Introduction
The Humanitarian Device Exemption (HDE) program offers an expedited regulatory pathway for medical devices aimed at treating or diagnosing rare diseases or conditions, which affect fewer than 8,000 individuals in the United States annually. This program is pivotal in facilitating the availability of innovative medical solutions to patients with limited treatment options by allowing device manufacturers to bring their products to market with reduced clinical trial requirements. While streamlining the regulatory process, the program ensures that devices still undergo rigorous evaluation to ensure compliance with quality standards.
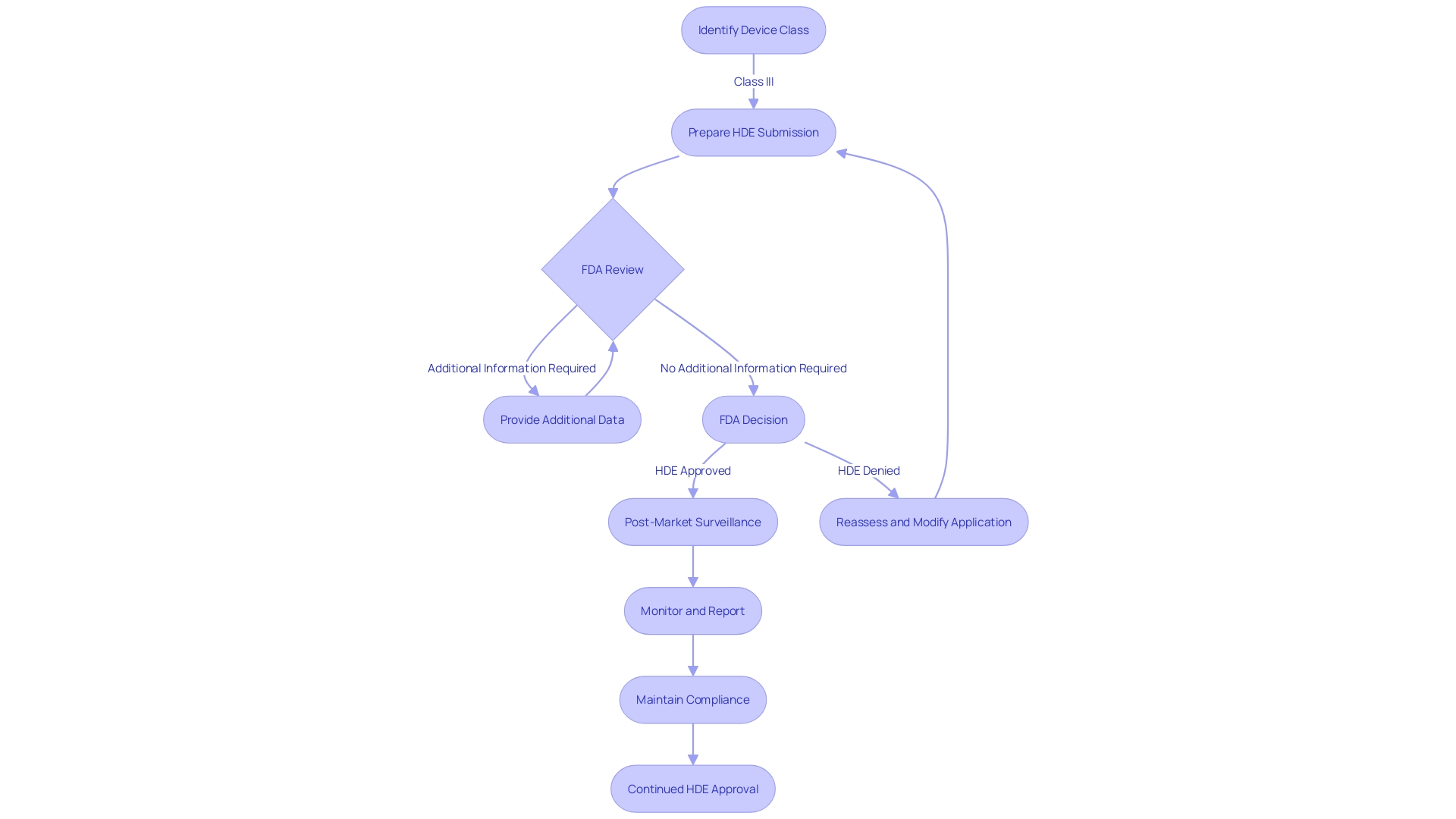
This article explores the criteria for HDE eligibility, the key elements of an HDE application, IRB approval and oversight requirements, emergency use of HDE devices, profit and use restrictions, the annual distribution number for profitable sales, a case study on navigating the HDE application process, and the challenges and considerations for HDE devices. Through these insights, we gain a comprehensive understanding of the complex landscape surrounding HDE devices and the importance of balancing innovation with patient safety.
Definition and Criteria for HUDs
To qualify for the Humanitarian Device Exemption (HDE) program, healthcare instruments must meet strict criteria. They should be designed for the diagnosis or treatment of conditions that affect fewer than 8,000 individuals annually in the United States. Importantly, there should be a lack of any comparable product on the market that deals with the same health problem. A case in point is the Impella Connect System, which includes components like the Impella Automated Controller (AIC) for critical care heart support, and its web-based user portal and hardware for remote monitoring. The system's capacity to provide timely, patient-specific notifications qualifies it as an instrument under Section 201(h) of the Act, highlighting the HDE's emphasis on innovative solutions for unmet health requirements. Furthermore, as the landscape of healthcare equipment evolves, the FDA's classification, coding systems, and guidance documents—such as those detailing the Breakthrough Devices Program—provide essential regulatory clarity to support the advancement and proper use of healthcare technologies.
