Introduction
The International Council for Harmonisation (ICH) serves as a global collaboration between regulatory authorities and the pharmaceutical industry, with the aim of establishing uniform guidelines for clinical trials and the approval processes of pharmaceutical products. These guidelines play a crucial role in ensuring the ethical execution of clinical research, safeguarding participant rights, and maintaining the integrity and quality of data collected. By standardizing methodologies and expectations across borders, the ICH mitigates challenges and facilitates the interpretation of results, ultimately influencing the development of medical treatments.
In addition to its role in clinical research, the ICH strives for greater uniformity in drug registration and development, aiming to harmonize regulations and enhance public health. The organization's commitment to data governance and advancements in clinical technology further underscore its dedication to patient safety, data integrity, and the future of medical research. With ongoing efforts to adapt to the evolving landscape of clinical trials, the ICH continues to provide clarity and direction that resonate with the needs of the industry, ensuring that clinical trials remain robust and results-driven.
What is the International Council for Harmonisation (ICH)?
The International Council for Harmonization (ICH) embodies a collaborative effort that converges the expertise of regulatory authorities and the pharmaceutical industry across the globe. Founded in 1990, Ich's foundational goal is to establish uniform guidelines for the conduct of clinical trials as well as the approval processes for pharmaceutical products. The coalition includes key stakeholders from the United States, Europe, Japan, and other significant regions, all working in concert to streamline and enhance the quality of clinical research worldwide.
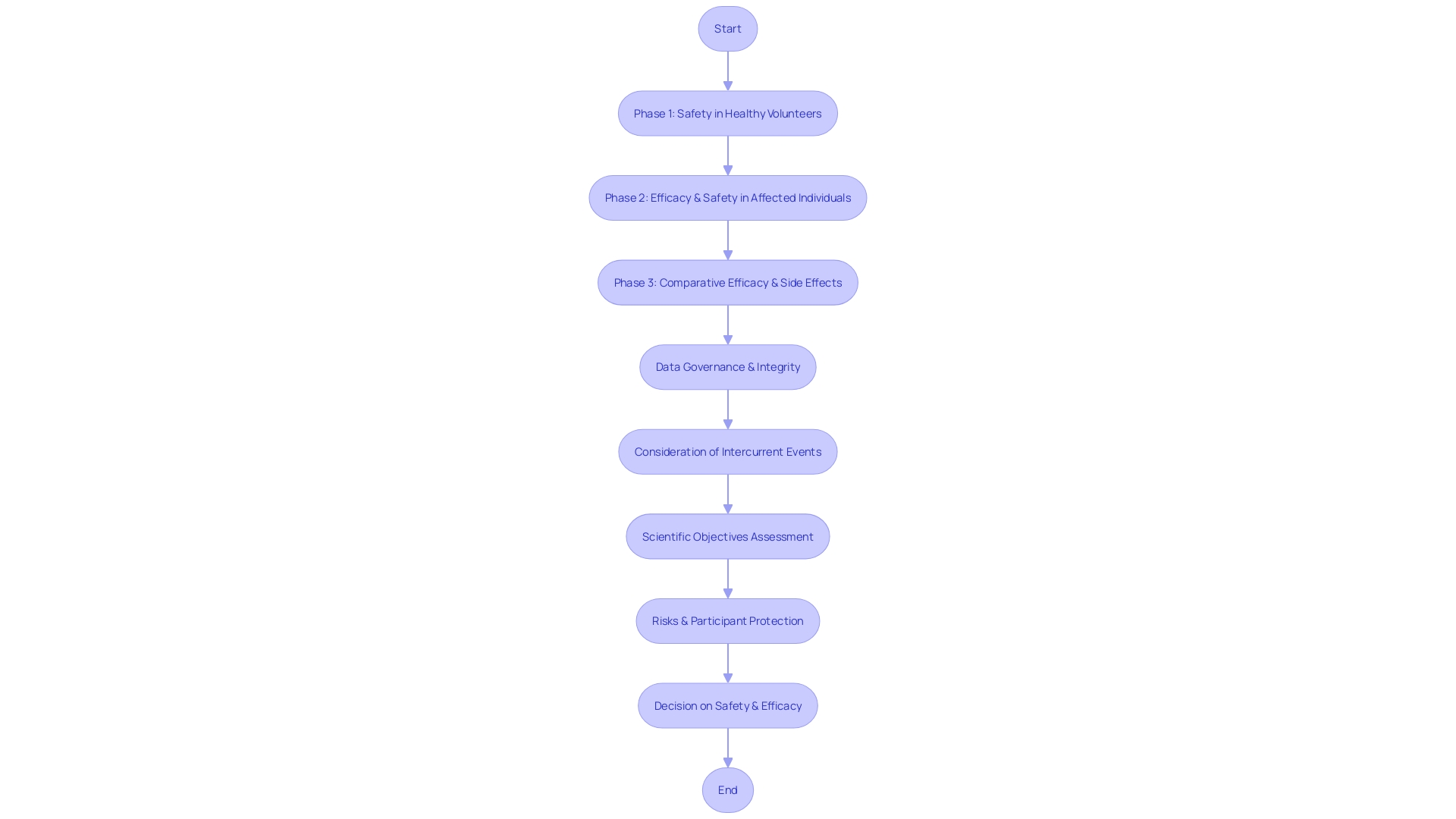
Clinical trials are essential in the medical field, serving as the conduit through which new treatments and interventions are tested for safety and efficacy. Each phase of these trials, from the initial safety assessments involving healthy volunteers to the larger-scale efficacy evaluations in affected populations, operates under stringent standards to ensure reliability and patient safety. The ICH guidelines play a pivotal role in guiding these trials, ensuring that the rights, safety, and well-being of participants are safeguarded while also securing the integrity of the data collected.
By standardizing methodologies and expectations across international borders, the ICH mitigates challenges such as those presented in the FLO-ELA trial. This trial highlighted the impact of intercurrent events, like surgery cancellations, on the analysis of clinical outcomes. In such cases, the ICH guidelines help determine the most pertinent research questions and the appropriate methods of analysis.
