Introduction
Navigating the complex regulatory landscape of medical device approval in the United States requires a comprehensive understanding of the 510(k) submission process. This critical premarket notification to the FDA is essential for manufacturers aiming to introduce new devices to the market, as it ensures adherence to stringent safety and effectiveness standards. The core of the 510(k) submission lies in demonstrating substantial equivalence to an already legally marketed device, a task that demands meticulous preparation and a deep understanding of both the subject device and its predicate.
This article delves into the key steps of the 510(k) submission process, from determining device eligibility to conducting necessary testing and preparing the final application. By highlighting the importance of detailed comparative analyses, robust quality management systems, and thorough documentation, it aims to guide manufacturers through each phase, significantly enhancing the likelihood of a successful submission. Additionally, the article sheds light on the FDA’s decision-making criteria, emphasizing the importance of clear communication and data-driven arguments focused on patient safety.
Understanding these elements is crucial for efficiently navigating the regulatory requirements and achieving market approval for new medical devices.
What is a 510(k) Submission?
A 510(k) filing acts as a premarket alert to the FDA, showing that a medical instrument is significantly comparable to an already legally marketed product. 'This process is crucial for manufacturers aiming to introduce their products into the U.S. market, as it ensures compliance with the FDA's stringent safety and effectiveness standards.'.