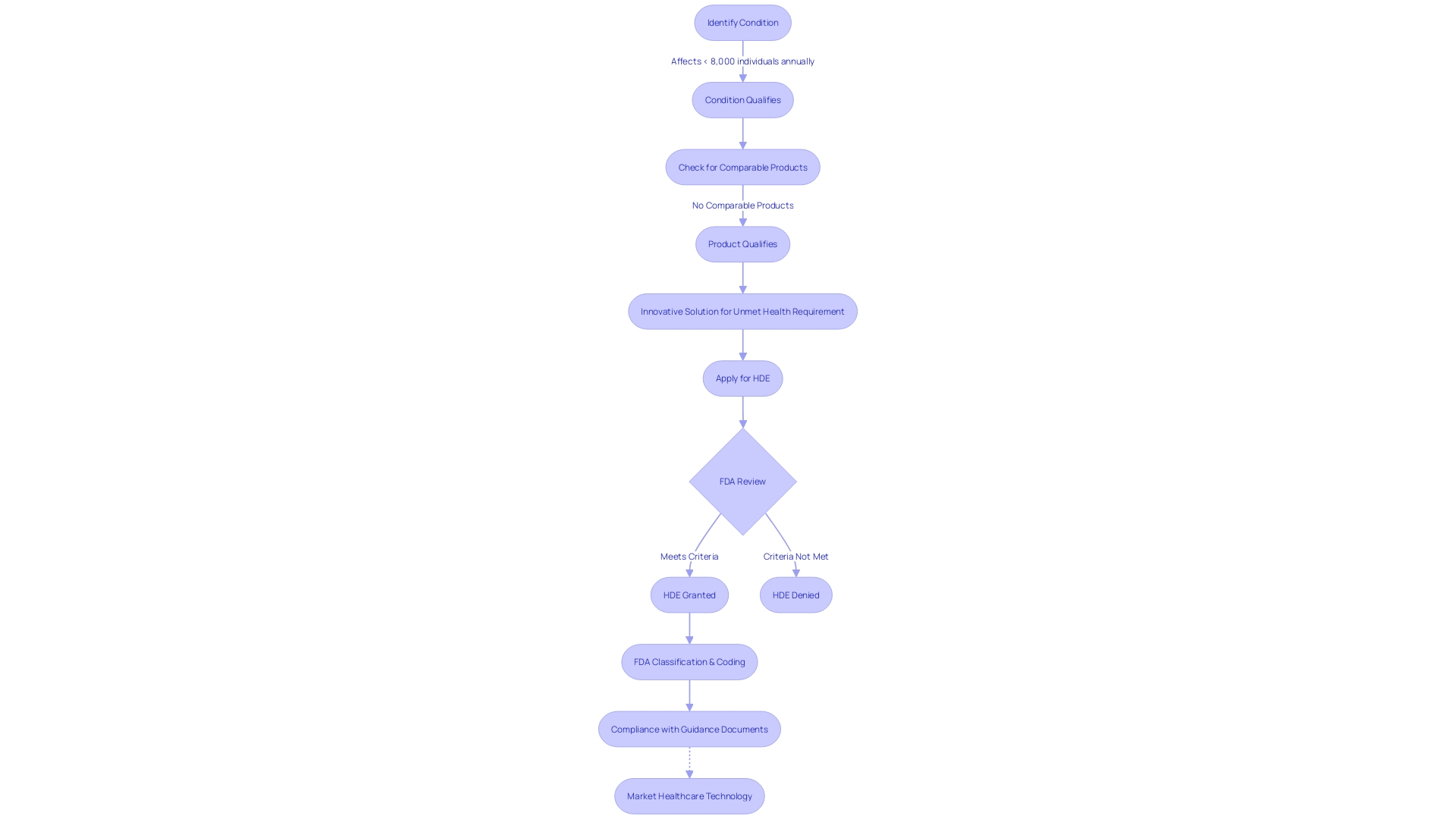
Humanitarian Device Exemption (HDE) Program Overview
The humanitarian Device Exemption (HDE) program offers an expedited regulatory pathway for healthcare instruments aimed at treating or diagnosing rare diseases or conditions, which affect fewer than 8,000 individuals in the United States annually. This program is crucial in enabling the availability of innovative solutions for patients with limited treatment options by permitting manufacturers to bring their products to market with reduced clinical trial requirements. This not only accelerates the advancement of specialized medical equipment but also aligns with the FDA's mission to ensure the safety, effectiveness, and security of medical devices. It's crucial to highlight that while the HDE program simplifies the regulatory process, it does not compromise on the quality standards that products must meet. These instruments, despite being for niche applications, still undergo rigorous evaluation to ensure they comply with current good manufacturing practice (CGMP) requirements, as mandated by the FDA's quality system regulation. The program highlights the FDA's dedication to promoting the progress of medical technology while guaranteeing patient safety and the efficiency of medical equipment.
