Introduction
Clinical Trial Organizations (CROs) play a crucial role in advancing medical research and expanding access to potentially life-saving clinical trials. In this article, we will explore the growing importance of CROs in the realm of clinical trials, including their expertise, benefits of partnering with them, case studies showcasing their impact, key factors to consider when selecting a CRO partner, and their overall influence on advancing medical research. Join us as we delve into the world of CROs and their pivotal role in shaping the future of healthcare.
The Growing Role of CROs in Clinical Trials
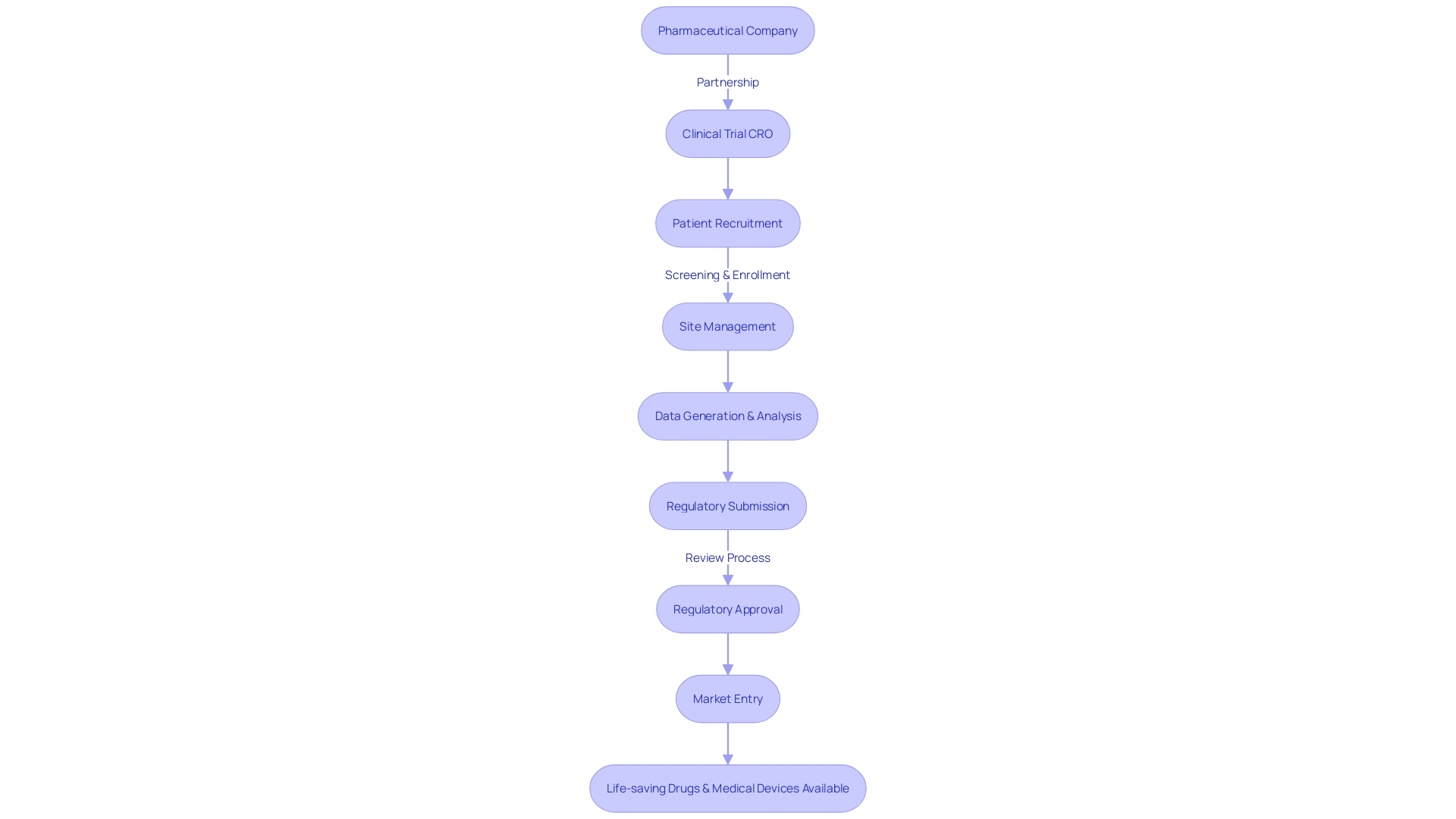
Clinical Trial Organizations (CROs) serve as pivotal partners in the quest to expand medical research, particularly in facilitating access to potentially lifesaving clinical trials for patients facing rare diseases with limited treatment options. For instance, consider a patient from a remote area in Pennsylvania diagnosed with an ultra-rare condition lacking FDA-approved therapies. Presented with the chance to join a clinical trial in Turkey, the patient confronts a maze of logistical challenges, including securing travel visas, navigating foreign documentation, and arranging international transportation.