'Significant similarity, a key element of the 510(k) application, indicates that the instrument has the same intended use and technological features as the predicate product, or any differences do not introduce new safety and effectiveness concerns.'. Statistics show that 75% of 510(k) submissions are initially rejected, with 85% of these rejections due to issues with demonstrating substantial equivalence during the scientific review. Therefore, a deep understanding of both the subject apparatus and its predicate is vital.
To achieve substantial equivalence, manufacturers should thoroughly understand the intended use, technological characteristics, and any potential risks associated with their product. This involves detailed comparisons with predicate devices, including reviewing research literature, clinical studies, and the FDA's Summaries of Safety and Effectiveness (SSEs). Recognizing and tackling these factors can greatly enhance the chances of a successful 510(k) filing.
Key Steps in the 510(k) Submission Process
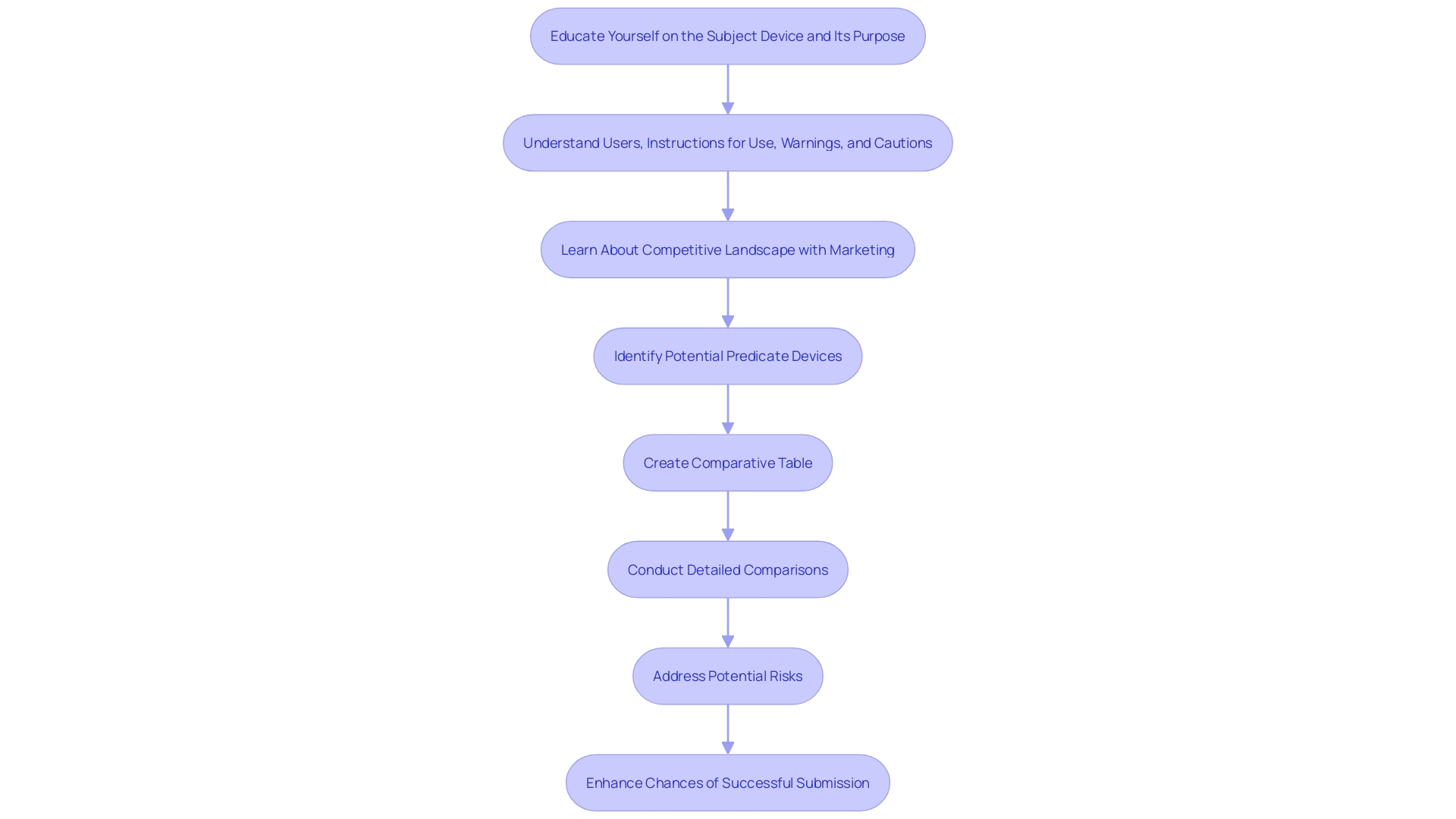
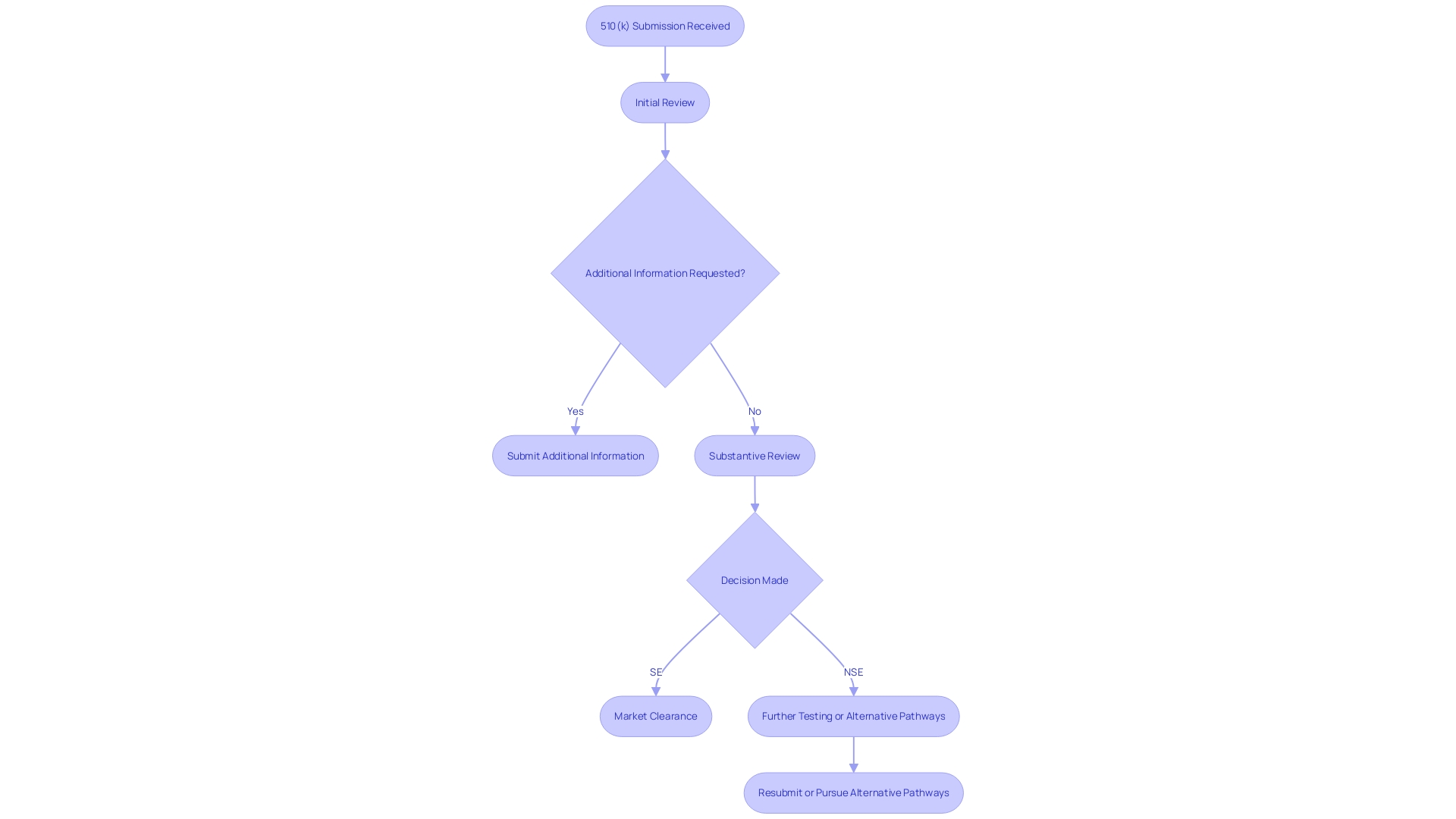
The 510(k) filing process involves several critical steps that ensure compliance with FDA requirements. Firstly, it is essential to determine if your equipment is eligible for a 510(k) submission. This involves understanding the tool's intended use and identifying similar items already on the market. Learning about the instrument, its users (clinicians, physicians, dentists, patients), and its guidelines for operation is essential.
Next, identifying a predicate instrument with the same intended use and similar technological characteristics is a pivotal step. According to FDA guidelines, substantial equivalence means that the device has the same intended use as the predicate device and either the same technological characteristics or different technological characteristics that do not raise new safety or effectiveness questions. It's noteworthy that 75% of 510(k) applications are initially rejected, with 85% of these rejections due to issues related to substantial equivalence during the scientific review.
Building a robust quality management system follows, ensuring that all processes meet FDA standards. This system includes comprehensive documentation and adherence to regulatory requirements, which are vital for the smooth progression of the application. Furthermore, performing essential testing and research is crucial to showcase the safety and effectiveness of the equipment. This information serves as the foundation of your entry, offering proof that your apparatus is as secure and efficient as a legally marketed product.
Ultimately, preparing the application for presentation requires meticulous attention to detail. This entails gathering all pertinent information, including comparative charts of predicate products, and confirming that the application is comprehensive and precise. By following these steps, you can enhance the likelihood of a successful 510(k) filing, navigating the regulatory landscape efficiently and effectively.