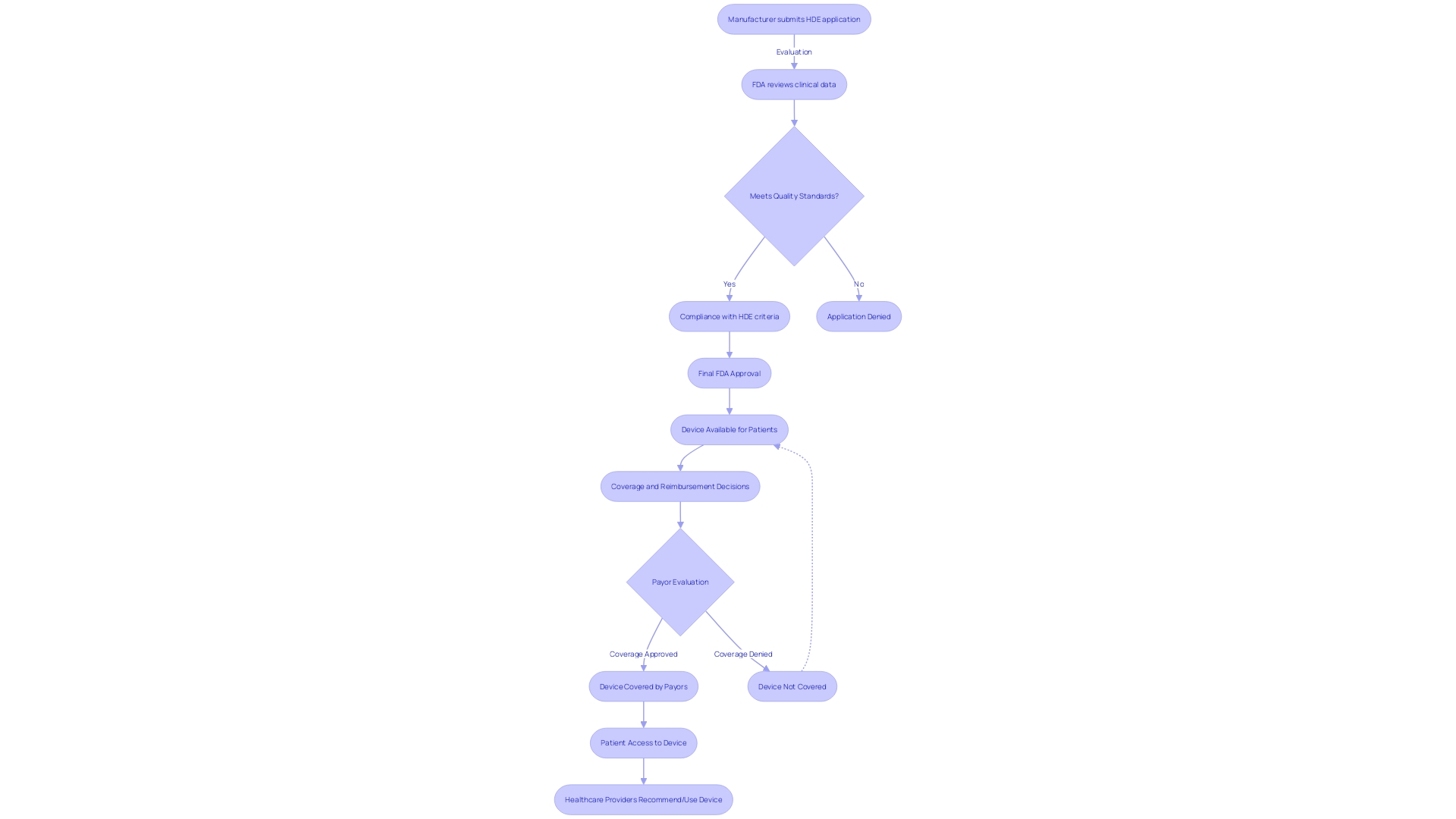
Key Elements of an HDE Application
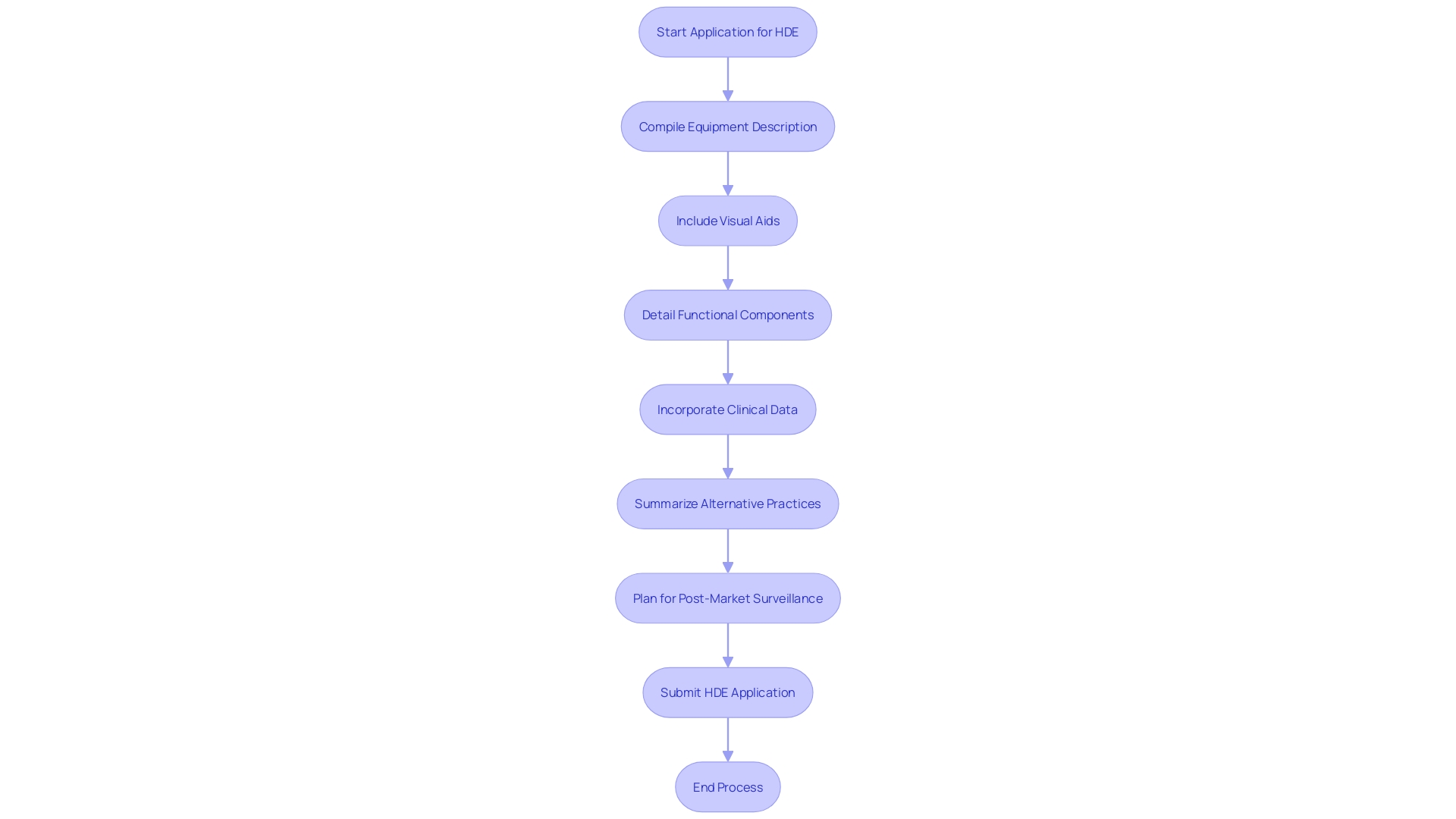
An application for Humanitarian Device Exemption (HDE) requires a comprehensive compilation of details to show the safety and potential benefits of the equipment. The application should include a comprehensive description of the equipment, accompanied by visual aids such as diagrams and specifications if applicable. It must outline the functional components or ingredients of the apparatus, especially if it comprises multiple physical entities.