This harmonization is crucial for the interpretation of results and ultimately influences the development of medical treatments.
Furthermore, the ICH's commitment to data governance, as underscored at the OCT Europe 2024 conference by Silvia Perez of AstraZeneca, emphasizes the necessity of protecting participant data and ensuring the reliability of trial results. In light of the evolving landscape of clinical research, such as the anticipated updates to the E6 GCP guidelines, ICH continues to provide clarity and direction that resonate with the needs of the industry, ensuring that clinical trials remain robust and results-driven.
Mission and Objectives of ICH
The International Council for Harmonization (ICH) strives for greater uniformity in the scientific and technical aspects of drug registration and development. By setting global guidelines focused on quality, safety, and efficacy, ICH aims to facilitate the swift and harmonized registration of pharmaceuticals across different territories. This endeavor is essential in a world where healthcare is inherently global and supply chains frequently transcend borders.
The drive for more consistent regulations can also be seen in the International Regulatory Cooperation for Herbal Medicines (IRCH), which since its establishment in 2006, has worked to enhance public health by improving regulations on herbal medicines. The IRCH, supported by the World Health Organization and health regulatory bodies worldwide, underscores the necessity of collaboration in safeguarding the integrity of pharmaceutical products. As the industry faces challenges such as complex and fragmented supply chains, particularly in low- and middle-income countries, and the infiltration of substandard or falsified medicines, initiatives like ICH and IRCH play a crucial role.
They work towards a global standardized system, ensuring product traceability and safety, ultimately protecting public health and reinforcing the robustness of healthcare systems.
Role of ICH in Medical Research
The International Council for Harmonization (ICH) is an essential entity in the realm of clinical research, setting forth guidelines and standards pivotal for ensuring the ethical execution of clinical trials. These standards are integral in safeguarding human participants and ensuring the integrity and quality of data collected. The guidelines span the breadth of clinical trials, addressing the design, conduct, monitoring, and reporting of studies, and are epitomized by the principles of Good Clinical Practice (GCP).