Step 1: Determine Eligibility for 510(k)
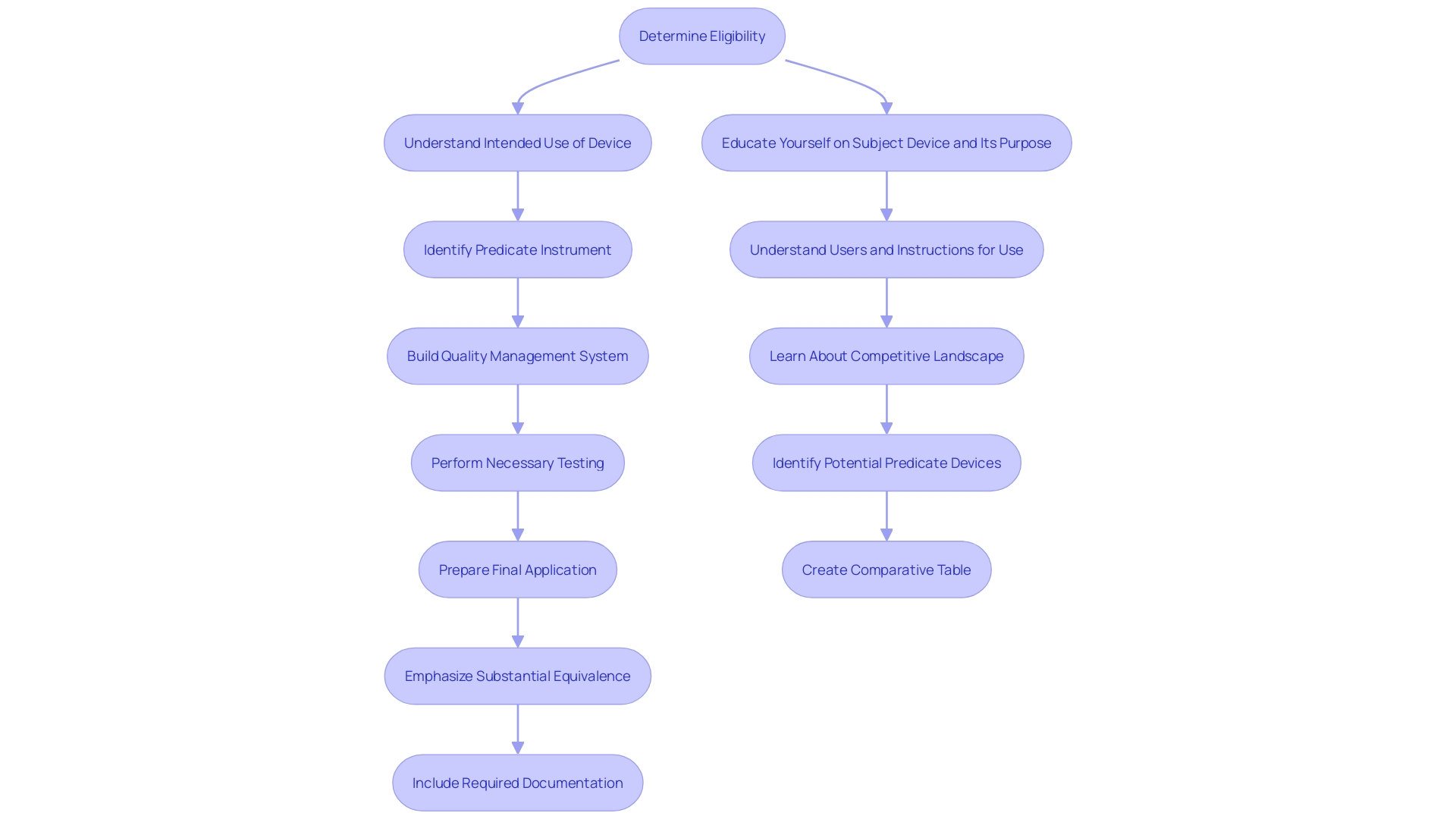
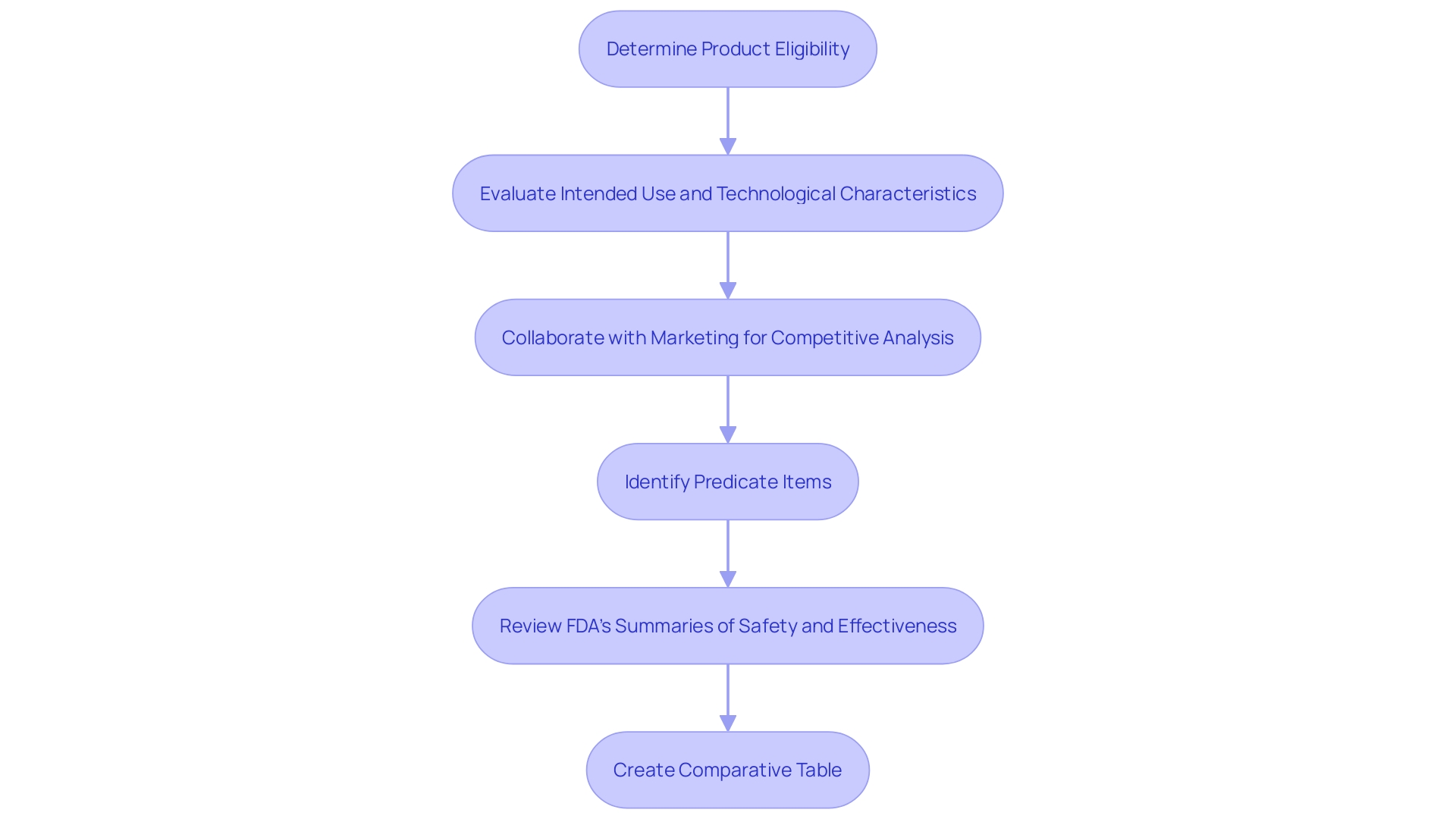
The initial step in the 510(k) submission process is determining if the product qualifies for this pathway. Instruments intended for human use and not exempt from 510(k) requirements must demonstrate substantial equivalence to a predicate instrument. This involves a thorough evaluation of the item's intended use and technological characteristics.
Learning about the topic and its function is essential. Aim to understand the users (clinicians, physicians, dentists, patients, etc.), and thoroughly review the instructions for use, including warnings and cautions. Work together with Marketing to understand the competitive environment and concentrate on rival products. 'Examine research literature, clinical studies, websites, brochures, sell-sheets, and labeling to identify potential predicate items with the same intended use and similar technological characteristics.'. Create a comparative table to aid in this process.
Once potential predicate items are identified, review the Summaries of Safety and Effectiveness (SSEs) available on FDA’s 510(k) database to evaluate similarities and differences. This comprehensive understanding is essential for a successful 510(k) submission, ensuring the product meets the FDA's substantial equivalence criteria.