The application also needs to encapsulate the conditions or diseases the apparatus intends to diagnose, treat, or mitigate, clarifying the apparatus's impact on the body's structure or function. This should be detailed along with a description of the patient demographics intended for its use. Moreover, the application should mention any FDA-assigned numbers for related legal medical instruments.
Clinical data, which confirms the safety and probable benefit of the apparatus, is a critical component of the submission. The data should be robust, possibly including real-time feeds or shared health information system data, as well as any modernized data exchange practices that contribute to public health, a crucial consideration as noted by Dr. Liz Kwo, Chief Commercial Officer at Everly Health.
Furthermore, the application should include a summary of alternative practices, offering a framework against which the benefits of the apparatus can be evaluated. A plan for ongoing post-market surveillance is essential to ensure the continued safety and effectiveness of the product after it enters the market. This plan should be efficient and secure to facilitate timely and accurate public health data exchange, aligning with the innovative approaches to data management across government and healthcare systems.
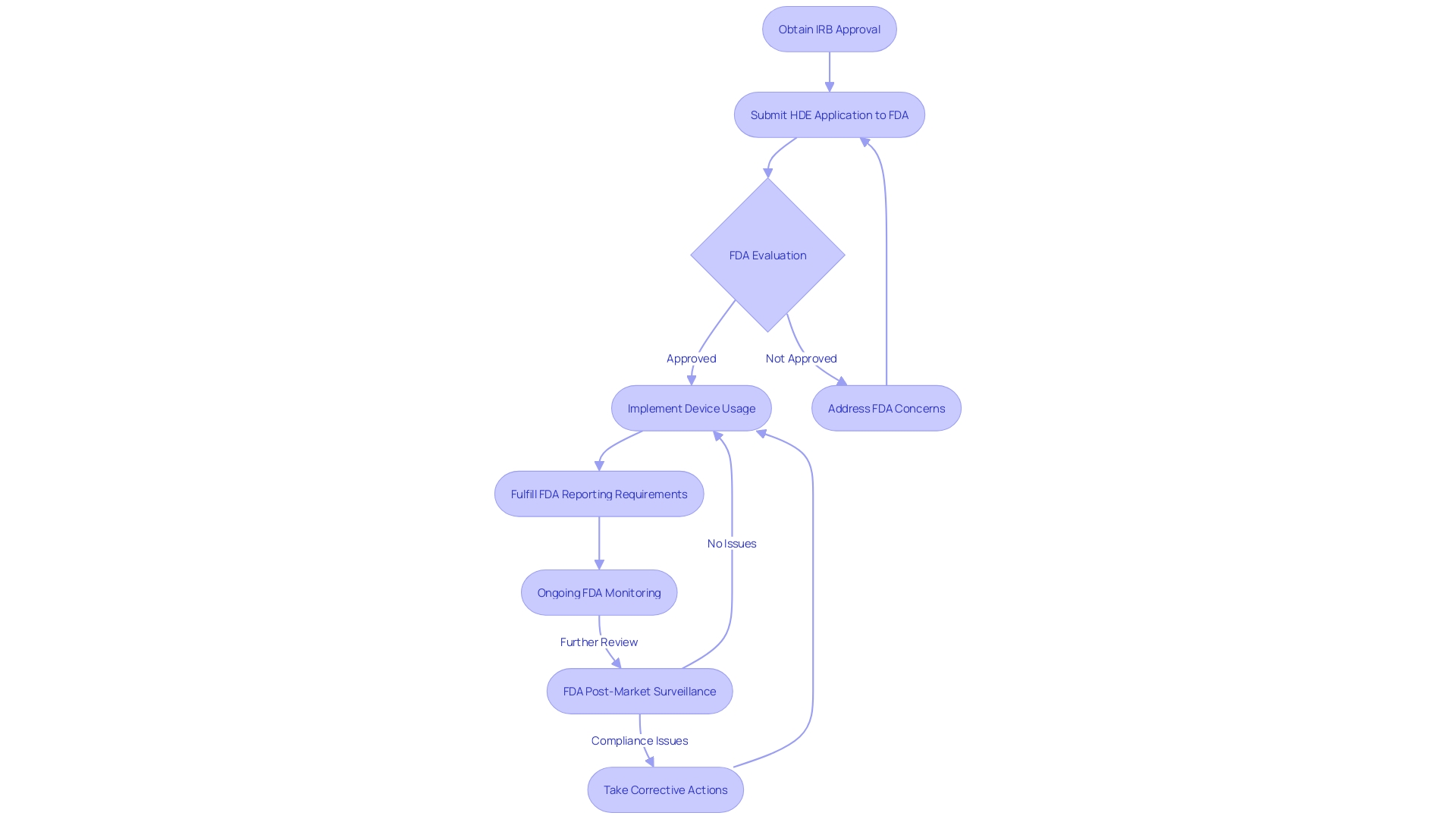
IRB Approval and Oversight Requirements
Securing Institutional Review Board (IRB) approval is the first critical step in the journey of a Humanitarian Device Exemption (HDE) application. The pivotal role of the IRB cannot be overstated as it safeguards the ethical treatment of human subjects in clinical research. Apart from this, the FDA remains watchful, with manufacturers obligated to adhere to a collection of reporting and monitoring requirements to guarantee the ongoing safety and efficacy of these products.
