Introduction
Contract Research Organizations (CROs) play a vital role in the field of clinical research, providing comprehensive solutions that span the entire pharmaceutical value-chain. From drug development and manufacturing to sales and marketing, CROs offer customized services to diverse clients such as pharmaceutical companies, medical device manufacturers, academia, and medical institutions.
Beyond their role as service providers, CROs also uphold ethical standards in clinical research and contribute to the success of trials through meticulous planning and strategic decision-making. This article explores the services offered by CROs, their importance in ensuring regulatory compliance, their adaptation and innovation during the COVID-19 pandemic, and the future of CROs in clinical research, particularly in the integration of artificial intelligence and machine learning.
The Role of CROs in the Research Process
Contract Research Organizations (CROs), such as CMIC Group which established the CRO business in Japan over three decades ago, are integral to the lifecycle of drug development. These organizations have evolved to provide comprehensive solutions that span the entire pharmaceutical value-chain.
From the early stages of drug development and manufacturing to sales, marketing, and even strategies for entering the Japanese market, CROss like CMIC offer customized services that cater to the diverse needs of their clients, including pharmaceutical companies, medical device manufacturers, academia, and medical institutions. The role of CROss extends beyond mere service providers; they are pivotal in ensuring that the ethical standards governing clinical research are upheld.
The recent shift in perspective on participant compensation underscores the importance of treating trial participants equitably. Fair treatment includes appropriate reimbursement for research-associated expenses, time commitment, and the risks involved in study participation, aligning with the compensation offered to those in public service roles like firefighters.