The role of CROss extends beyond traditional research support; they bridge the gap between patients and ground-breaking medical studies, no matter the geographic barriers. These companies marshal a multidisciplinary team of experts, ranging from clinical research associates to data analysts, all dedicated to the meticulous orchestration of clinical trials. Their collective efforts ensure that even the most complex international studies are conducted with precision and in accordance with the highest standards, thereby advancing the frontier of medical science and offering hope to patients worldwide.
Benefits of Partnering with a CRO
Clinical Trial Organizations (CTOs) offer a wealth of advantages to entities in the pharmaceutical, biotechnology, and medical device sectors. These benefits are multifaceted and address several critical aspects of clinical trial management:
- Expertise and Specialization: CTOs are equipped with a profound understanding of the intricacies of clinical research, ensuring that trials meet regulatory standards and compliance.
Their specialization facilitates the management of intricate clinical studies and the navigation of complex regulatory landscapes. 2. Resource Accessibility: With a plethora of resources at their disposal, including cutting-edge facilities and technologies, CTOs can streamline patient recruitment and enhance the quality of data collection.
This comprehensive access bolsters the efficiency of clinical trials and helps to expedite their timelines. 3. Cost-Effective Solutions: By engaging with CTOs, companies can alleviate the financial burden of maintaining infrastructure and training personnel.
CTOs deliver flexible and scalable services that allow for the prudent allocation of research budgets. 4. Risk Management: Through rigorous quality assurance and control measures, CTOs mitigate risks associated with clinical trials.
Adherence to regulatory guidelines is paramount, reducing the probability of costly errors or deviations from compliance. 5. Accelerated Market Entry: The proficiency of CTOs in streamlining the clinical trial process contributes to a swifter introduction of new medical innovations to the market.
This expedited timeline is particularly crucial for patients awaiting novel treatments, as highlighted by the scenario of a patient in rural Pennsylvania needing access to a clinical trial in Turkey. The complexities of cross-border participation underscore the importance of CTOs in facilitating global patient involvement. The evolution of clinical trial design has emphasized the balance between scientific excellence and operational efficiency, as noted by industry experts.
The insights from Treehill's advisory experience reveal that meticulous planning and attention to detail can significantly enhance the decision-making process in clinical trials. As regulatory bodies like the FDA and EMA continue to oversee medical devices with varying levels of risk, the role of CTOs in navigating these processes becomes increasingly valuable, ensuring that high-risk devices, such as class three, undergo thorough evaluation before reaching the public. This comprehensive approach to clinical trial management by CTOs is essential for advancing medical research and delivering effective treatments to patients in need.
CROs in Action: Case Studies
Several case studies highlight the significant impact that Clinical Trial CROss have had on advancing medical research. These case studies demonstrate how CROss have contributed to the development and approval of life-saving drugs and medical devices.
Here are a few examples:1. Case Study 1: XYZ Pharmaceuticals partnered with a CRO to conduct a Phase III clinical trial for a new cancer treatment.
The CRO's expertise in patient recruitment and site management resulted in faster enrollment and reduced dropout rates. The trial successfully demonstrated the efficacy and safety of the treatment, leading to FDA approval.
- Case Study 2: ABC Biotech collaborated with a CRO to conduct a multinational clinical trial for a novel medical device. The CRO's extensive network of investigators and sites facilitated recruitment from diverse patient populations. The trial generated robust data, supporting the device's effectiveness and paving the way for global market entry. These case studies illustrate how CROss bring valuable capabilities and resources to clinical trials, enabling the successful execution of complex research projects.
