Introduction
FDA clearance is a vital step in the process of bringing medical devices to market in the United States. This rigorous evaluation, overseen by the U.S. Food and Drug Administration, ensures that devices are safe and effective for their intended use. The FDA classifies devices into three categories based on risk level, with Class III devices carrying the most significant risk and requiring a thorough review process.
The most common pathway to clearance is the Premarket Notification, or 510(k), process, where devices are compared to previously cleared devices. However, concerns have been raised about the adequacy of this process, as some devices cleared through 510(k) have led to patient harm. In contrast, the Pre-Market Approval (PMA) pathway involves more comprehensive examination, including clinical trial data.
Staying up to date with regulatory requirements is crucial for manufacturers and healthcare professionals. The FDA's commitment to public health is evident in their recent publication of standards for direct-to-consumer drug advertisements. As the medical device industry continues to grow, understanding and navigating FDA regulations effectively is of utmost importance.
What is FDA Clearance?
FDA clearance is an essential milestone in the pathway to bringing medical devices to market in the United States. This process, overseen by the U.S. Food and Drug Administration, involves rigorous evaluation to ensure that a medical device is safe and effective for its intended use. The FDA classifies medical devices into three categories, each reflecting a different level of risk to patients. Class I devices pose the least risk, while Class III devices, such as life-sustaining implantable devices, carry the most significant risk and thus require a more comprehensive review process.
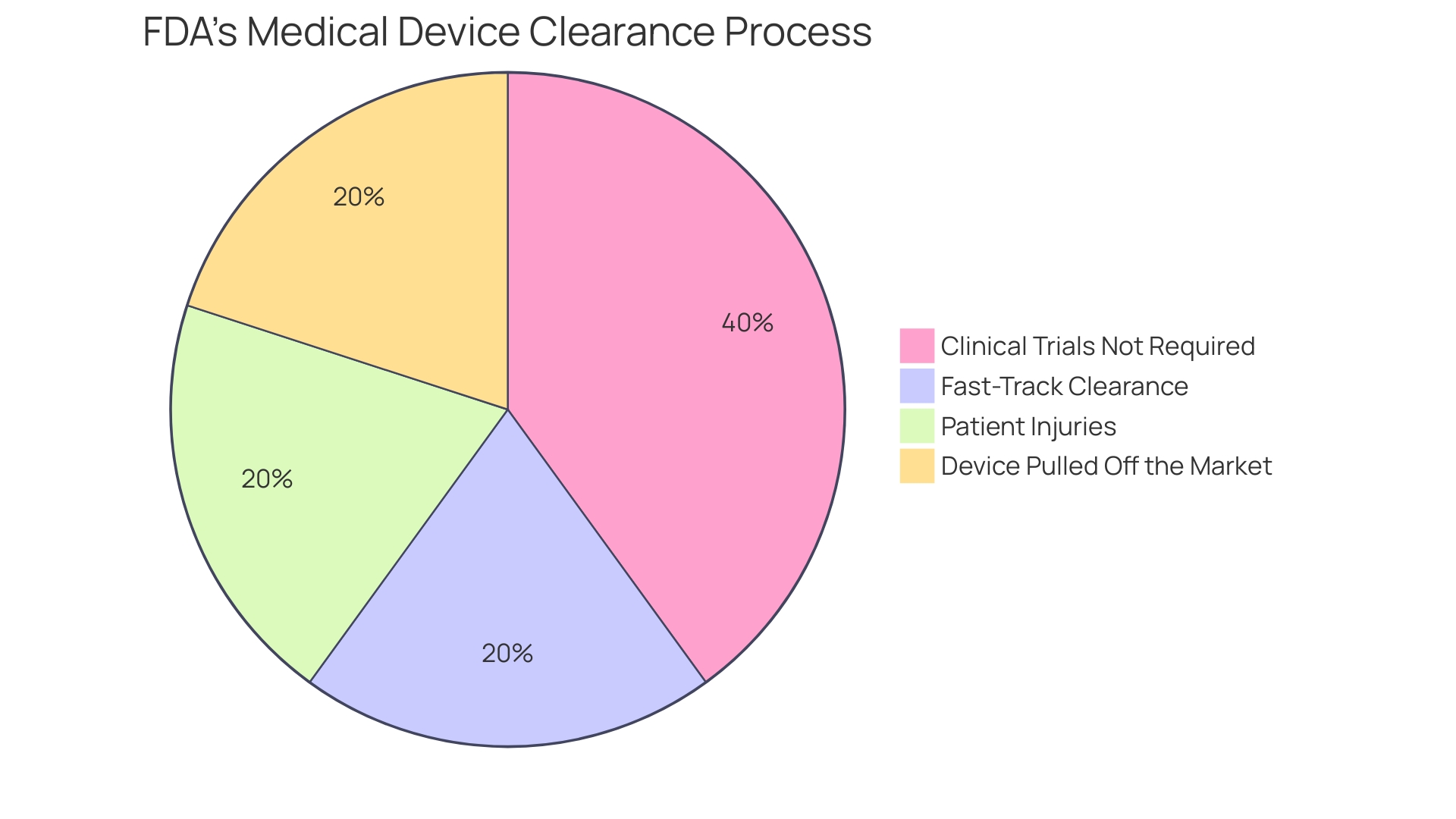
When a medical device is classified, manufacturers must navigate one of several regulatory pathways to obtain market authorization. The most common pathway is the Premarket Notification, also known as the 510(k) process, where a device is compared to a similar, previously cleared device, known as a predicate. This process can be less demanding than others, as clinical trials are not always a prerequisite, a fact highlighted in the 2018 documentary 'The Bleeding Edge.' However, some devices cleared via the 510(k) pathway have led to patient harm due to insufficient clinical testing.