The governing environment for healthcare equipment is intricate and multi-tiered, with the FDA classifying instruments into three categories according to the potential harm they may cause to patients. High-risk class three products, such as life-sustaining implantables, are subject to the most stringent regulatory scrutiny and lengthy approval processes, reflecting their critical nature. Despite the robust regulatory framework, challenges such as navigating the nuances of different classifications and registration pathways—be it 510(k), PMA, or De Novo—persist.
Moreover, the FDA's role extends to overseeing the myriad ethical, legal, and social dimensions emerging from the technology's use. Through case studies, the agency not only examines the governance of these technologies but also the market incentives and intellectual property considerations that shape their evolution. These real-world examples highlight the intricacy of ethical dilemmas encountered in today's healthcare technology industry.
In the pursuit of a cross-sectoral governance framework, the FDA's responsibilities also involve ensuring public health by confirming the safety, security, and efficacy of healthcare equipment. This commitment is reflected in efforts to simplify regulatory pathways, a movement that gained momentum during the COVID-19 pandemic, to address urgent healthcare needs swiftly. As the industry evolves, particularly in digital health and personalized medicine, regulatory processes are being recalibrated to keep pace with innovation while safeguarding patient safety.
Emergency Use of HUDs
In urgent scenarios where patients face serious or life-threatening conditions and no alternative treatments are available, the FDA authorizes the emergency use of Humanitarian Device Exemption (HDE) devices. This crucial provision ensures that healthcare practitioners can access and utilize advanced equipment to provide the best possible care when every second counts. To start the emergency use of HDE tools, healthcare providers must comply with strict conditions established by the FDA to ensure patient safety and tool efficacy. As an example, the advanced Impella Connect System, created for crucial cardiac support, is an instance of an HDE apparatus that enables remote monitoring of the heart's performance, offering essential, timely information to healthcare professionals. It's crucial to mention that the FDA, as a division of the U.S. Department of Health and Human Services, is dedicated to ensuring the safety and efficacy of such healthcare instruments. As regulations and product information can vary widely from country to country, it's crucial for healthcare providers to follow the guidelines specific to the United States. Healthcare professionals and consumers are encouraged to report any issues encountered with healthcare tools under an EUA to the FDA’s MedWatch program. These reports are invaluable for the continuous assessment of equipment safety. Adverse event reporting for manufacturers under an EUA is also mandated, with specific requirements detailed within each EUA, following the regulatory guidelines outlined in 21 CFR Part 803.
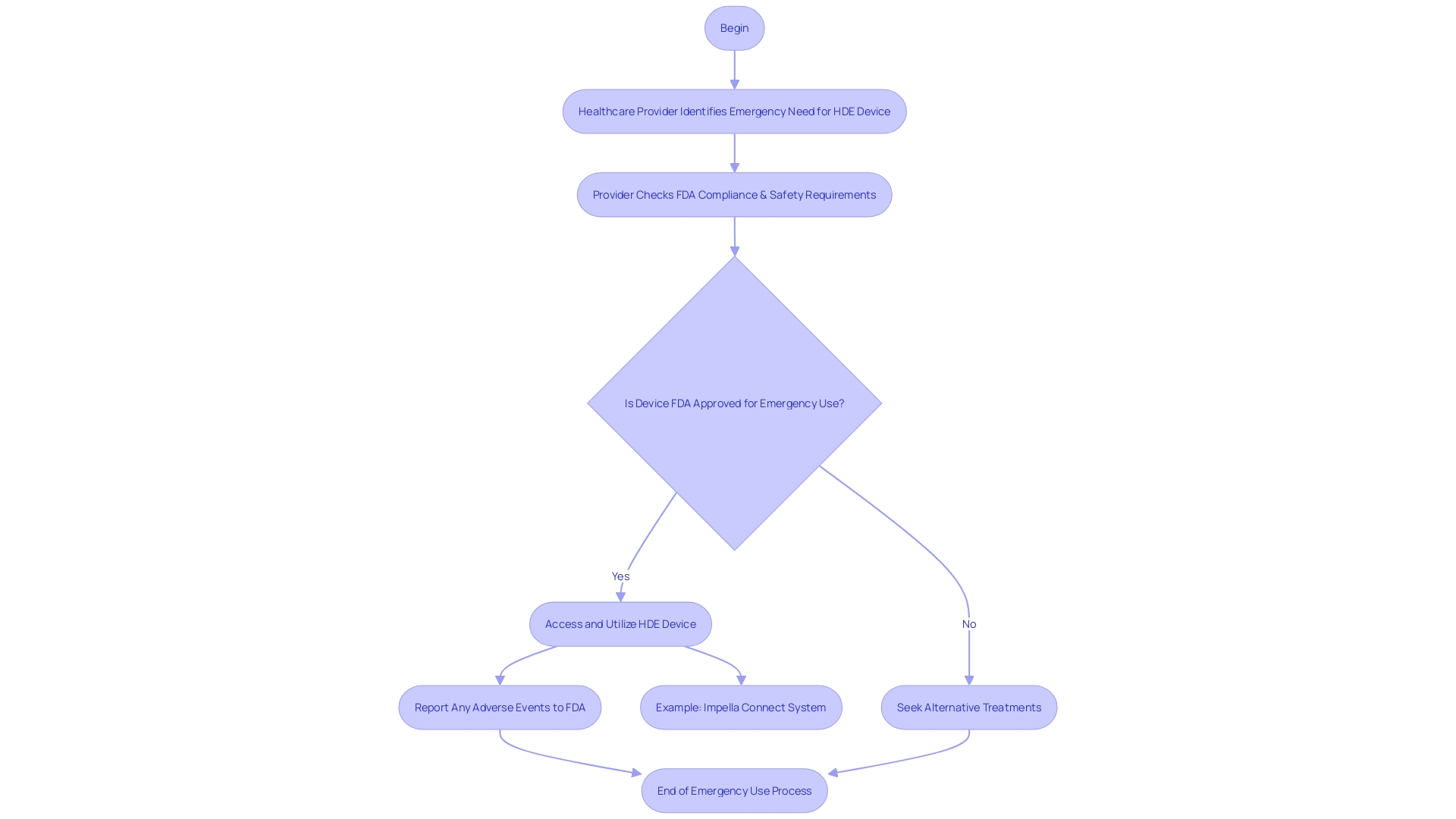
Profit and Use Restrictions for HDE Devices
Humanitarian Exemptions (HDE) are different from traditional medical tools in that their distribution and pricing are strictly regulated. In particular, HDE products cannot be sold at a price that exceeds the costs associated with their research, development, and fabrication. Moreover, healthcare facilities or professionals must either obtain an Investigational Device Exemption (IDE) or have an approved HDE application to gain access to these instruments. This regulatory framework ensures that such technology is used ethically and in a manner that prioritizes patient care over profit.
