Introduction
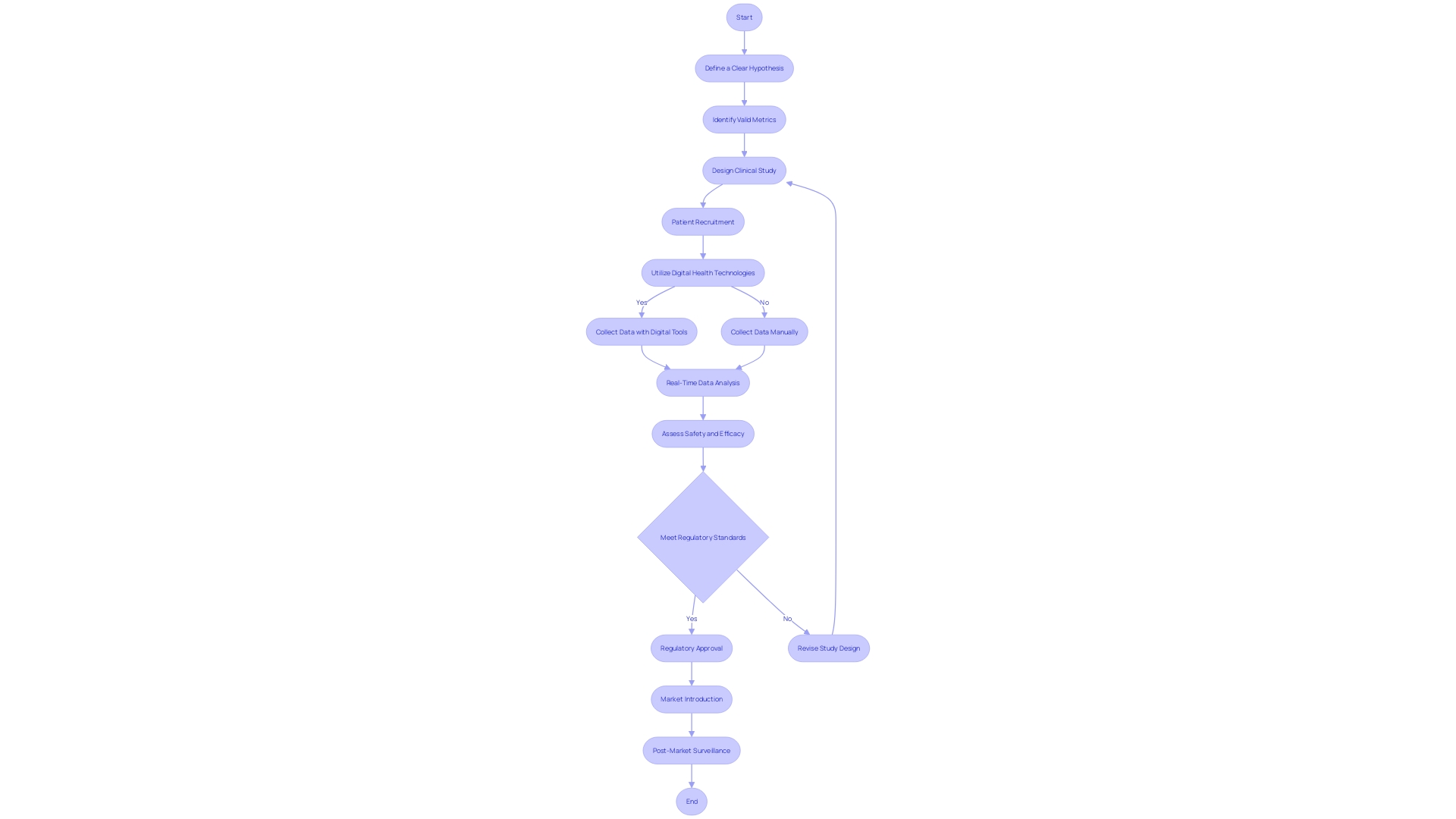
The landscape of digital health and MedTech is experiencing a surge in innovation, particularly in medical device development. To ensure the safety and efficacy of these devices, rigorous clinical studies are essential. These studies not only meet regulatory requirements but also provide valuable data on device performance and potential risks and benefits.
The FDA categorizes devices into classes based on risk, and the approval process can vary. Collaborations between regulatory agencies and industry stakeholders are crucial for improving the efficiency of these processes. Clinical trials for medical devices are complex and multi-phase, aiming to evaluate safety and effectiveness.