In contrast, the Pre-Market Approval (PMA) pathway is typically reserved for Class III devices and involves a more thorough examination, including clinical trial data, to affirm the device's safety and efficacy. The De Novo process, a less commonly used route, is an option for low to moderate risk devices that do not have a predicate.
Staying abreast of regulatory requirements is crucial for medical device manufacturers and healthcare professionals alike. The FDA's commitment to public health is evidenced by their recent publication of standards for direct-to-consumer drug advertisements, ensuring information is conveyed in a clear, understandable manner. Additionally, the medical device industry is experiencing rapid growth, with innovations like 3D printing poised to generate significant revenue by 2026, further emphasizing the importance of navigating FDA regulations effectively.
Differentiating FDA Clearance vs. FDA Approval
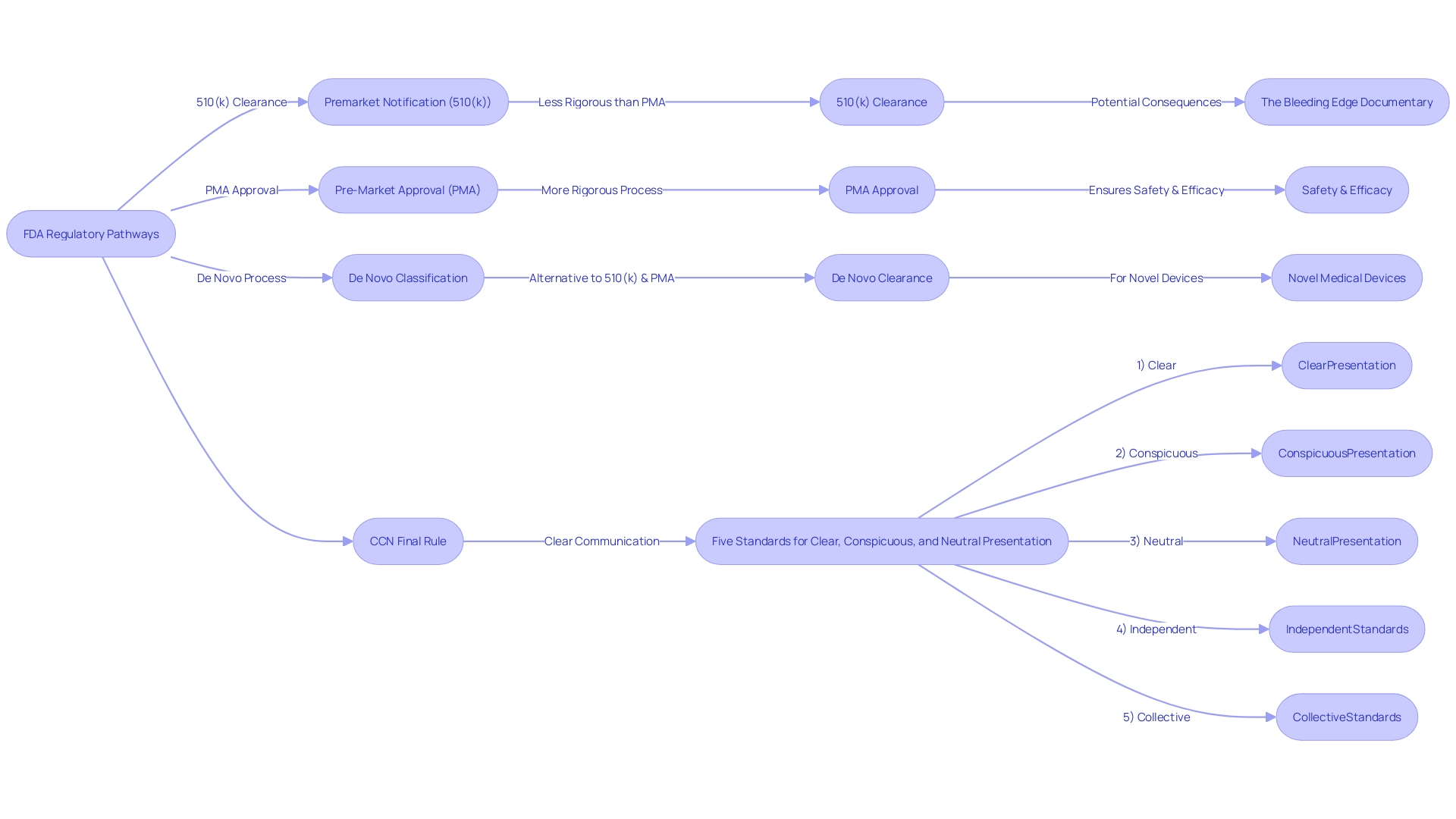
Understanding the nuances between FDA clearance and FDA approval is crucial for professionals in the medical device industry. The FDA categorizes medical devices into three classes, based on the level of risk they pose to patients, which dictates the regulatory pathway a device must follow before it can be marketed in the United States. Devices may undergo Premarket Notification (510(k)), Pre-Market Approval (PMA), or the De Novo process, with each pathway having distinct requirements and implications.