The concept of Health Institution Exemptions (HIE), also referred to as 'in-house manufacturing,' allows healthcare organizations to create and use healthcare equipment customized to specific clinical needs without complying with the complete range of regulatory requirements that commercial manufacturers encounter. This tradition developed over time to enable clinicians to invent and modify tools within the boundaries of their practice. HIEs are applicable within entities primarily engaged in patient care, such as hospitals, public health institutes, and laboratories, but do not extend to wellness-focused businesses like spas or gyms.
In accordance with His, the MHRA Northern Ireland guidelines allow the transfer of healthcare equipment between health institutions, each of which must independently assert their exemption. It's significant to observe, however, that such products cannot be sold or transferred to other legal entities. Furthermore, any healthcare device—even those produced under HIE—must comply with the General Safety and Performance Requirements as indicated in Annex I of the MDR. While the specific criteria for an 'appropriate' Quality Management System (QMS) may vary based on the instrument and its intended use, ISO 13485 is generally considered a suitable standard.
While the pricing and distribution of HDE instruments are controlled, the broader healthcare technology market is influenced by a myriad of factors including therapeutic area, procedural complexity, reimbursement levels, and the competitive landscape. Market intelligence from GlobalData reveals that pricing strategies, sales targets, and regional factors also significantly impact the final selling price of healthcare equipment. For instance, large healthcare institutions may negotiate discounts based on volume, which can lead to price variations between hospital settings and other healthcare facilities. These insights highlight the intricacy of pricing for healthcare equipment and the significance of strategic financial planning within the healthcare sector.
Annual Distribution Number (ADN) for Profitable Sales
The Food and Drug Administration (FDA) implements proactive measures to oversee the distribution of healthcare tools, particularly those that come under the Humanitarian Device Exemption (HDE). When a piece of equipment under HDE becomes profitable, the FDA determines an Annual Distribution Number (ADN), which caps the quantity of equipment that can be distributed annually. Adherence to this cap is crucial for manufacturers to retain their HDE status. The creation of the ADN is impacted by a range of factors including the intricacy of the procedure the tool is designed for, the level of market competition, and the lifecycle of the product. Furthermore, the market dynamics of healthcare equipment are greatly influenced by manufacturers' strategic pricing choices and discount strategies, similar to those implemented by companies such as Stryker, which are based on internal strategies and sales targets. These factors, together with geographical and institutional pricing variations, emphasize the intricacy of the healthcare equipment marketplace.