One of the key challenges in clinical research is the management of 'intercurrent events,' which are occurrences that can affect the assigned treatment or the clinical trial's outcomes. For example, in the FLO-ELA trial, which compared two fluid delivery methods in emergency bowel surgery, the occurrence of surgery cancellations is a significant intercurrent event. Such events necessitate a robust methodological approach to ensure that the research question remains reflective of real-world scenarios and that the results are interpreted correctly.
The ICH guidelines are increasingly important as the landscape of clinical research evolves, particularly with the emergence of real-world data. The ongoing efforts under ICH M14 to harmonize guidelines for post-approval non-interventional studies exemplify the organization's commitment to adapting to the changing research environment. This initiative reflects a recognition of both the value and the growing availability of real-world data, aiming to develop guidelines that will streamline regulatory processes and enhance efficiency.
With the ongoing pursuit of more nuanced and relevant research questions, as seen in the heart failure study by BigData@Heart, the Ich's role becomes ever more critical. This study highlighted the significance of considering subpopulations in clinical trials and observational studies to ensure that treatments are applicable to the real-world patient population. Such considerations are crucial in addressing past oversights, such as the underdiagnosis and undertreatment of heart disease in women, and represent a forward-thinking approach to disease and outcome definitions.
In summary, the ICH's guidelines and the principles of GCP provide a framework that supports researchers in designing studies that are both scientifically robust and ethically sound, reflecting a commitment to advancing medical research in a manner that is responsible, inclusive, and reflective of diverse patient needs.
Good Clinical Practice (GCP) Guidelines and Their Impact
Good Clinical Practice (GCP) represents an international ethical and scientific quality benchmark for designing, conducting, recording, and reporting clinical trials that involve human subjects. Developed by the International Council for Harmonization (ICH), these guidelines are crucial in upholding the rights, safety, and well-being of trial participants, as well as ensuring the authenticity and reliability of trial data. Regulatory authorities worldwide demand adherence to GCP as a gold standard for accepting clinical research data.
GCP is part of a broader family of regulations and quality guidelines known collectively as 'GxP', where 'G' stands for 'good' and 'xP' refers to the various practices including manufacturing (GMP) and laboratory (GLP) standards. Together, these practices aim to safeguard the quality and safety of products and the integrity of data in life science industries.
The significance of GCP guidelines is underscored by statistics showing the substantial influence of clinical trials on patient care and medical practice. High-quality clinical trials, which are integral to advancing medical knowledge and improving patient outcomes, are predicated on robust design and transparent reporting mechanisms. The integration of clinical trials with clinical practice, as discussed in a special communication in JAMA, highlights the need to overcome the siloed nature of traditional clinical trial designs to enhance their scope and impact.
Case studies, such as the DAPA-MI study, exemplify the innovative use of registry data in randomized controlled trials (RCTs) to achieve pragmatic, cost-effective, and efficient trial designs while maintaining scientific rigor. Furthermore, the MHRA, an executive agency of the Department of Health and Social Care, exemplifies the commitment to ensuring that medical interventions are both effective and safe.
Ultimately, GCP serves not only to protect participants but also to instill confidence in the medical community and the public that clinical research findings are credible and that medical interventions are based on solid evidence. This is vital in an era where the transparency and integrity of clinical research are increasingly scrutinized and where ethical considerations are paramount.
Harmonization of Clinical Trials Across Countries
The International Council for Harmonization (ICH) has been instrumental in aligning clinical trial protocols across various countries, streamlining the multinational clinical trial process and expediting the creation of new pharmaceuticals. Through ICH guidelines, there is a reduction in redundant testing and regulatory hurdles, facilitating the mutual acceptance of clinical data among international regulatory bodies. For instance, the DAPA-MI trial utilized an innovative R-RCT design, leveraging real-world registry data with the structured rigor of randomized trials, to efficiently assess cardiovascular outcomes.