The FDA's 510(k) clearance is often misconstrued as 'approval'; however, this process primarily assesses whether a new device is substantially equivalent to an existing, legally marketed device. Unlike the more rigorous PMA, which demands extensive clinical trial data to confirm a device's safety and efficacy, a 510(k) clearance can sometimes be obtained without such trials. This was highlighted in the 2018 documentary 'The Bleeding Edge', which brought to light instances where the 510(k) process may have been inadequate to prevent patient harm.
Moreover, the FDA's recent advancements, such as the 'Direct-to-Consumer Prescription Drug Advertisements: Presentation of the Major Statement' final rule, emphasize the agency's commitment to making health information more accessible and understandable to the public. Such efforts ensure that the benefits and risks of products are clearly communicated.
In the realm of pharmaceuticals, the FDA's Center for Drug Evaluation and Research (CDER) plays a pivotal role, taking approximately 12 to 15 years to scrutinize and approve new drugs, ensuring they are safe and effective. The approval comes after thorough examination by independent experts, unlike the clearance process for medical devices.
As the medical device industry evolves, with technologies such as 3D printing anticipated to generate significant revenue, understanding the differences between FDA terms like 'Registered', 'Cleared', 'Approved', and 'Granted' becomes imperative. These distinctions are not just academic but have substantial implications for how medical devices are brought to market and monitored for safety.