Key Factors to Consider When Selecting a CRO Partner
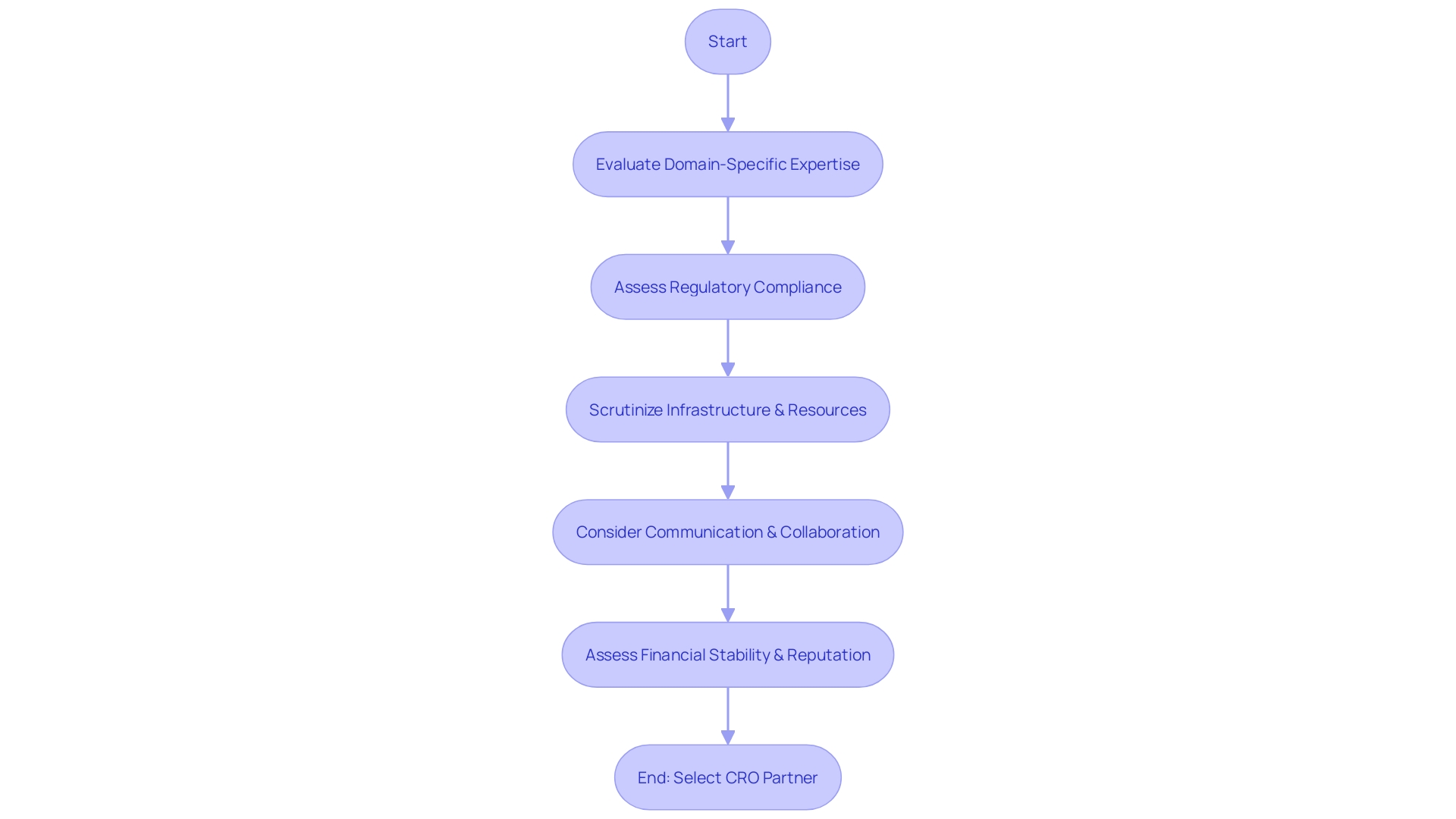
The selection of a Clinical Trial Contract Research Organization (CRO) partner is a pivotal decision that can significantly influence the outcome of a research project. When evaluating potential CROss, it is essential to consider their domain-specific expertise, particularly in managing clinical trials that pertain to your area of therapeutic interest.