Such innovative approaches in trial design are part of the broader movement towards harmonization and improved efficiency in clinical research.
Additionally, recent revisions to the Clinical Trials Information System (CTIS) portal, driven by user feedback, have enhanced transparency and accessibility of trial information. The removal of publication deferrals and the focus on essential documents in clinical trials underscore the commitment to making clinical research more open and efficient. These changes align with the goal of ICH to streamline research processes, enabling faster publication and simplifying user interaction with the CTIS portal.
Moreover, the collaboration between a Finnish lab and a Canadian CRO exemplifies the harmonization efforts by ICH, as it shows how international partnerships can navigate local regulations while benefiting from diverse patient populations and expertise. Such international cooperative efforts underscore the significance of ICH's role in facilitating efficient clinical research.
In light of these developments, the evolving landscape of clinical trials is moving towards greater incorporation of digital technologies and real-world evidence, as highlighted by ongoing discussions within the industry. As per the Bioworld Insider Podcast, AI is set to revolutionize drug development, with digital clinical trials anticipated to significantly reduce study times. The harmonization of guidelines for post-approval studies, as discussed under ICH M14, will further streamline the use of real-world data, enhancing the efficiency and effectiveness of pharmacovigilance.
These collective efforts demonstrate a commitment to overcoming the challenges of clinical trial variability and technological limitations. By addressing the need for harmonized assessment criteria, the ICH continues to foster global convergence in medical research, making safe and effective treatments more rapidly available to patients worldwide.
Advancements in Clinical Technology and Data Management
The International Council for Harmonization (ICH) has acknowledged the transformative impact that clinical technology and sophisticated data management have on enhancing the efficiency and quality of medical research. Leveraging electronic data capture systems and electronic signatures, ICH guidelines are setting the stage for the integration of advanced technological tools into clinical trials. These innovative strides not only streamline the entire research process but also fortify data integrity and enable real-time monitoring of trials, which is crucial for timely decision-making and ensuring patient safety.
Reflecting on real-world applications, health systems such as WakeMed have initiated digital transformation projects that underscore the necessity of structured healthcare data and streamlined operations. By forming specialized teams, they have successfully identified optimal practices and established care pathways that leverage analytics and dashboards for outcome evaluation, thus exemplifying the ICH vision in practice.
Moreover, the Veterans Affairs' Corporate Data Warehouse and the development of the Health Data and Analytics Platform signify the potential of data management in enhancing healthcare delivery. This scalable, cloud-native enterprise data analytics platform aims to harness big data projects and facilitate clinical decision support, aligning with the Ich's objective of improving research methodologies through technology.
Exciting advances in the field further underscore the role of technology in clinical settings. For instance, the introduction of novel technologies for patient monitoring, as supported by industry experts, promises a significant leap in patient care efficiency, potentially altering pre-hospital and in-hospital care practices.