In summary, navigating the FDA's regulatory landscape requires a clear understanding of the specific processes for drugs and devices, the importance of device classification, and the implications of the chosen regulatory pathway. Comprehending these aspects is essential for ensuring the safety and efficacy of medical products and their compliance with FDA standards.
The 510(k) Clearance Process
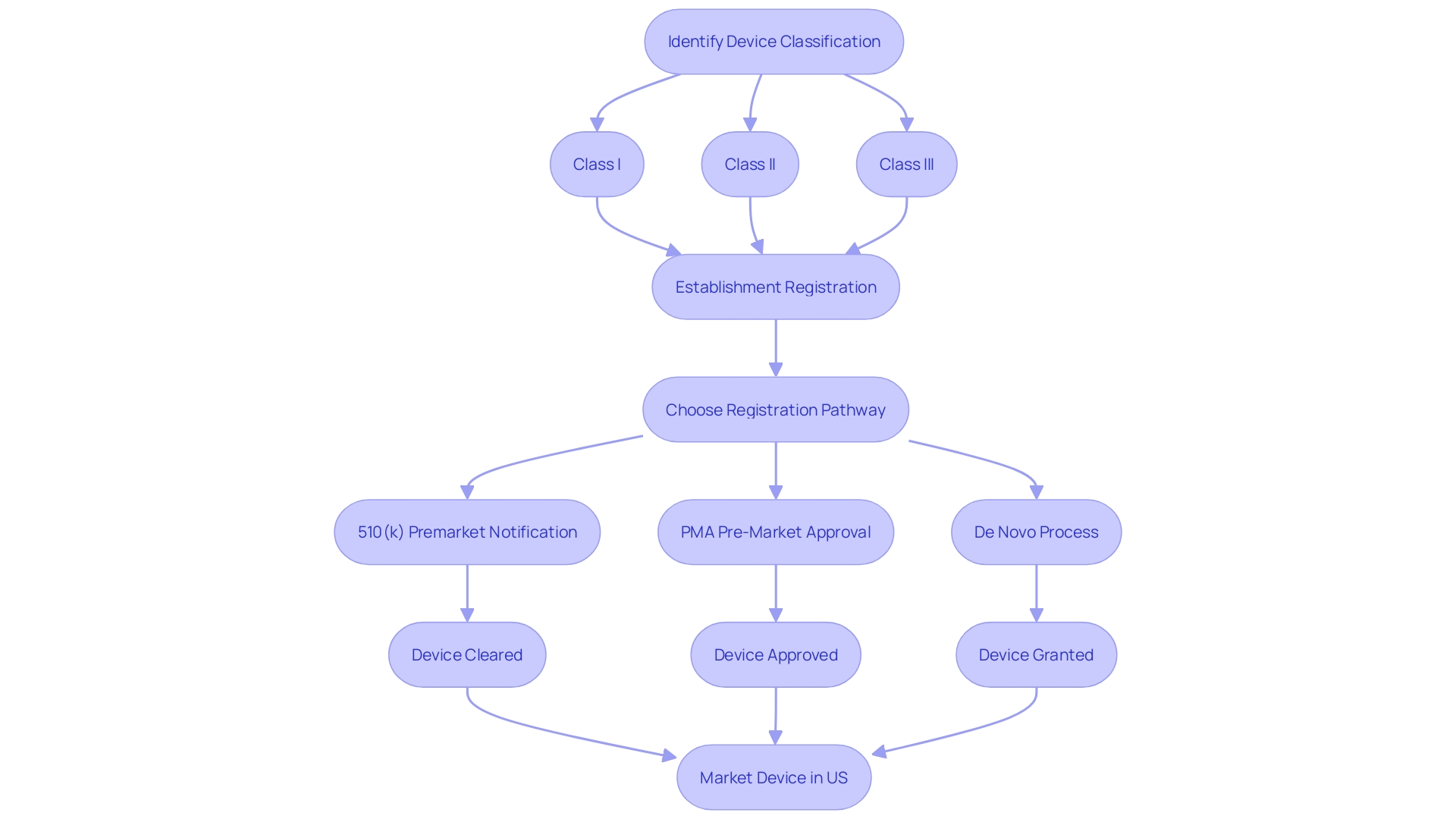
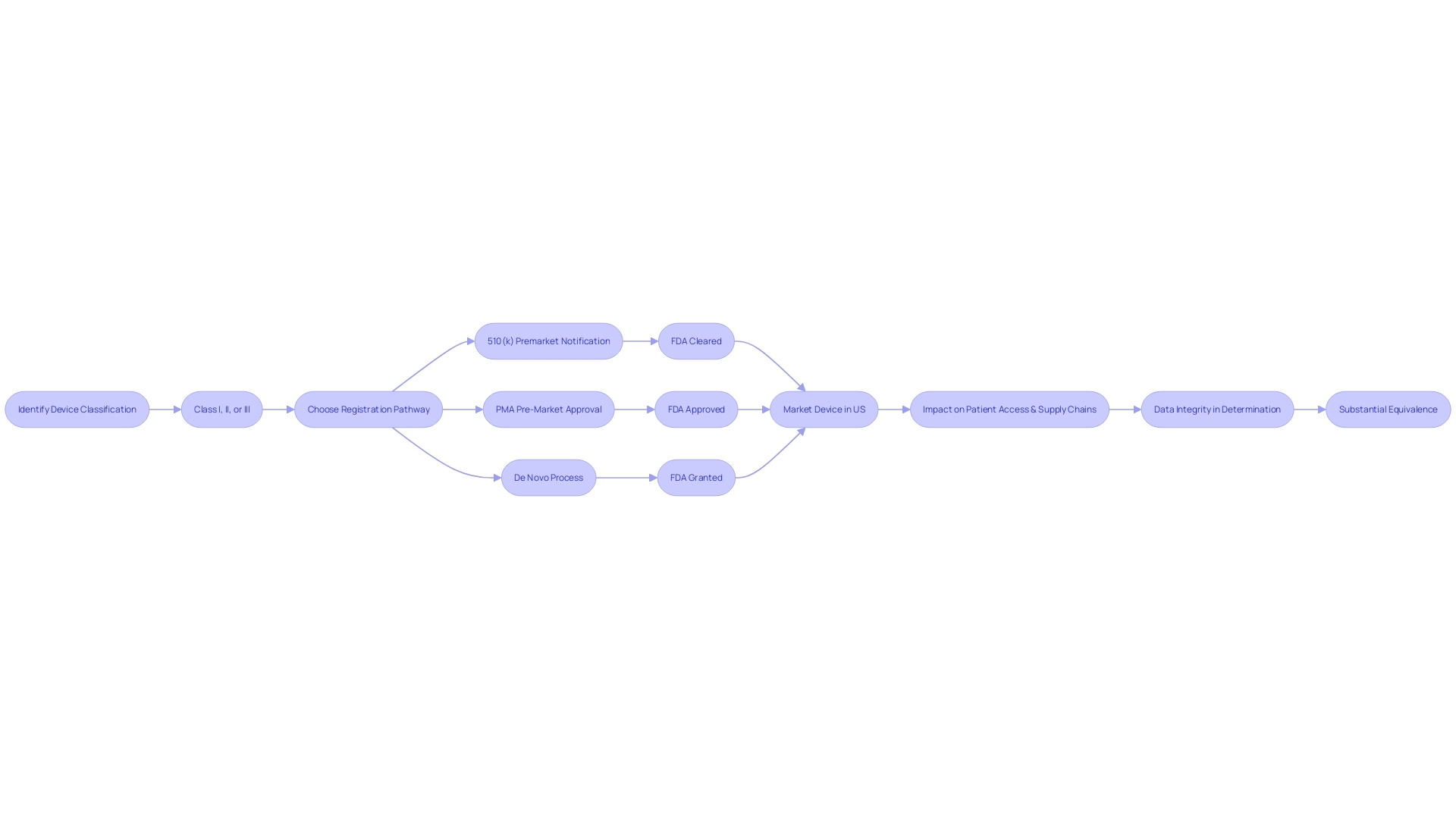
Navigating the FDA's regulatory landscape for medical devices requires a clear understanding of the classification and clearance processes. Medical devices are categorized into three classes by the FDA based on the level of risk they pose to patients, with Class I representing the lowest risk and Class III the highest. To bring a medical device to the U.S. market, it must be FDA Cleared, Approved, or Granted through one of the regulatory pathways: Premarket Notification (510(k)), Pre-Market Approval (PMA), or the De Novo process. The 510(k) clearance is a prevalent route for devices that demonstrate substantial equivalence to a legally marketed predicate device, meaning they have the same intended use and similar technological characteristics without raising new questions of safety or effectiveness. This process, while scrutinized in the documentary 'The Bleeding Edge' for allowing devices to bypass clinical trials, remains a critical pathway for introducing new medical technologies that are comparable to existing ones. Regulatory professionals must distinguish between terms like Registered, Cleared, Approved, and Granted to ensure proper compliance and safeguard patient health. The recent focus on the 510(k) clearance, including legislative and regulatory discussions, highlights the ongoing evolution of FDA oversight, aiming to balance patient safety with innovation in the medical device sector.
