withstand the test of time, both in content and in digital form, to maintain the value and usability of the data for future research and discoveries.
- Storage and Accessibility
Protecting participant privacy and confidentiality is paramount in the realm of clinical research and healthcare. It is critical to ensure that any consent forms, which outline the nature of the participant's involvement and the data being shared, are stored securely and only accessible to authorized personnel. Consent processes should include full disclosure of the activity's nature and be conveyed in a manner that participants can comprehend.
Adhering to this principle, a study on participant preferences at a speed-dating event, as published in Psychological Science, serves as a case in point. Researchers ensured the confidentiality of over 300 participants by anonymizing data, removing personal identifiers such as ages and ethnicities, and sharing only aggregate data. Such measures are in line with the guidelines suggested by the European Commission, which emphasize the critical nature of ethics in research. The Commission is actively seeking to update policy documents like the European Charter for Researchers to address the dynamic landscape of European research and innovation.
Furthermore, the integration of technology in consent processes is becoming increasingly important. A study published in the journal Computer Communications outlined a blockchain-based personal data management platform that employs smart contracts to manage consent, which once agreed upon, are immutable and adhere to the European Union's General Data Protection Regulation (GDPR) principles. This advancement exemplifies the need for innovative solutions to protect participant data in a technologically evolving environment.