Moreover, insights from industry experts reveal that decisions made early in the clinical trial process can significantly impact the study's outcome. Treehill's advisory experience highlights that meticulous planning and strategic decision-making, particularly in the early phases of a trial, are critical. CROs are now recognized for their ability to meticulously optimize each phase of the trial, akin to strengthening each link in a chain, ensuring the robustness of the entire research process. This optimization often leads to more effective trial outcomes, as informed by the analysis of over 1,200 data points indicating that better-prepared decisions can enhance the success rate of clinical studies.
Services Offered by CROs
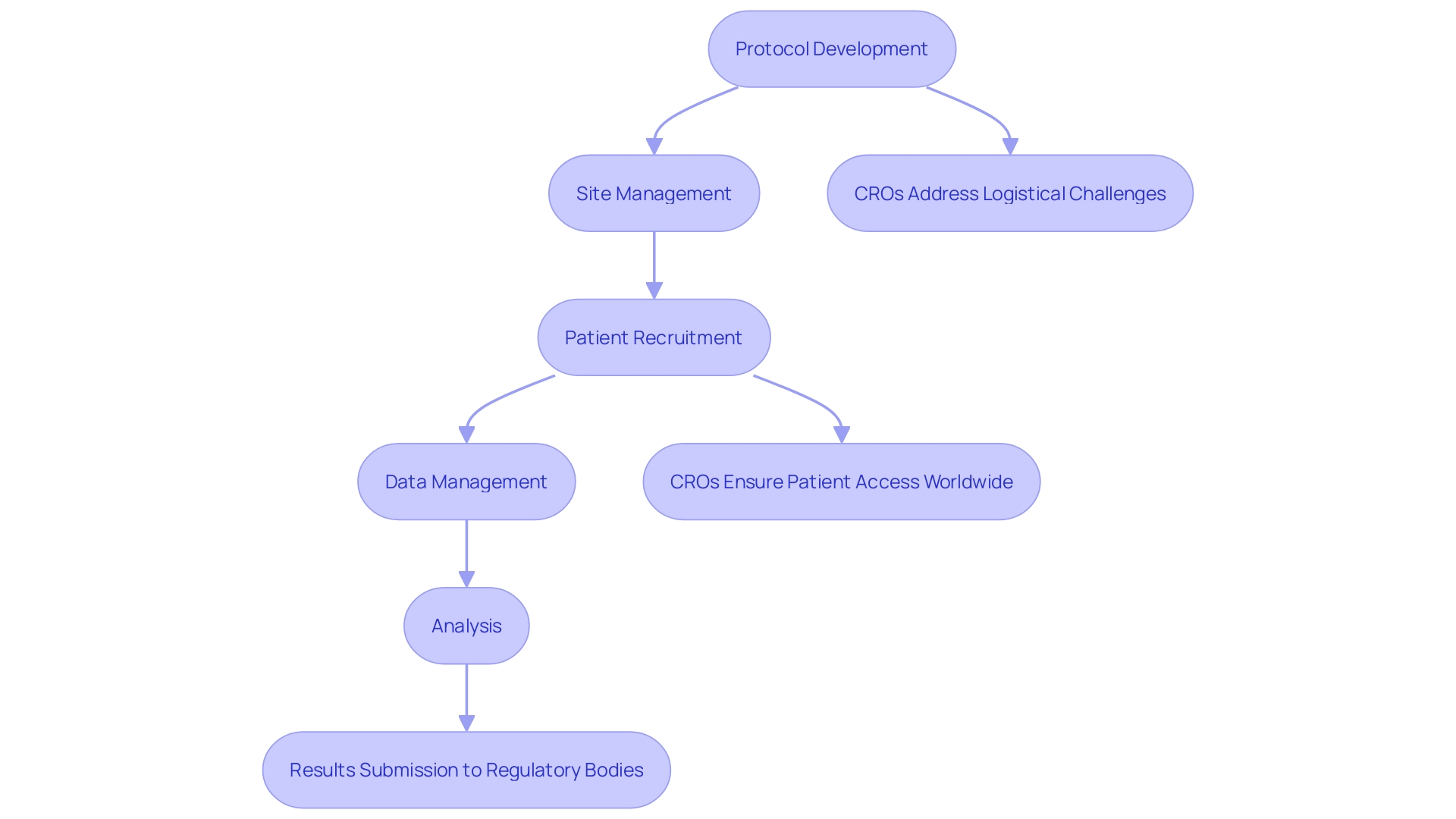
Contract Research Organizations (CROs) are pivotal in the intricate landscape of clinical research, offering a suite of services that address every phase of the trial process. These organizations bring together skilled professionals such as project managers, clinical research associates, and regulatory experts to ensure clinical trials are conducted efficiently and effectively.
Their expertise is not limited to trial execution; they also specialize in protocol development, site management, patient recruitment, as well as data management and analysis. A prime example is CMIC Group, Japan's first and largest CRO, which has revolutionized the industry by providing end-to-end solutions that span the entire pharmaceutical value-chain.
From developing and manufacturing contracts to facilitating market entry in Japan, CMIC is an exemplar of how CROs can adapt to meet specific client needs, ensuring that new therapies are delivered to the market expediently and cost-effectively. Furthermore, CROss play an essential role in addressing the complexities of global clinical trials. For instance, a patient from rural Pennsylvania with an ultra-rare disease may face the daunting task of international travel to participate in a trial in Turkey. In such cases, CROss can be instrumental in navigating the logistical challenges, removing barriers to participation and ensuring patients can access potentially lifesaving treatments without undue stress over visas, travel arrangements, or language barriers.
The Importance of CROs in Ensuring Regulatory Compliance
Clinical Research Organizations (CROs) are essential in advancing medical interventions, ensuring compliance with regulatory standards set by authorities such as the FDA and ICH. They navigate the complex regulatory landscape, securing all necessary authorizations and ensuring research phases meet ethical and quality benchmarks. This diligence is critical to maintaining the integrity of research and participant welfare.