Ensuring Patient Safety
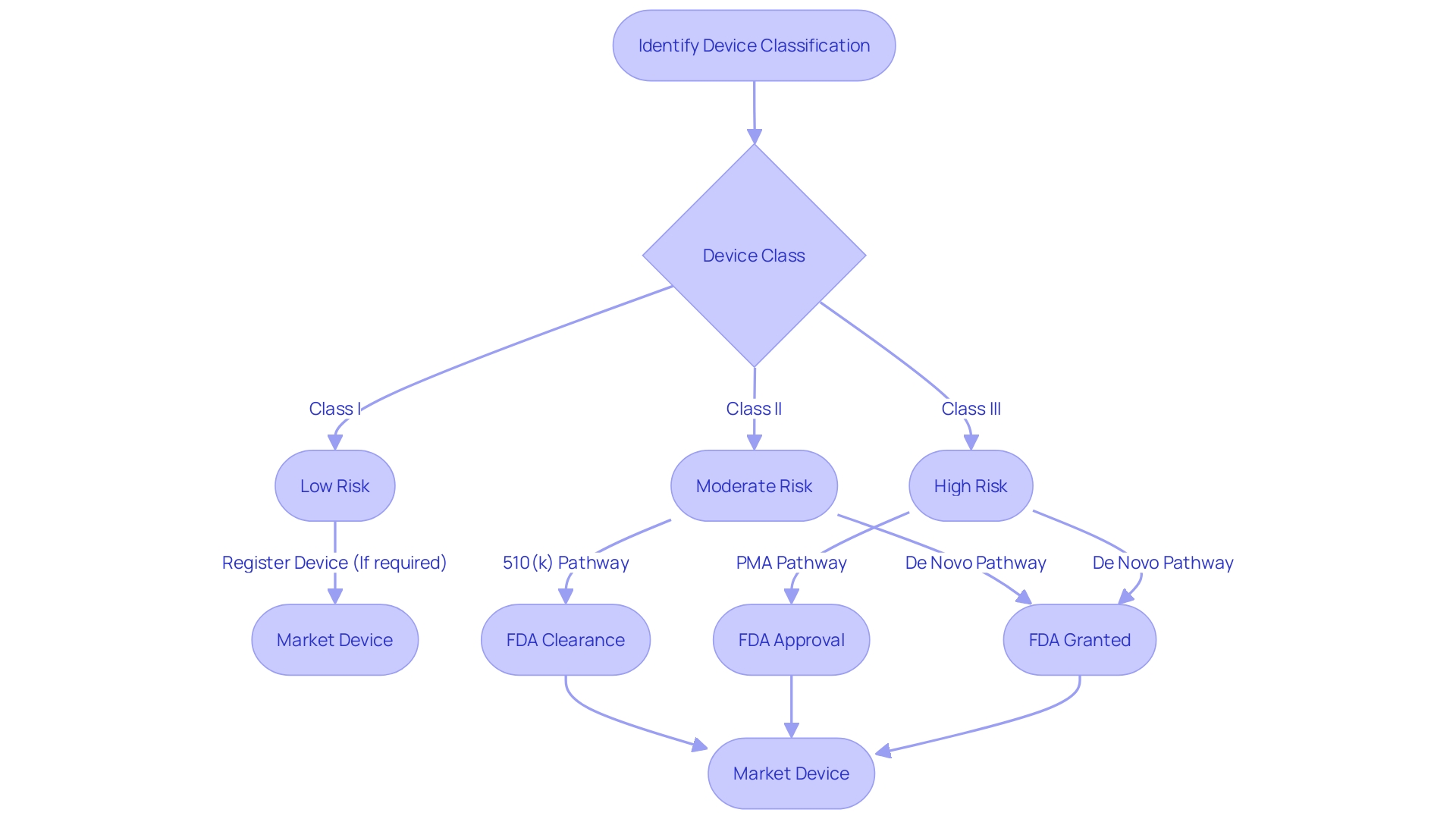
Navigating the complexities of FDA clearance is essential for ensuring that medical devices meet rigorous safety and effectiveness standards. The FDA classifies medical devices into three risk-based categories. Class I devices pose the lowest risk and often do not require a premarket submission to the FDA. Class II devices require a 510(k) Premarket Notification, which involves demonstrating that the device is substantially equivalent to a legally marketed device that is not subject to PMA. Class III devices, which include life-supporting or life-sustaining devices, require Pre-Market Approval (PMA), the most stringent type of device marketing application. The De Novo process is an alternative pathway for low to moderate risk devices that are novel and do not have a valid predicate. Understanding the distinction between these pathways—Registered, Cleared, Approved, and Granted—is critical for compliance and patient safety.