They provide real-world feedback and evidence to refine device design and functionality. The importance of clinical trials cannot be overstated, as they uncover unforeseen health information and contribute to advancing medical device efficacy. However, challenges such as poor trial design and lack of diversity among participants need to be addressed.
Ethical considerations, regulatory aspects, and the impact of clinical trials on medical innovation are also significant factors to be considered. Despite the challenges, the integration of technology into clinical trials is transforming the landscape, facilitating swifter evaluations and robust data collection. With the growing trend towards digitalization, the need for rigorous clinical trials becomes paramount in the evolving field of digital therapeutics.
The Role of Clinical Studies in Medical Device Development
The landscape of digital health and MedTech is experiencing a surge in innovation, particularly in medical instrument development. These instruments must go through extensive studies to evaluate their safety and effectiveness. These studies are not only a regulatory requirement but are also essential in ensuring that the equipment can be safely integrated into patient care routines. The data collected from clinical studies are invaluable, shedding light on instrument performance and illuminating any potential risks and benefits.
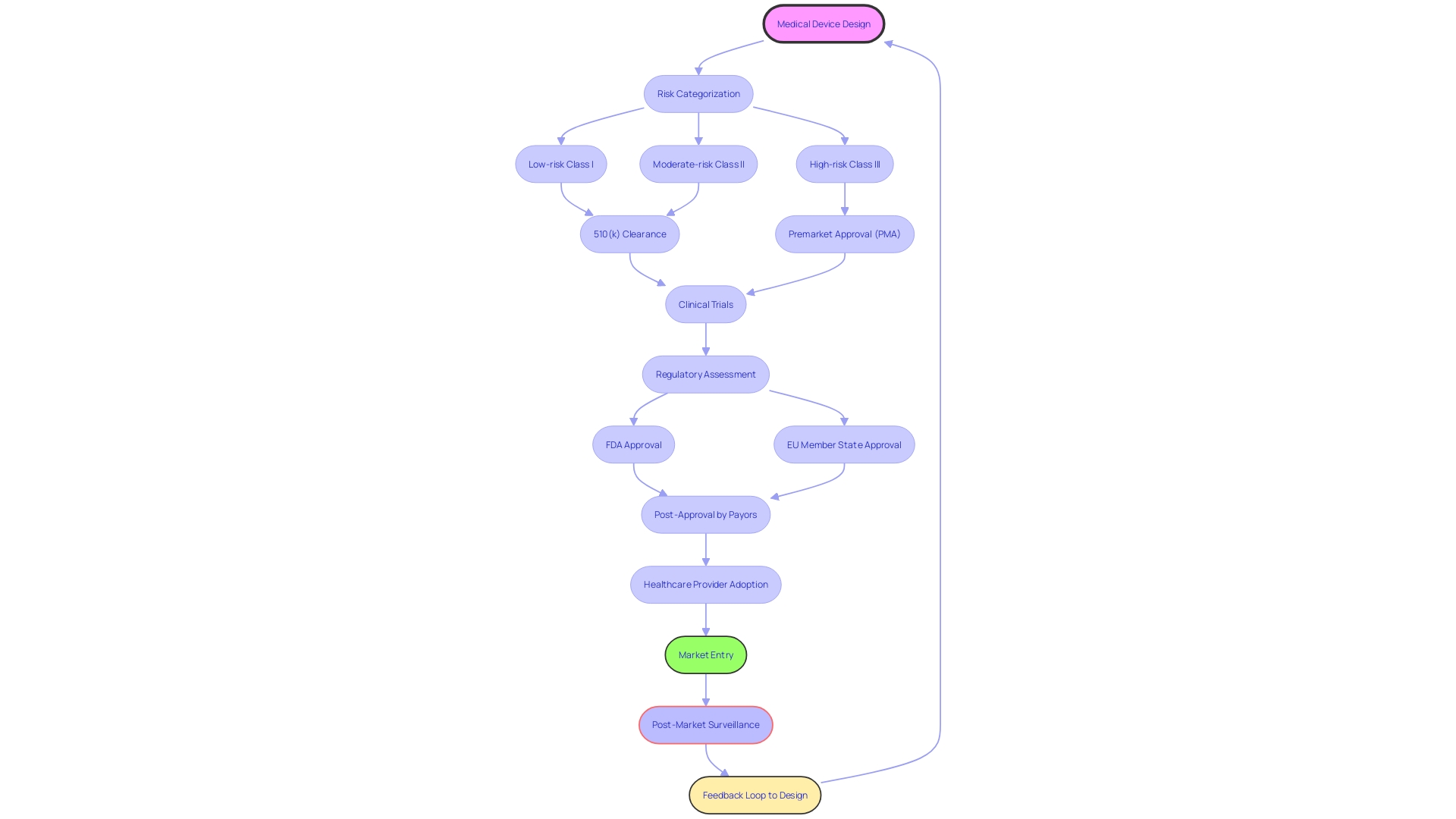
Clinical trials are essential in the regulatory process for medical equipment. In the United States, the FDA classifies instruments into categories based on risk, and the approval time can vary. For instance, low-risk class one and two instruments may only necessitate a 510(k) clearance, while high-risk class three instruments, such as pacemakers, go through a more rigorous approval process. In Europe, healthcare apparatus are typically controlled at the member state level, with the EMA participating in the process. Recent attempts have been made to simplify these channels, with the goal of speeding up the approval of technologies that fulfill pressing healthcare requirements, particularly in the rapidly advancing fields of digital health and personalized medicine.