For instance, a CRO with a history of handling studies related to rare diseases would be invaluable for a trial involving patients with unique medical conditions, such as an individual in rural Pennsylvania suffering from an ultra-rare disease with no FDA-approved treatments. Regulatory compliance is another cornerstone of a successful clinical trial.
The chosen CRO must not only be well-versed in the regulatory landscape but also have a robust track record of adhering to these standards to prevent any research setbacks. As articulated by industry advisors, decisions made years prior can have a lasting impact on the study's integrity, necessitating a meticulous approach to compliance early on.
Additionally, the infrastructure and resources at the CRO's disposal warrant thorough scrutiny. This includes evaluating their facilities, state-of-the-art technologies, and the breadth of their investigator networks, to ensure they can adeptly manage the complexities of the study, such as cross-border logistics that may arise when trials span multiple countries.
Communication and collaboration are the linchpins of a harmonious partnership with a CRO. Opt for a CRO that is committed to transparency and fosters a cooperative relationship, which is fundamental for navigating the multifaceted nature of clinical trials. Lastly, consider the financial stability and reputation of the CRO. Ensuring that the organization has the necessary resources to sustain the study throughout its course is vital for uninterrupted research progression. By meticulously appraising these elements, companies can forge a partnership with a CRO that not only aligns with their research objectives but also enhances the probability of a trial's success.
The Impact of CROs on Advancing Medical Research
Clinical Trial Contract Research Organizations (CROs) are pivotal in the transformation of medical research, impacting the field in several profound ways. Firstly, they accelerate the research trajectory, streamlining the clinical trial process from patient recruitment to data analysis.
This efficiency is crucial for the rapid development of new therapies, potentially offering life-altering treatments to patients with urgent needs. For instance, a patient with a rare condition in rural Pennsylvania may find hope in a trial conducted overseas, but faces daunting logistical challenges.
CROs can facilitate such global collaborations, not only expanding access to trials but also ensuring diverse patient demographics which enrich the robustness of research findings. Moreover, CROss uphold the highest data quality standards, employing sophisticated data management and rigorous quality control measures.
This ensures the reliability of trial outcomes, fostering evidence-based medical advancements. As the healthcare industry harnesses the power of AI and ML, CROss are at the forefront, integrating these technologies to personalize treatments and enhance trial efficiency. The vision of a 'self-driving' clinical trial is becoming more tangible, promising exponential improvements in pharmacological research and patient care. Additionally, CROs serve as a bridge, providing resources and expertise that may be scarce within individual institutions, thereby enabling the successful completion of intricate research endeavors. The collective expertise and resourcefulness of CROss are instrumental in delivering innovative treatments to the market swiftly and ensuring improved health outcomes for patients worldwide.
Conclusion
Clinical Trial Organizations (CROs) play a crucial role in advancing medical research and expanding access to potentially life-saving clinical trials. They bridge the gap between patients and ground-breaking medical studies, regardless of geographic barriers.
CROs bring expertise, specialization, resource accessibility, cost-effective solutions, risk management, and accelerated market entry to clinical trial management. Several case studies highlight the significant impact CROs have had on advancing medical research by contributing to the development and approval of life-saving drugs and medical devices.
When selecting a CRO partner, it is important to consider their domain-specific expertise, regulatory compliance track record, infrastructure and resources, communication and collaboration approach, financial stability, and reputation. These factors ensure a successful partnership that aligns with research objectives and enhances the probability of a trial's success.
CROs accelerate the research trajectory by streamlining the clinical trial process from patient recruitment to data analysis. They uphold high data quality standards and integrate technologies like AI and ML for personalized treatments and enhanced trial efficiency. CROs serve as a bridge by providing resources and expertise that enable the successful completion of intricate research endeavors. In conclusion, CROs are pivotal in shaping the future of healthcare by advancing medical research, delivering innovative treatments to the market swiftly, and ensuring improved health outcomes for patients worldwide. Their impact is profound and essential in the quest for medical advancements.