The structured progression of clinical trials, from phase one focusing on safety in healthy volunteers to phase two assessing safety and efficacy in affected individuals, relies on CROss for meticulous execution. As clinical research incorporates AI and ML, CROss are key in merging innovation with patient safety, adhering to evolving guidelines to keep treatments within ethical and regulatory boundaries. Recognizing the importance of participant contribution, CROss advocate for fair compensation for the risks and involvement of trial participants, aligning with ethical standards that honor their role in public health advancements.
In Latin America, bioaccess™ exemplifies the role of a dedicated CRO, specializing in a range of study types including pilot studies, first-in-human (FIH), early-feasibility studies (EFS), pivotal studies, and post-market clinical follow-up studies (PMCF). With over 20 years of expertise in medtech, bioaccess™ offers a tailored approach to expedite the advancement of medical devices. Additionally, bioaccess™ supports regulatory submissions, such as securing study approval from Colombia's Ministry of Health (INVIMA), streamlining the process for clients like Flow-FX to commence studies with confidence and compliance.
CROs and the COVID-19 Pandemic: Adaptation and Innovation
Clinical Research Organizations (CROs) have been at the forefront of addressing the myriad of challenges posed by the COVID-19 pandemic in the realm of clinical research. With traditional in-person clinical activities being disrupted, CROss have rapidly pivoted to remote monitoring and data collection techniques.
This shift has been essential in ensuring that clinical trials could persist notwithstanding restrictions on face-to-face interactions. Furthermore, CROss have harnessed technology to streamline virtual patient recruitment and engagement, a move that has been crucial in preserving the momentum of research amidst global health crises.
In the wake of the pandemic, the complexity of regulatory components, such as those detailed in the US Food and Drug Administration Form 1572, have come into sharp focus. The form, which outlines the need for identifying facilities for study activities and data collection, has highlighted the importance of adaptive strategies in clinical research.
This includes the utilization of local clinical laboratories and research facilities, which contribute significantly to the collection and generation of clinical data. The insights gleaned during the COVID-19 pandemic have underscored the necessity for innovative approaches in combating communicable diseases. These strategies include personalized medicine tailored to individual patient needs, the integration of systems medicine and data science, and the refinement of public health science to inform policies and address health disparities. As the pandemic has already resulted in approximately 20 million deaths globally, the role of CROs in advancing medical research through such innovative strategies has never been more critical.
The Future of CROs in Clinical Research
Clinical Research Organizations (CROs) are on the cusp of a transformative era, driven by the integration of artificial intelligence (AI) and machine learning (ML) in healthcare. The deployment of automated technologies, including digital workflows and data digitization, is not only enhancing the personalization of patient treatments but also revolutionizing the speed and efficiency of clinical trials.
The burgeoning volume of health data paves the way for the advent of self-driving clinical trials, which heralds a future of significantly enhanced pharmacological capabilities, organizational efficiency, and patient care. The potential for AI and ML to deliver unprecedented accuracy in clinical research is a testament to the innovative trajectory of CROss. As the digital landscape of healthcare expands, CROss will be instrumental in harnessing these technologies to streamline the drug development process, maintain stringent regulatory compliance, and fortify the efficacy of clinical research initiatives.
Conclusion
In conclusion, Contract Research Organizations (CROs) are crucial players in clinical research. They provide comprehensive solutions, uphold ethical standards, and ensure regulatory compliance. CROs make strategic decisions to optimize trial outcomes and offer a wide range of services, from protocol development to data management.
During the COVID-19 pandemic, CROs have adapted through remote monitoring and virtual patient engagement, ensuring research continuity. Looking ahead, the integration of artificial intelligence (AI) and machine learning (ML) will revolutionize clinical trials. CROs will play a pivotal role in harnessing these technologies to streamline drug development processes and fortify the efficacy of clinical research initiatives.
In summary, CROs are essential partners in advancing medical interventions while maintaining regulatory compliance. Their adaptability during the pandemic demonstrates their ability to overcome challenges. With the integration of AI and ML, CROs are driving innovation in healthcare and shaping the future of clinical research.