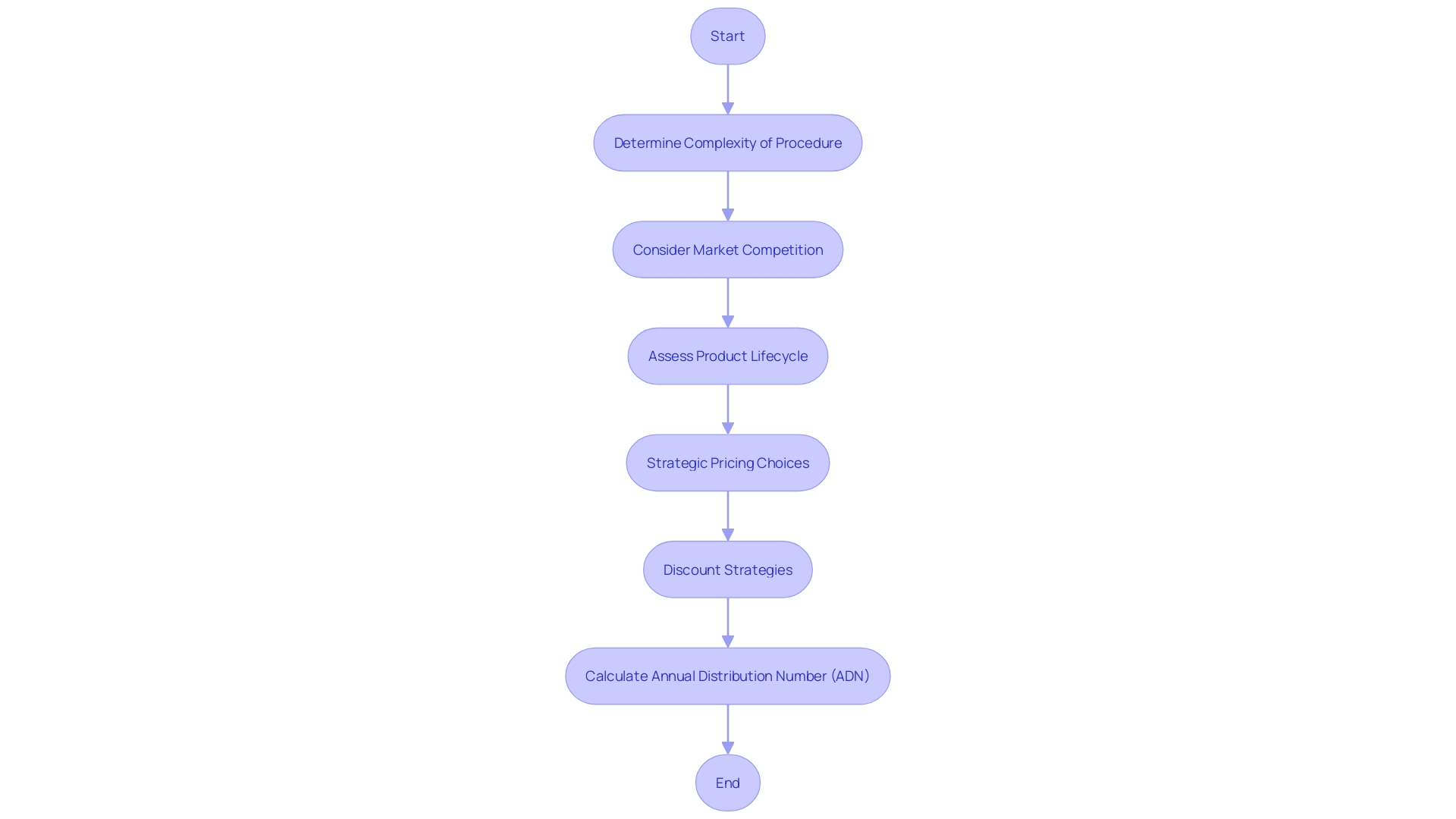
Case Study: Navigating the HDE Application Process
Exploring the journey of healthcare innovation approval is a complex process that highlights the challenges and successes of innovation in healthcare. One illustrative example is the pathway to obtaining Humanitarian Device Exemption (HDE) approval. Through the lens of a case study, we delve into the experiences of a company as they navigate the labyrinth of regulatory requirements. The initial steps involve a request submitted by a clinician or department to the Digital Service Team, who conducts a thorough assessment to determine if the technology is secure, compliant, and not redundant within the trust. An interesting revelation from this process, as noted by an NHS representative, is the discovery of existing technologies within the organization which were previously unknown, thus underscoring the importance of internal communication and resource awareness.
When considering the wider healthcare framework, it is essential to acknowledge the significant function of healthcare technology, especially in rural regions where populations face substantial health challenges in affluent nations. For instance, the United States stands 29th among OECD nations, with life expectancy at its lowest in two decades, highlighting the urgent need for quality healthcare solutions. This fact speeds up the importance of streamlined approval processes for instruments that can deliver lifesaving care to these underserved communities.
The case study further reveals the multi-staged journey of a project from conception to a working system. Soeren, a project leader, shares their strategic approach which included initial analysis, design customization, and the development of alternatives, demonstrating a comprehensive and tailored pathway to innovation.
Furthermore, the dynamic advancements in the industry, like the development of Renewstable® hydrogen power plants and digital twin technologies, demonstrate the progress in health and technological fields. Such innovations hold the potential to revolutionize energy production and maritime operations, respectively, showcasing the intersection of healthcare and technology.
In terms of regulatory understanding, the FDA classifies instruments into three categories, with class three instruments requiring the most rigorous approval due to their critical role in sustaining life. Roughly 10% of healthcare equipment belong to this category, emphasizing the intricacy of HDE approval for tools that are vital for patient care.
The emphasis on improving regulatory pathways, particularly considering the COVID-19 pandemic, highlights the changing approval landscape for healthcare technology. Organizations such as the FDA and EMA are leading the way in these transformations, impacting the time it takes to approve products based on their intricacy and the health conditions they target. The cooperative endeavors between regulatory agencies and industry stakeholders are crucial in accelerating the availability of technologies that meet unfulfilled healthcare requirements, with a notable emphasis on digital well-being and individualized treatment.