The 510(k) process has faced scrutiny for its approach that allows certain devices to bypass clinical trials by proving similarity to existing products. As illustrated in the 2018 documentary 'The Bleeding Edge,' this has sometimes resulted in devices being fast-tracked despite significant differences from their predicates, leading to patient harm.
Recent news reflects the gravity of FDA oversight. For instance, Justin Green, Assistant Commissioner for Criminal Investigations at the FDA, emphasized the risks of distributing unapproved medical devices. The FDA's Office of Criminal Investigations ensures the safety of medical products by enforcing the Federal Food, Drug, and Cosmetic Act (FDCA). In a sobering reminder of the risks, a recall notice for a Medtronic device was issued too late for a Missouri man who was found deceased, highlighting the critical nature of timely FDA intervention.
With the global orthopedic market projected to expand from $47.6 billion in 2022 to $60.2 billion by 2030, the stakes for ensuring the safety and effectiveness of medical devices are higher than ever. This underscores the importance of FDA clearance not only for compliance but also for maintaining public trust and safeguarding patient outcomes.
Benefits for Manufacturers
Achieving FDA clearance is a pivotal step for medical device manufacturers, as it signifies a green light to enter the market and provides a mark of quality and safety to healthcare professionals and patients. The FDA categorizes medical devices into three classes, with class I representing low-risk devices and class III high-risk devices. The clearance process, such as the 510(k) pathway, is critical for class I and II devices and compares the new device to an existing, legally marketed predicate device. The 510(k) process has raised some concerns, as highlighted in the 2018 documentary 'The Bleeding Edge', which revealed that certain devices were fast-tracked without the need for clinical trials, sometimes resulting in patient harm.
The FDA’s clearance not only facilitates market entry but also contributes to a manufacturer's reputation, underlining their dedication to compliance with rigorous regulatory standards. This can have direct financial implications, as insurers often require FDA clearance for reimbursement, and it can also impact a company’s valuation and prospects in scenarios such as acquisitions or IPOs.
While the clearance process aims to ensure safety and effectiveness, it is not without its challenges and risks. Manufacturers must navigate the complexities of FDA terms, and even experienced regulatory professionals can find the landscape perplexing. Companies need to understand the distinctions between terms such as 'Registered', 'Cleared', 'Approved', and 'Granted' to successfully manage their regulatory strategies.
The importance of FDA clearance is also evident in the business development world, where leaders recognize the value of regulatory compliance as a factor in successful medical device exits. However, it is essential to note that despite FDA clearance, other factors such as revenue strategies, patient outcomes, and market acceptance play a critical role in a device's success, as evidenced by statements from companies like Vivos, highlighting the uncertainties and risks even after approval.
In conclusion, FDA clearance is crucial for medical device manufacturers, but it is just the beginning of a journey that requires careful navigation of the regulatory landscape, strategic business planning, and a focus on both patient safety and market dynamics.
Examples of FDA Cleared Devices
FDA clearance is a critical aspect of ensuring that medical devices used for patient care meet established safety and efficacy standards. The clearance process encompasses devices varying from diagnostic apparatuses like blood glucose monitors, vital imaging technologies such as MRI machines, to essential surgical equipment, and life-sustaining implantable devices such as pacemakers and artificial joints.
For instance, the FDA's review of the Impella Connect System - a device comprising both software and hardware components designed to work with the Automated Impella Controller for temporary ventricular support - underscores the rigorous evaluation that devices undergo. This system, which includes a remote monitoring feature for critical care, illustrates the stringent criteria that software functions within medical devices must meet to gain FDA premarket authorization.
Another poignant example is the HeartMate 3 Left Ventricular Assist System, which serves as a pivotal aid for patients with severe left ventricular heart failure. This device can be used in various contexts: as a bridge while waiting for a heart transplant, as support to facilitate heart recovery, or as a long-term alternative when a transplant is not feasible. Its role in replacing the pumping function of the left ventricle exemplifies the type of high-risk Class III medical devices that undergo a comprehensive approval process due to their significant role in sustaining life.
Recent advancements in medical technology, such as the integration of automated insulin delivery systems with continuous glucose monitoring technology, reflect the evolving landscape of FDA-regulated devices. These developments demonstrate the FDA's ongoing commitment to safeguarding public health while allowing for innovation in the medical device sector.