Current collaborations between regulatory agencies and industry stakeholders are critical in improving the efficiency of these processes. While the FDA assesses the safety and effectiveness of medical equipment, other entities such as payors and healthcare providers determine coverage, payment, and usage post-approval. The information provided to the FDA may not always match the information required by payors, which can result in delays in patient access to new equipment.
Moreover, the journey from design to production is intricate and demands meticulous attention. Working with Contract Manufacturers (CM) requires a thorough understanding that developing a single prototype is distinct from mass production. An assessment by the manufacturer is crucial to evaluate the readiness of the equipment for production. Comprehending the intricacies of the approval process and the following stages to market entry is crucial for the growth and implementation of instruments in digital health and MedTech.
Phases of Medical Device Clinical Trials
Clinical trials for medical instruments are a intricate and multi-stage process designed to assess the safety and efficacy of these instruments. During the exploratory phase, the attention is on evaluating the initial safety and effectiveness of the apparatus with a small group of participants. This stage is essential for determining whether the equipment can progress to more extensive testing.
Following stages broaden the participant pool and increase the stringency of testing, with the goal of gathering extensive data on the performance. These stages are crucial for improving the design, manufacturing process, and functionality based on real-world feedback and evidence.
An example is the step-by-step approach to the introduction of equipment, where the initial release to the market does not overwhelm the production process, enabling necessary modifications based on the response from the market and findings from patient care. This flexible approach is crucial in the healthcare technology sector, where the initial iteration of a product may require prompt subsequent updates to address omitted features or design modifications prompted by user input and evaluation outcomes.
The insights from experienced professionals like Chris, a biomedical engineer with 13 years in the space, highlight the significance of a scalable supply chain, especially after Phase Two trials, to accommodate potential production ramp-ups.
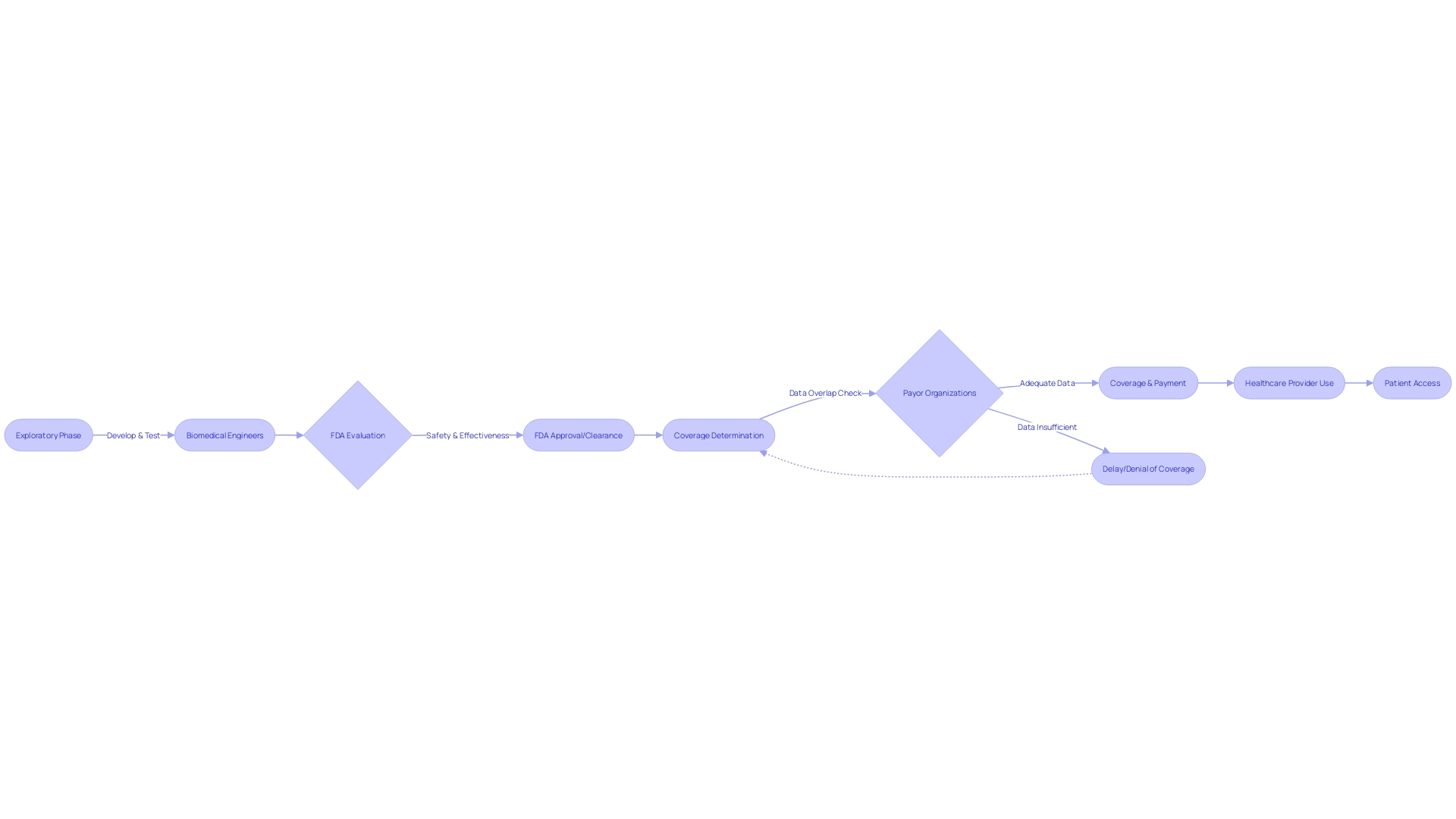
Furthermore, the interaction between FDA endorsement and healthcare payor choices emphasizes the intricacy of bringing a healthcare tool from experimental examinations to the hands of patients. The FDA assesses medical devices for safety and effectiveness, but payor organizations make independent decisions on coverage and reimbursement, which may not always align with FDA submissions, leading to delays in patient access.