To ensure compliance with regulatory standards and ethical guidelines, it is essential to document the source of data, whether it stems from surveys, assessments, or observations, and specify how the data will be cleaned and curated before sharing. The level of data aggregation and the format of documentation, whether XML, CSV, or PDF, must be considered, along with any standards for metadata or data collection.
In summary, the secure storage and restricted access to consent forms, alongside the meticulous anonymization of participant data, are non-negotiable aspects of maintaining privacy and confidentiality. These practices are vital in fostering public trust and ensuring that data remains available for future research and discovery, thus contributing to the advancement of medical knowledge and the improvement of patient outcomes.
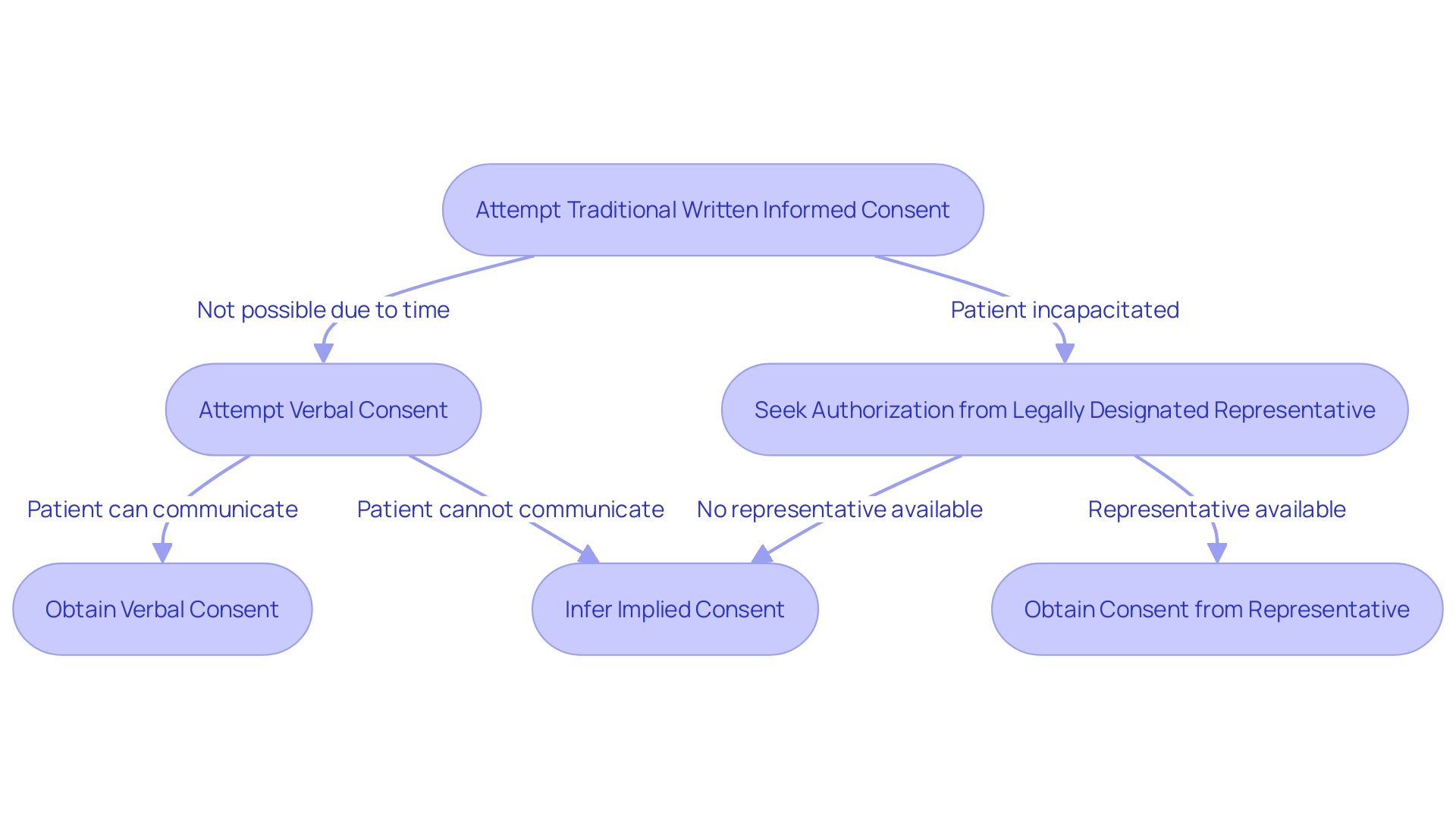
Informed Consent in Urgent or Emergency Situations
In situations where the immediacy of medical intervention overrides the ability to obtain traditional written informed consent, healthcare professionals may resort to alternative methods. Verbal consent, while not the norm, is sometimes necessary when time is critical, and the patient can still communicate their agreement. Implied consent may be inferred from the patient's behavior in emergency circumstances when they are unable to provide verbal consent but require immediate care. In scenarios where the patient is incapacitated or otherwise unable to consent, seeking authorization from a legally designated representative becomes the course of action. These methods uphold the ethical principle of patient autonomy and ensure that care is provided respectfully and responsibly, aligning with the consensus that patient dignity and informed participation are paramount in healthcare.