The FDA, belonging to the U.S. Department of Health and Human Services, is charged with the protection of public health by ensuring the safety and security of a wide array of products, from human and veterinary drugs to the nation's food supply and medical devices. It is through this lens of rigorous evaluation and oversight that the FDA continues to fulfill its mission, approving a diverse range of new medical devices and drugs each year, thereby offering new treatment options and advancing healthcare for the public.
Misusing FDA Terminology
Accurate use of FDA terminology is critical when it comes to medical devices. For instance, the term 'FDA cleared' refers to a specific regulatory pathway, typically the 510(k) process, which is often utilized for devices that are not entirely new but are substantially equivalent to existing products. On the other hand, 'FDA approved' implies a device has undergone the more rigorous Pre-Market Approval (PMA) process, usually reserved for high-risk devices that are novel and require more extensive data to support their safety and efficacy.
A case in point is the Impella Connect System, which includes both software and hardware components designed to aid in critical care by providing remote monitoring of ventricular support devices. This system is subject to premarket authorization as its functions fall under the definition of a medical device per section 201(h) of the Federal Food, Drug, and Cosmetic Act. These functions include providing patient-specific medical information to detect life-threatening conditions and generating crucial alarms to notify health care providers.
Moreover, the integrity of the data submitted to the FDA for device clearance is paramount. Recent trends have shown an alarming increase in unreliable data submissions, especially from third-party test labs based in China and India. Data integrity issues can prevent the FDA from determining substantial equivalence, ultimately hindering the marketing authorization of medical devices, which negatively impacts patient access and supply chains.
The World Health Organization defines medical devices broadly, encompassing a range of products from simple tools like tongue depressors to complex machinery like diagnostic software. The FDA classifies these devices into three levels based on the risk they pose to patients, which dictates the appropriate registration pathway—be it 510(k), PMA, or De Novo. Each classification and registration pathway has specific implications for how a device can be marketed and used in patient care.
The distinctions between FDA terms such as 'Registered,' 'Cleared,' 'Approved,' and 'Granted' are not merely semantic; they reflect varying levels of review and evidence required by the FDA. These terms should be used precisely to communicate the status and ensure compliance with regulatory requirements. Transparency in these communications is vital, as it directly affects risks and patient outcomes, needing a clear explanation or 'explainability' for the decisions made.
In the documentary 'The Bleeding Edge,' the FDA's 510(k) clearance process is explored, highlighting that not all devices require clinical trials before being cleared for use. This pathway allows for fast-tracking devices that are similar to already approved products but has led to instances of patient harm due to inadequate review. As of March 2024, there are over 85,000 510(k) application summaries available, indicating the scope and complexity of the clearance process. The documentary's findings spurred changes and increased scrutiny of the clearance process, underscoring the importance of robust regulatory oversight for medical devices.
Resources and Guidance
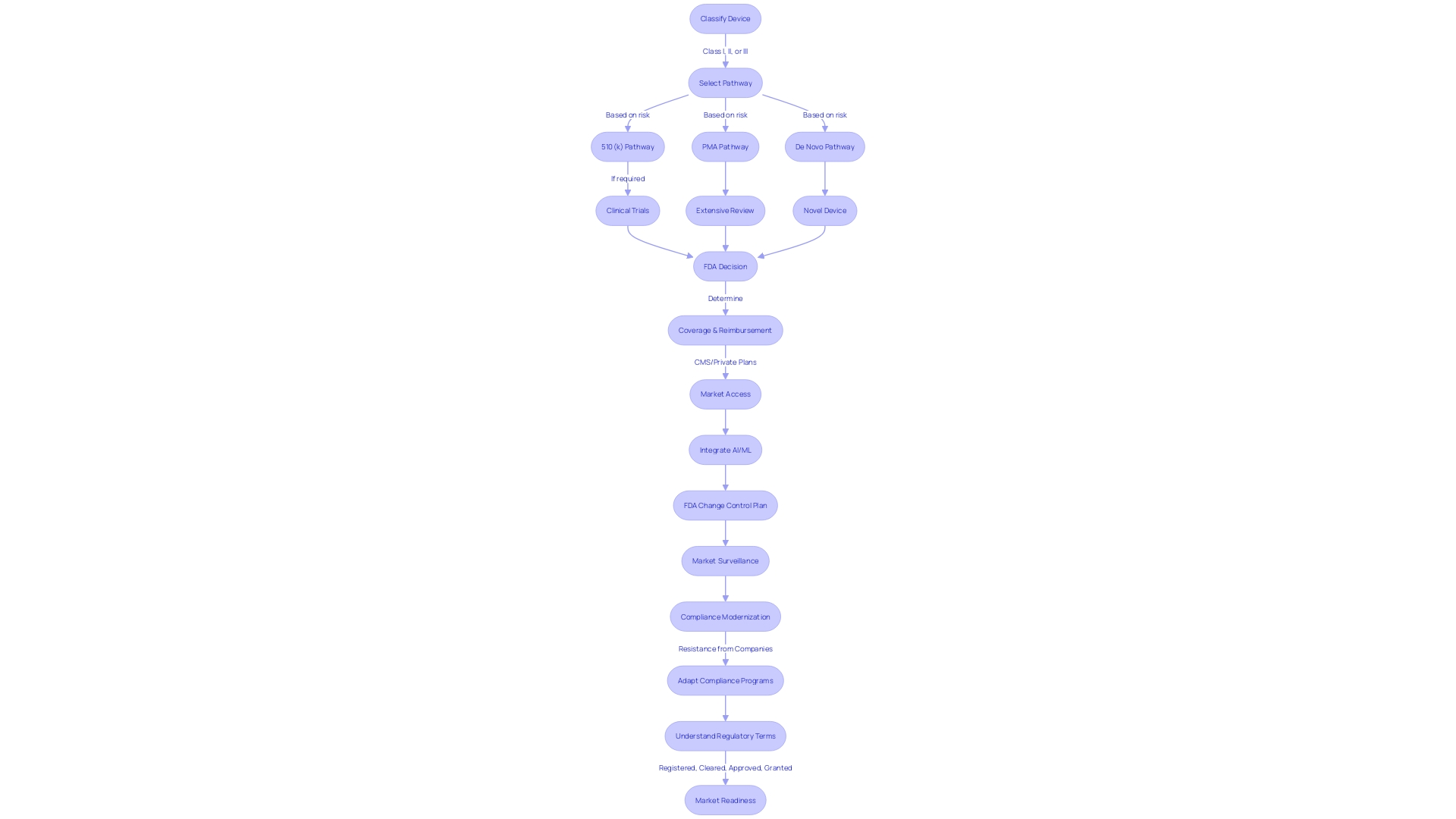
Navigating the FDA clearance process for medical devices is a critical step for manufacturers. To begin, it's essential to accurately classify your device; the FDA categorizes medical devices into three classes based on the level of risk they pose to patients. Once classification is determined, the pathway to registration—be it 510(k) Premarket Notification, Pre-market Approval (PMA), or the De Novo process—must be selected according to the device's classification and associated risks.