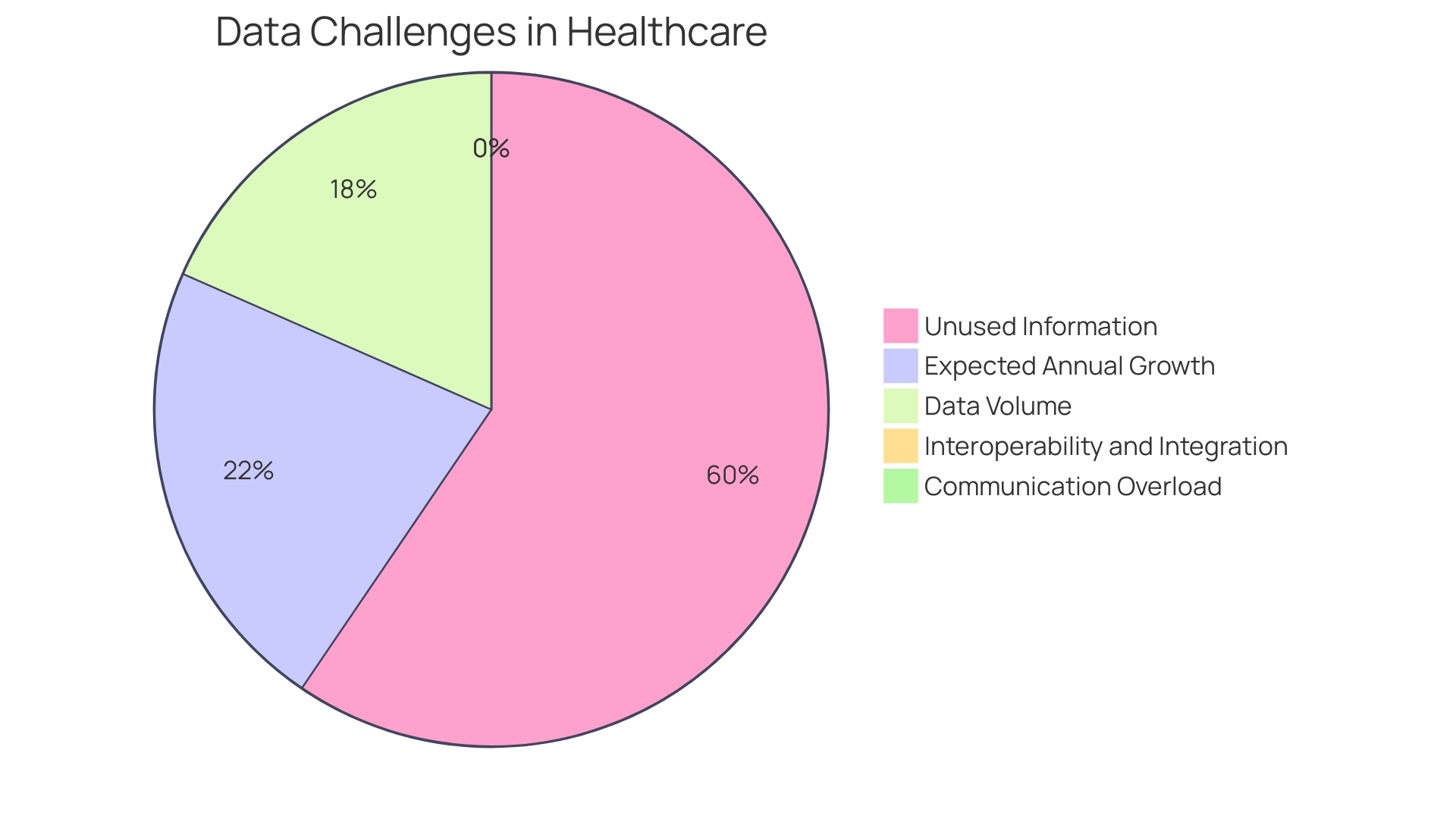
Furthermore, the pressing need for a comprehensive healthcare data management system is evident, as nearly one-third of the world's data volume is generated by the healthcare industry, growing at a rate of 36% annually. Ich's emphasis on technology in clinical trials resonates with the industry's direction towards organizing data around patients and improving health outcomes.
The advent of digital patient engagement tools, wearable devices, and sensors has revolutionized clinical trials by enabling faster outcome assessments and more reliable data. This technological pivot aligns with the insights from healthcare providers who recognize that a vast majority of patient data remains underutilized, signaling a missed opportunity to transform it into actionable insights for quality healthcare delivery.
In conclusion, the ICH's foresight in integrating contemporary technology and data management practices into clinical research is not only paving the way for enhanced trial efficiency and data integrity but also mirrors the broader healthcare industry's shift towards leveraging data to meet patient expectations and improve overall health outcomes.
Benefits of ICH Guidelines for Patient Safety and Data Integrity
The application of ICH guidelines in clinical research ensures the protection of trial participants while fostering the production of high-quality data. These guidelines are pivotal in safeguarding participant rights and promoting data integrity, thus facilitating well-informed decisions on the safety and efficacy of new pharmaceuticals. The FLO-ELA trial illustrates the complexities of clinical research, highlighting the need for thoughtful consideration of intercurrent events—occurrences that can alter a patient's treatment course—when designing and analyzing studies.