Step 4: Conduct Device Testing and Studies
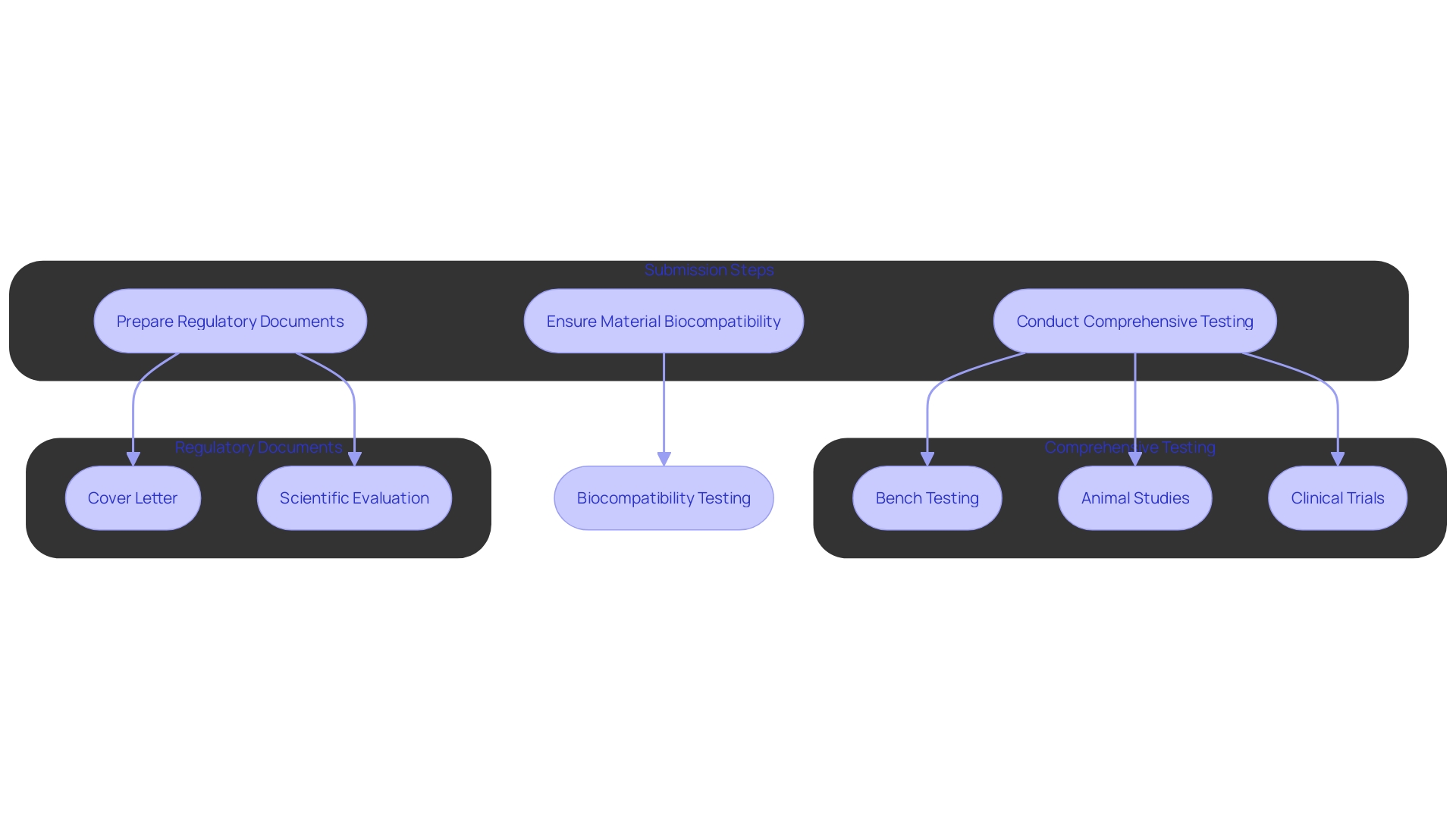
Before submitting a 510(k) application, manufacturers must conduct comprehensive testing and studies to gather data that demonstrates the product’s safety and effectiveness. 'This process may involve bench testing, animal studies, or clinical trials, depending on the classification of the apparatus and associated risks.'. For example, the FDA’s Materials and Chemical Characterization Program at the Center for Devices and Radiological Health (CDRH) plays a crucial role in ensuring the biocompatibility and biostability of materials utilized in medical equipment. The synthesis, processing, and fabrication of these materials are scrutinized to determine their suitability for specific applications.