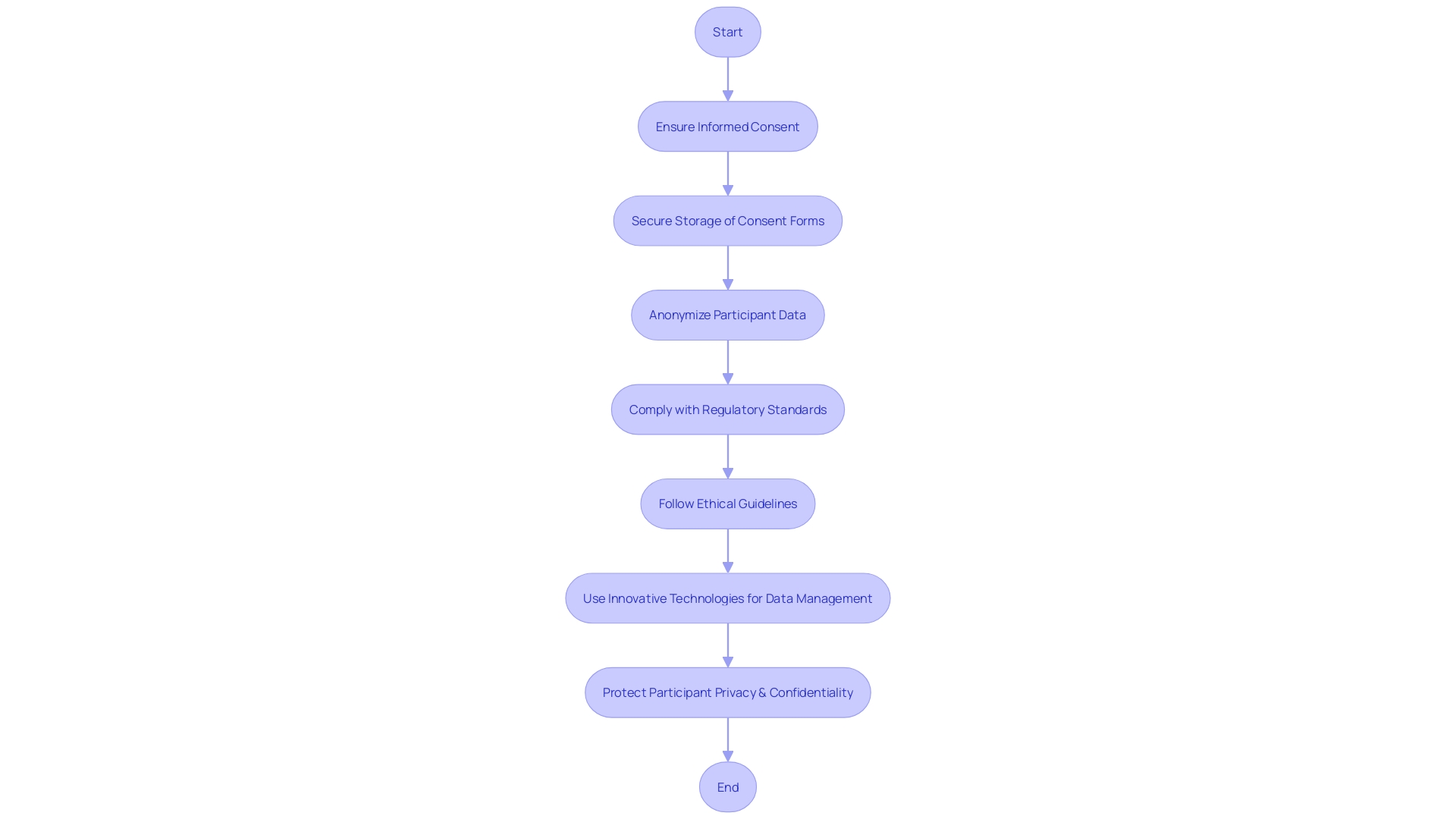
Conclusion
In conclusion, clear communication is crucial in the informed consent process for ethical and legal medical practice. The article emphasizes the importance of various components, including describing the research or procedure, outlining risks and benefits, presenting alternatives, ensuring confidentiality, respecting voluntariness and the right to withdraw, providing compensation for injury, offering contact information for questions and concerns, addressing additional costs and consequences of withdrawal, disclosing new findings and commercial use, considering vulnerable populations, optimizing the process, using visual aids, assessing participant comprehension, documenting and revising consent forms, obtaining participant and witness signatures, maintaining version control and dates, and ensuring secure storage and accessibility.
To optimize the process, healthcare professionals and researchers should prioritize clear and accessible information, using visual aids and plain language. Adequate time for decision-making and meaningful dialogue with participants are essential. Documentation and revision of consent forms should be meticulous, and privacy and confidentiality must be upheld.
In urgent or emergency situations, alternative methods like verbal or implied consent may be necessary to ensure timely care while respecting patient autonomy.
By addressing these aspects, healthcare professionals and researchers can ensure an effective informed consent process that upholds ethical and legal standards, respects patient autonomy, and promotes clear communication.