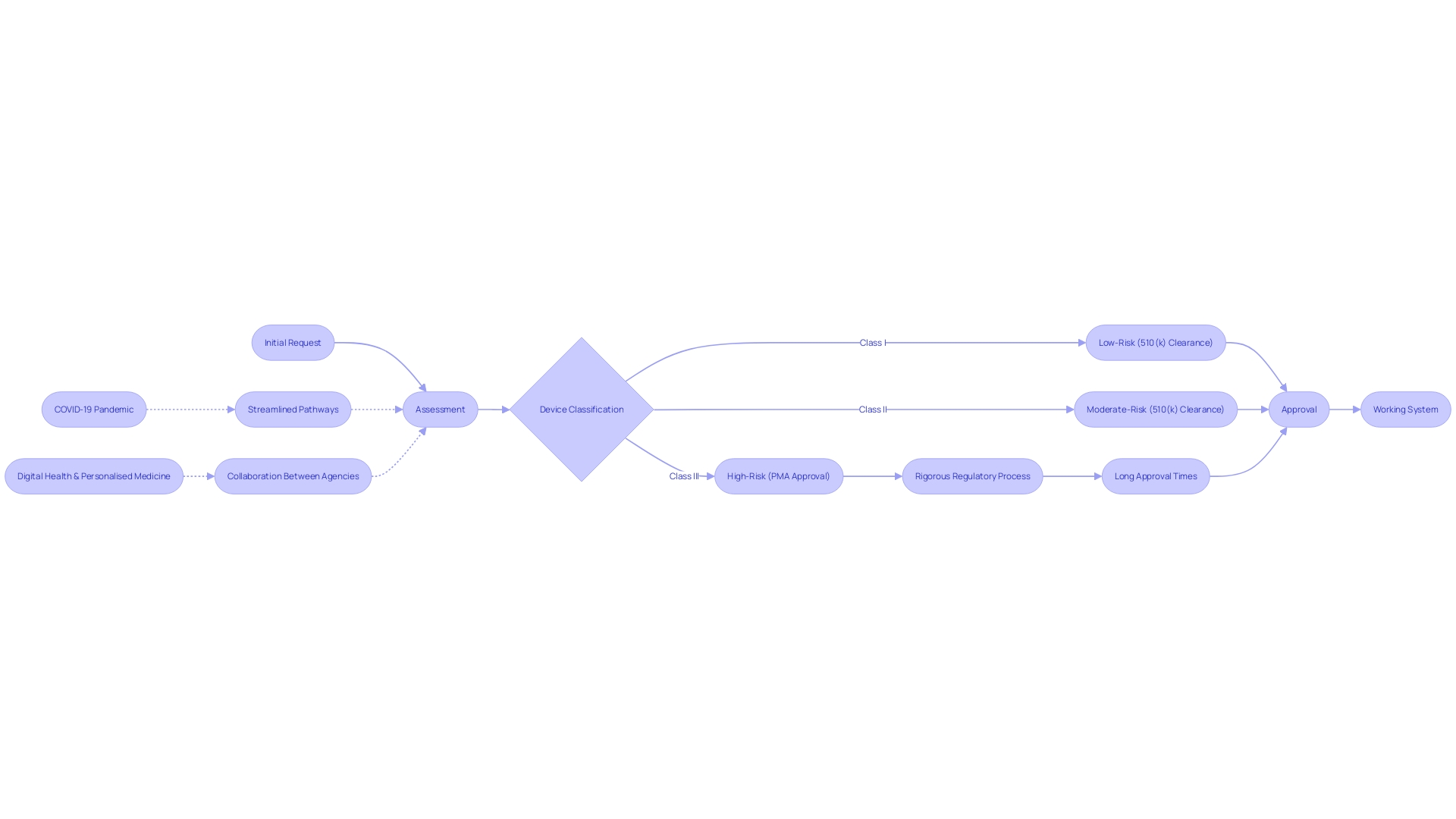
Challenges and Considerations for HDE Devices
Securing a Humanitarian Device Exemption (HDE) demands meticulous attention to a number of stringent requirements. One must consider the comprehensive collection of clinical evidence, adept navigation through the intricate regulatory landscape, and the establishment of robust post-market surveillance mechanisms. To illustrate the complexities involved, consider the comparison to California utilities' efforts in mitigating catastrophic wildfire risks. They encountered uncertainties in risk levels, costs, and effectiveness of mitigation strategies, similar to the uncertainties manufacturers face when seeking HDE approval.
For instance, as expressed by Dr. Liz Kwo of Everly Health Solutions, the significance of early detection in healthcare demonstrates the crucial requirement for thorough post-market surveillance of healthcare tools to safeguard public health. Likewise, the instance of Medtronic's Symplicity blood pressure procedure, which obtained approval in more than 70 countries, illustrates the worldwide magnitude at which regulatory processes for healthcare equipment operate and the importance of acquiring HDE approval in broadening a device's scope.
The healthcare equipment sector, as emphasized by the World Health Organization, covers more than 10,000 varieties of appliances. This diversity requires a nuanced understanding of both human aspects and equipment factors. Taking into account the classification of healthcare tools by the FDA into three categories based on risk, category three tools—which encompass life-sustaining implants such as pacemakers—demand a particularly stringent HDE approval procedure due to their high stakes.
The FDA's main responsibility is to evaluate the safety and efficiency of healthcare equipment for the US market, however, the information submitted for FDA approval may not match the information payors require for coverage determinations, which can result in possible delays or rejections in accessing the equipment, as indicated by the FDA. This underscores the need for strategic planning and comprehensive data analysis to meet the criteria for HDE approval.
To conclude, the pursuit of HDE approval is a challenging yet crucial endeavor for medical device manufacturers, demanding a multifaceted approach that considers clinical data, regulatory navigation, post-market oversight, and the intricacies of medical device diversity.
Conclusion
In conclusion, the Humanitarian Device Exemption (HDE) program provides an expedited regulatory pathway for medical devices treating rare diseases or conditions. It allows manufacturers to bring innovative products to market with reduced clinical trial requirements while ensuring compliance with quality standards. The key elements of an HDE application include a comprehensive device description, clinical data, alternative practices, and post-market surveillance plans.
Securing Institutional Review Board (IRB) approval is crucial for ethical treatment of human subjects in clinical research. In urgent medical scenarios, the FDA authorizes the emergency use of HDE devices, ensuring timely access to advanced medical solutions. HDE devices are subject to profit and use restrictions, with pricing strictly regulated to prioritize patient care over profit.
Navigating the HDE application process requires assessments, communication, and strategic planning. The evolving regulatory landscape, particularly during the COVID-19 pandemic, emphasizes the need for efficient approval processes to address urgent medical needs swiftly.
Securing HDE approval presents challenges that demand attention to clinical evidence, regulatory navigation, and post-market surveillance. The complex landscape of medical devices, the significance of class three devices, and the need for comprehensive data analysis contribute to the complexity of the process.
In summary, the HDE program balances innovation and patient safety, enabling the availability of innovative medical solutions for rare diseases or conditions. Its criteria, application process, oversight requirements, emergency use provisions, and profit and use restrictions provide a comprehensive understanding of the complex landscape surrounding HDE devices.