The significance of these studies cannot be overstated, as they form the backbone of the 510(k) submission, establishing the product's equivalence to a legally marketed predicate. 'According to a 2018 FDA study, more than 1.7 million injuries and 83,000 deaths over a decade were potentially linked to medical equipment, underscoring the critical need for rigorous testing and validation.'.
Furthermore, industry specialists emphasize the changing environment of regulatory requirements, which influences the creation and market introduction of medical tools. Efficient preparation of regulatory and safety documents is essential to meet these increasing requirements, minimizing delays and errors. Practical approaches to adapt and thrive amidst changing regulatory landscapes are vital, as evidenced by firsthand experiences and survey responses from professionals in the field.
Understanding FDA Decisions: Substantial Equivalence (SE) and Not Substantially Equivalent (NSE)
After the 510(k) submission is reviewed, the FDA issues a decision based on the data provided. If the FDA determines the item to be substantially equivalent (SE) to a legally marketed product, it grants clearance, allowing the product to be marketed. Conversely, a not substantially equivalent (NSE) decision indicates that the product does not meet the required criteria, potentially necessitating additional testing or an alternative regulatory pathway such as the Pre-Market Approval (PMA) or De Novo process. Understanding these outcomes is crucial for manufacturers to effectively navigate subsequent steps and ensure compliance with regulatory standards.
Tips for a Successful 510(k) Submission
To significantly increase the chances of a successful 510(k) submission, it is crucial to thoroughly prepare all documentation and conduct comprehensive testing. Start by acquiring a thorough comprehension of the subject tool, its intended users—including clinicians, physicians, and patients—and its instructions for use, including any warnings and cautions. Work together with marketing groups to examine the competitive environment, concentrating on comparable products. Examine research literature, clinical studies, websites, brochures, and labeling to identify potential predicate items with the same intended use and similar technological characteristics. Creating a comparative table can be highly beneficial.
Once potential predicate devices are identified, examine the Summaries of Safety and Effectiveness (SSEs) available on the FDA’s 510(k) database to evaluate similarities and differences. Maintaining clear communication with the FDA throughout the process is imperative. Collaborating with regulatory advisors and utilizing input from pre-filing meetings can offer valuable insights and improve the quality of your application.
According to industry experts, the FDA prioritizes patient health and safety above all else. Ensure that any arguments made during the submission process are data-driven and centered around patient welfare. The evolving regulatory landscape demands that professionals adapt and thrive by ensuring compliance and efficiency. This approach not only improves the likelihood of approval but also aligns with the FDA's mission to protect public health.
Conclusion
Navigating the 510(k) submission process is essential for manufacturers seeking to introduce medical devices to the U.S. market. This process hinges on demonstrating substantial equivalence to legally marketed devices, which requires a comprehensive understanding of both the new device and its predicate. Given that a significant percentage of submissions are initially rejected, meticulous preparation, including thorough comparative analyses and robust documentation, is critical for success.
The key steps in the 510(k) submission process include determining device eligibility, identifying appropriate predicate devices, and building a strong quality management system. Conducting necessary testing and studies is paramount, as this data supports claims of safety and effectiveness. By adhering to these steps and maintaining clear communication with the FDA, manufacturers can enhance their chances of obtaining clearance for their devices.
Ultimately, the emphasis on patient safety and rigorous compliance with regulatory standards cannot be overstated. Manufacturers must prioritize data-driven arguments that focus on patient welfare throughout the submission process. By doing so, they not only improve the likelihood of approval but also align with the FDA's commitment to protecting public health.