To exemplify the difficulties encountered in medical experiments, contemplate the scenario where a Phase I oncology study encompassed 52 patients distributed across 105 sites, emphasizing the logistical intricacies and the need for flexibility and adaptability in research administration. Such scenarios highlight the necessity for thorough planning and resource allocation to guarantee success.
In the digital health and MedTech fields, the progression of healthcare tools is a careful process from creation to market launch, involving a delicate equilibrium between innovation, patient safety, and compliance with regulations.
Importance of Clinical Trials for Patient Safety and Efficacy
The significance of experiments in the advancement and evaluation of healthcare tools cannot be exaggerated. These experiments are not only a pathway to comprehend the interaction of objects with the human body but also a medium for discovering unexpected health information that can potentially save lives. As highlighted by the experience of Barbara, a participant in a research study, unexpected findings led to the identification of heart problems that were otherwise asymptomatic. This situation, made possible by her enrollment through The New Normal—an online platform that connects participants with research opportunities—underscores the dual benefits of medical device research: advancing medical device efficacy while simultaneously enhancing individual health awareness.
As per the World Health Organization, the field of experiments faces difficulties, such as inadequate experiment layout and a shortage of variety among participants. Information from 2022 shows a noticeable difference, with 27,133 experiments carried out in wealthy nations compared to 24,791 in nations with lower and middle incomes, causing worries about global disparities in medical research. By integrating medical research studies within regular healthcare services and prioritizing country-driven research and development, these studies can become more accessible and fair, ultimately expediting the availability of secure and efficient medical interventions.
The intricacy of managing trials is reflected in the operational aspects, where even well-established protocols can undergo amendments months into a project due to the dynamic nature of the research environment. This complexity is exemplified by a Phase I oncology study involving 52 patients across 105 sites. Such intricacies highlight the need for meticulous resource allocation and adaptability within medical research management.