The 510(k) clearance process, specifically, is intended for devices that are substantially equivalent to a legally marketed device that is not subject to PMA. Interestingly, not all devices undergoing the 510(k) process require clinical trials, which was a point of examination in the 2018 documentary 'The Bleeding Edge'. This has led to discussions on the sufficiency of the process, as certain fast-tracked devices have been associated with patient injuries.
After a device is FDA Cleared, Approved, or Granted via the De Novo process, it's up to other entities, such as CMS and private health plans, to decide on coverage and reimbursement, which may not always align with FDA's safety and effectiveness data. This disconnection can result in delays or denials in device accessibility for patients.
Furthermore, as the medical device industry evolves with the integration of AI and machine learning, the FDA is adapting by introducing predetermined change control plans for AI- and ML-enabled devices. However, there's resistance from medtech companies to modernize compliance programs due to concerns over automation and its impact on patient safety. Overcoming this resistance is crucial for fostering innovation while maintaining compliance.
The FDA's role is paramount in protecting public health by ensuring the safety, effectiveness, and security of medical devices. Understanding the nuances between the terms Registered, Cleared, Approved, and Granted is pivotal for regulatory professionals, as each term signifies different levels of regulatory scrutiny and requirements for bringing a device to the U.S. market.

Conclusion
In conclusion, FDA clearance is a vital step in bringing medical devices to market in the United States. The FDA classifies devices based on risk level, with Class III devices requiring a more thorough review process. The common pathway to clearance is the 510(k) process, which compares devices to previously cleared ones.
However, concerns have been raised about its adequacy, as some cleared devices have caused harm. The Pre-Market Approval (PMA) pathway involves more comprehensive examination, including clinical trial data.
Accurate use of FDA terminology is critical. The 510(k) process has faced scrutiny for allowing devices to bypass clinical trials. Understanding terms like "Registered," "Cleared," "Approved," and "Granted" is important for compliance and patient safety.
FDA clearance is pivotal for manufacturers, as it signifies market entry and demonstrates compliance with regulatory standards. It has financial implications and impacts a company's reputation. However, clearance is just the beginning and requires careful navigation of the regulatory landscape.
Ensuring patient safety is paramount. The FDA classifies devices based on risk and the 510(k) process has faced scrutiny for inadequate review. Recent news highlights the risks of distributing unapproved devices and the importance of timely FDA intervention.
FDA clearance is important for compliance, public trust, and patient outcomes. The FDA's review of devices like the Impella Connect System and the HeartMate 3 Left Ventricular Assist System demonstrates rigorous evaluation.
Navigating the FDA clearance process requires accurate classification, selecting the appropriate pathway, and understanding coverage and reimbursement considerations. The FDA is adapting to the integration of AI and machine learning. Overcoming resistance to compliance modernization is essential for innovation and patient safety.
In conclusion, FDA clearance is crucial for bringing safe and effective medical devices to market. Understanding the regulatory landscape and complying with FDA standards are essential for manufacturers and healthcare professionals. The FDA plays a crucial role in safeguarding public health and ensuring patient safety.