Such meticulous attention to detail is critical for interpreting the true impact of a medical intervention. Recent updates to the European GCP guidance, incorporating a dedicated section on data governance, underscore the importance of evaluating scientific objectives and associated risks, ensuring the protection of participant data, and maintaining the integrity of clinical trial processes. These developments reflect an ongoing commitment to the principles of patient safety and data integrity, which are central to the success of clinical research.
Future Implications for Medical Research and Clinical Trials
The International Council for Harmonization (ICH) plays a pivotal role in sculpting the future of medical research and the rigor of clinical trials. As we step into an era brimming with novel technologies and therapeutic methodologies, ICH is tasked with the continuous development and revision of guidelines that will meet the evolving challenges that these advancements entail. The harmonization efforts of ICH are instrumental in maintaining the integrity and safety of clinical research, which, in turn, enables the discovery of efficacious treatments and the progression of medical science.
One of the profound impacts of ICH's work is evident in projects like BigData@Heart. This consortium has united experts across sectors to redefine disease and outcome definitions using existing data, thereby enhancing the precision and relevance of clinical research. In the realm of heart failure research, for instance, a study published in the European Journal of Heart Failure has highlighted the necessity of considering sex differences in randomized clinical trials (RCTs).
This research showed significant variations in outcomes when comparing data across RCTs and observational registries, underlining the importance of Ich's mandate to ensure that clinical trials reflect the diversity of patient populations and address previously overlooked factors such as gender disparities.
The importance of harmonization is further underscored by the upcoming ICH M14 guideline, which aims to standardize post-approval studies using real-world data. This initiative recognizes the growing availability of such data and seeks to optimize its use across international borders, reducing inefficiencies for both sponsors and regulators.
Amidst these developments, the work of the ICH is not just about setting standards—it is about creating a unified vision for clinical research that aligns with global health objectives. This was reflected in the recent clinical trial forum that convened an international assembly of experts with the goal of bolstering clinical trial capacities and enhancing the quality and coordination of research.
The ICH's commitment to balancing innovation with patient safety is also evident in the regulatory landscape surrounding emerging technologies like AI and ML. As regulatory bodies scrutinize the risks associated with these advancements, ICH guidelines are vital in guiding the life sciences industry through the compliance labyrinth, ensuring that patient safety remains at the forefront of innovation.
Moreover, the transition from directives to regulations in the European Union showcases ICH's role in facilitating a more cohesive regulatory environment. By streamlining application and assessment processes, the ICH contributes to a more unified approach to clinical trials across Member States, ultimately leading to better health outcomes.
In essence, the ICH's contributions are far from static; they are dynamically evolving with the healthcare landscape, fostering a global ecosystem where medical research can thrive and patients can reap the benefits of safe, effective, and well-regulated treatments.
Conclusion
The International Council for Harmonisation (ICH) plays a crucial role in ensuring ethical clinical research and the development of safe medical treatments. Their guidelines standardize methodologies across borders, facilitating result interpretation and mitigating challenges. With a focus on data governance and clinical technology, the ICH prioritizes patient safety and data integrity.
By aligning clinical trial protocols internationally, the ICH streamlines the process and accelerates the creation of new pharmaceuticals. Their guidelines reduce redundant testing and regulatory hurdles, making treatments more accessible worldwide. Embracing advancements in technology and data management, the ICH enhances research efficiency and data accuracy.
The application of ICH guidelines safeguards trial participants and promotes high-quality data generation. Their commitment to harmonization, data integrity, and technological progress drives responsible medical research and addresses emerging challenges. Continual guideline development ensures research integrity and enables the discovery of effective treatments.
In summary, the ICH's global collaboration, dedication to safety and data integrity, and focus on harmonization and technological advancements shape the future of medical research and the delivery of safe treatments.