The U.S. Food and Drug Administration (FDA) highlights the importance of effective, well-structured research as a foundation of public health. Dependable information from such research is essential for well-informed FDA decision-making regarding the safety and effectiveness of healthcare equipment. Efforts to align human subject protection regulations with the Common Rule and to streamline clinical research reflect the FDA's dedication to safeguarding participant rights while promoting advancements in healthcare.
Nevertheless, the path of a medical instrument from experimentation to cure is not without obstacles. As the FDA assesses the safety and effectiveness of equipment, there is a potential misalignment with the data requirements of payors, which can result in delays or denials in coverage, payment, and usage post-approval. This misalignment underscores the complexities of translating clinical trial results into patient care, as highlighted by the AMA.
The urgency of addressing safety in healthcare apparatus is underpinned by the sobering statistics of more than 1.7 million injuries and 83,000 deaths potentially linked to medical devices over a decade in the United States. The FDA's dynamic postmarket surveillance system aims to identify and rectify safety concerns, strengthening the oversight of healthcare instruments. Nonetheless, challenges in funding and patient identification persist, prompting continued efforts to enhance the surveillance infrastructure.
To summarize, while experiments for healthcare tools are essential in shaping patient treatment choices, they also uncover crucial health insights, promote the well-being of populations through fair research, and require thorough management and regulatory oversight to guarantee the safety and welfare of patients.
Regulatory Aspects of Medical Device Clinical Trials
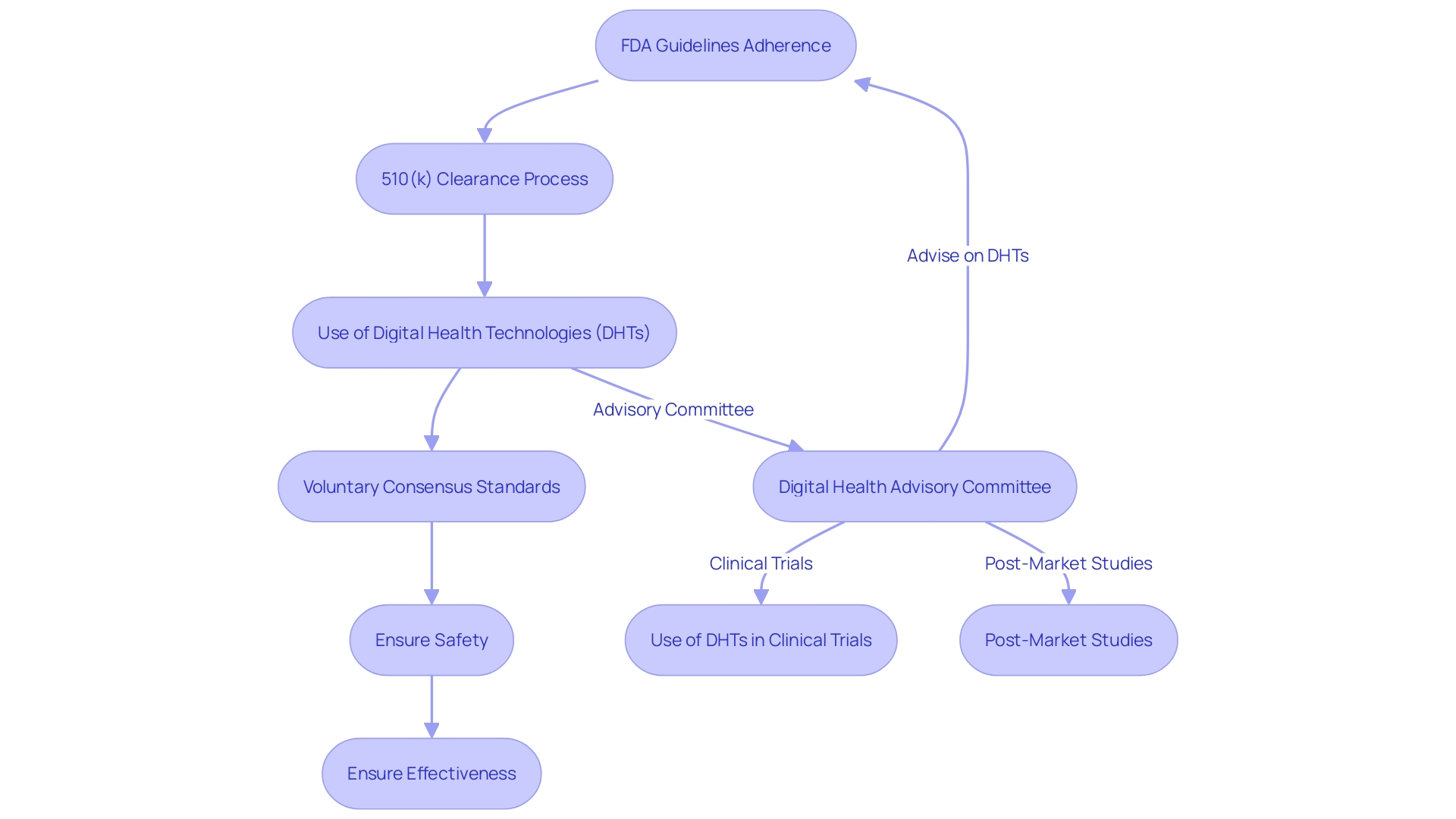
Guaranteeing the safety and effectiveness of healthcare tools is a crucial duty, and the FDA has a crucial function in the regulatory supervision of trials in the MedTech field. Adherence to FDA guidelines is not just about compliance; it's about safeguarding participants and securing the integrity of the research. For example, the FDA's 510(k) clearance process, which permits certain products to be fast-tracked to the market due to resemblance to previously authorized items, has been under scrutiny. It is significant to mention, nevertheless, that not all medical tools necessitate trials—a reality emphasized by the documentary 'The Bleeding Edge' which scrutinizes the intricacies of the FDA's clearance process.
To navigate these complexities, a thorough understanding of the FDA's regulatory framework is essential. This includes a clear definition of the Context of Use (COU) for computational models in regulatory submissions, ensuring that they address specific questions or concerns pertinent to the research. The FDA's recent final guidance on the use of Digital Health Technologies (DHTs) in investigations is a testament to the evolving landscape. DHTs, which can vary from software to sensors, must satisfy the definition of a tool and comply with requirements for data retention and protection. The guidance also delineates the process for selecting a DHT and rationalizing its use in investigations.
Moreover, the FDA emphasizes the importance of voluntary consensus standards, developed by Standards Development Organizations, which uphold principles of transparency, stakeholder participation, and due process. These standards contribute significantly to regulatory quality and innovation, providing a framework for technologies that facilitate patient access to new equipment.
In the field of healthcare equipment and clinical trials, the FDA's endorsement is equivalent to a product's efficiency and safety. As explained by professors Dhruva, Kesselheim, and Redberg, this trust in the FDA's approval process is a cornerstone of healthcare. However, the evidence supporting new drugs and equipment can vary, which raises questions about physician awareness of regulatory changes. The initial guidance for sponsors of medical experiments on the establishment and operation of data monitoring committees is a step towards enhancing the monitoring procedures of research studies, ensuring that the safety and efficacy of healthcare instruments are rigorously evaluated.
Ethical Considerations in Conducting Clinical Trials
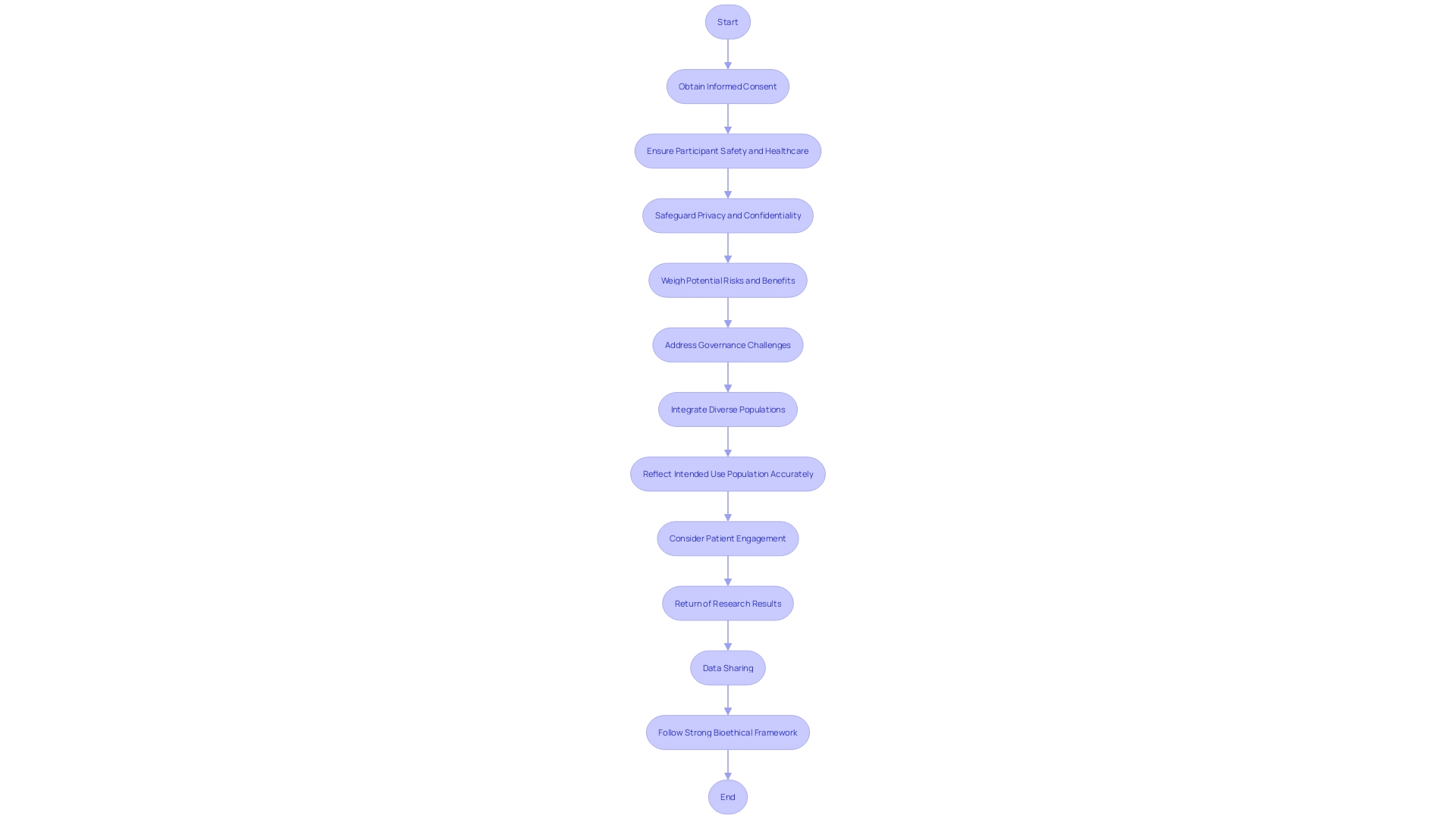
In the field of device clinical experiments, the protection of participant safety and the ethical use of technology are of utmost importance. As the landscape of digital health and MedTech rapidly evolves, the ethical, legal, and social implications are increasingly complex. Informed consent is a cornerstone of ethical research, requiring clear communication with participants about the risks, benefits, and the nature of the study. Privacy and confidentiality must be strictly safeguarded, and participants must receive suitable healthcare throughout the experiment.
Experiments must weigh potential dangers against the expected advantages of a new device, aiming to reduce damage while striving for the greatest positive influence. Real-world case studies, such as those involving mature but evolving technologies, shed light on the governance challenges and ethical issues that shape the trajectory of emerging medical technologies. These include considerations around market incentives, intellectual property, and the dynamic regulatory environment.
Recent reports demonstrate the intricacies involved in medical experiments, with examples of a Phase I oncology research involving 52 patients across 105 sites, emphasizing the colossal undertaking of overseeing such experiments. Furthermore, the emergence of real-world data provides a fresh opportunity for integrating diverse populations into medical experiments, as exemplified by the UK's NHS adhering to recommendations from NICE.
The FDA's 2022 to 2025 Strategic Priority: Advancing Health Equity, emphasizes the requirement for studies to reflect the intended use population accurately. This includes addressing disease burden, physiological considerations, and the specific technology in question. The planning of experiments in digital health must consider patient engagement, the return of research results, and data sharing, while navigating the ethical terrain of using digital health and real-world data.
As we stand on the verge of an AI revolution in medicine, the motivation for medical experiments differs among various markets. In the UK, for instance, the government requires a higher proof requirement before healthcare facilities can obtain AI products, encouraging companies to conduct experiments.
These diverse considerations require a strong bioethical framework to direct the progress and implementation of health instruments in research, guaranteeing that advancements in health technology are achieved responsibly and equitably.
Challenges and Solutions in Medical Device Clinical Trials
Navigating the diverse terrain of healthcare instrument experiments necessitates dealing with a group of obstacles, from participant recruitment to regulatory compliance. Innovative strategies such as leveraging online platforms like The New Normal have proven successful in enlisting participants, as demonstrated by Mehta's research team, which saw an influx of healthy volunteers. Compelling patient stories, like that of Barbara who discovered her heart condition through participation in a study, emphasize the double advantage of progressing healthcare technology and adding to personal health insights.
Effective communication and education strategies play a pivotal role in ensuring participant adherence to study protocols. For example, Chris, a biomedical engineer with more than ten years in the field of healthcare equipment, emphasizes the significance of comprehensive protocol understanding, which can be accomplished by closely engaging with study teams at investigator meetings.
Data management in research studies for medical devices has been transformed with advanced tools, as emphasized by experts like Etienne Nichols, who brings extensive experience from manufacturing to product development. The carefulness needed in moving from design to production resonates in the data analysis phase of medical experiments, where accuracy and uniformity are crucial.
Collaboration with regulatory authorities is another cornerstone for successful clinical trials. The FDA plays a vital role in assessing the safety and efficacy of healthcare equipment, and its approval is a prerequisite for payors to make coverage and reimbursement determinations. However, the FDA's regulatory process, which classifies equipment from low-risk class one to high-risk class three, requires extensive data that may not always align with payor requirements, potentially delaying patient access to new technologies.
Efforts have been made to enhance approval processes, especially for items that cater to urgent healthcare requirements, by implementing more efficient regulatory pathways and fostering better cooperation among stakeholders. The COVID-19 pandemic has catalyzed these efforts, emphasizing the need for efficiency in areas like digital health and personalized medicine.
In the rapidly evolving MedTech landscape, the market is poised for significant growth, with projections estimating a rise from $788 million in 2022 to $1.9 billion by 2033. This growth path emphasizes the significant significance of overcoming the inherent obstacles in healthcare equipment experiments to promote innovation and enhance patient results.
The Impact of Clinical Trials on Medical Innovation
In the field of digital health and MedTech, clinical experiments act as the foundation for healthcare innovation, offering crucial understanding into the safety and efficacy of novel healthcare tools. These experiments are carefully organized, producing data that not only clears the path for regulatory approval but also enhances the collective medical knowledge, resulting in improved treatments and patient care. The incorporation of technology into these experiments has been revolutionary, enabling faster assessments and strong data through the implementation of digital patient engagement tools, wearable devices, and sensors. Such advancements ensure precise data collection, optimize patient adherence, and minimize errors, offering real-time insights that are promptly available for analysis. Moreover, the present scenario of experiments is being influenced by different factors like market incentives and intellectual property rights, all while addressing ethical, legal, and social concerns. With the digital therapeutics sector emerging as a promising field, particularly in the management of chronic diseases and neurological conditions, the need for rigorous testing becomes paramount. Despite challenges such as data privacy and ethical concerns, the surge in wearable technology users, which reached 1.1 billion in 2022, reflects a growing trend towards the digitalization of clinical trials. This trajectory is backed by an ever-evolving governance ecosystem, both in the United States and internationally, that seeks to frame a cross-sectoral governance framework for emerging technologies in health and medicine.
Conclusion
In conclusion, clinical trials are crucial for developing and assessing medical devices in the digital health and MedTech landscape. They ensure safety, efficacy, and provide valuable data on device performance and risks. Collaboration between regulatory agencies and industry stakeholders is vital for efficient processes and faster patient access to new devices.
Clinical trials have complex phases that evaluate safety and effectiveness. Challenges like poor trial design and lack of participant diversity must be addressed for comprehensive research. Ethical considerations, regulatory aspects, and the impact on medical innovation are also important factors.
The integration of technology is transforming clinical trials, enabling faster evaluations and robust data collection. Digital tools and wearable devices optimize data management, patient adherence, and minimize errors. The growing trend towards digitalization and wearable technology highlights the need for rigorous clinical trials in digital therapeutics.
In summary, clinical trials shape treatment options, uncover health insights, and advance medical device efficacy. They require meticulous management, regulatory oversight, and ethical considerations. Technology integration drives innovation and improves patient outcomes.
Rigorous clinical trials are paramount in ensuring the safety, effectiveness, and accessibility of medical devices in the evolving digital health and MedTech landscape.




