Overview
The article focuses on the significant growth and potential of the Latin America clinical research market, highlighting its diverse patient populations, cost-effectiveness, and efficient regulatory processes as key advantages. It supports this by detailing increased investments in the region, the vital role of Clinical Research Organizations (CROs) in overcoming challenges, and the growing collaboration among stakeholders, all of which enhance the feasibility and appeal of conducting clinical trials in Latin America.
Introduction
Latin America is emerging as a critical hub for global clinical research, characterized by its diverse patient demographics, cost-effective solutions, and increasingly favorable regulatory environments. Countries like Brazil, Mexico, and Argentina are at the forefront, demonstrating robust healthcare infrastructures that not only attract significant investments but also facilitate the rapid expansion of Clinical Research Organizations (CROs).
With annual expenditures in clinical trials soaring from a few million to over $50 million, the region is witnessing unprecedented growth and collaboration, exemplified by initiatives such as the partnership between bioaccess™ and Caribbean Health Group.
However, this promising landscape is not without its challenges, including regulatory complexities and language barriers, which necessitate a strategic approach to harness the full potential of clinical trials in Latin America. As the region continues to evolve, understanding the intricacies of conducting research here becomes essential for stakeholders aiming to improve healthcare outcomes and address global health disparities.
The Importance of Latin America in Global Clinical Research
Latin America is quickly positioning itself as a crucial participant in the Latin America clinical research market, fueled by its varied patient populations, cost-effectiveness, and efficient regulatory processes. Noteworthy countries such as Brazil, Mexico, and Argentina boast robust healthcare infrastructures that facilitate the swift growth of Clinical Research Organizations (CROs). Recent years have witnessed a significant increase in investment, with spending in the research sector soaring from $3-4 million to more than $50 million each year.
This growth is emphasized by important collaborations, such as the partnership between bioaccess™ and Caribbean Health Group, aimed at positioning Barranquilla as a premier destination for medical studies, supported by Colombia's Minister of Health. However, Medtech companies encounter various obstacles, including:
- Regulatory challenges
- Language barriers
- Fragmented resources
These challenges can hinder effective collaboration and implementation of research studies. As more medical studies are delegated to the Latin America clinical research market, the commitment to enhancing healthcare access aligns with the necessity for investigations that address health inequalities.
The area's distinctive demographic variety enables scholars to acquire findings that are more universally applicable, enhancing the quality and relevance of medical studies. Moreover, as of 2000, all examined medical study locations in Peru have successfully adhered to regulations, indicating a solid basis for future medical investigation efforts. The expansion of medical studies in the Andean Region of Latin emphasizes the importance of addressing these challenges and embracing a solution-oriented strategy to close the gaps in healthcare, ultimately enhancing health standards throughout the area.
Navigating Challenges and Opportunities in Latin American Clinical Trials
Carrying out medical studies in the Latin America clinical research market, particularly in Colombia, requires maneuvering through a complicated environment marked by multiple obstacles and considerable benefits. While researchers may face:
- Language barriers
- Diverse regulatory frameworks
- Cultural differences impacting participant recruitment and retention
Colombia presents unique opportunities. The nation offers extensive management services for studies, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Project oversight
- Particular reporting needs such as study status, inventory, and serious and non-serious adverse occurrences
All of which enhance the process.
Furthermore, the Colombian National Food and Drug Surveillance Institute (INVIMA) plays a crucial role in the regulatory landscape, overseeing medical device approval and classification as a Level 4 health authority by PAHO/WHO. Hospitals in Colombia are permitted to carry out studies involving pharmaceutical drugs only after they have undergone a stringent ICH/GCP certification process. These regulatory efficiencies contribute to the overall competitive advantages of conducting first-in-human studies in Colombia and play a crucial role in the Latin America clinical research market, including:
- Cost savings (over 30% compared to North America)
- Rapid approval timelines (90-120 days for IRB/EC and MoH reviews)
- Access to a diverse patient population across more than 18,000 hospitals
Moreover, the strong R&D tax benefits and government funding further improve the feasibility of medical studies. By fostering strong relationships with local stakeholders and employing bilingual staff, researchers can significantly improve communication and participant engagement, thereby enhancing recruitment and retention efforts. The area's rising production of scientific publications, especially in oncology, highlights its expanding significance in the global research landscape.
As the Global Clinical Trials Support Service Market grows, the Latin America clinical research market becomes a promising space for medical investigations, provided that researchers strategically tackle inherent challenges.
The Role of Clinical Research Organizations in Latin America
The success of clinical studies in the Latin America clinical research market heavily relies on Clinical Research Organizations (CROs), which provide a thorough range of vital services including:
- Regulatory compliance
- Feasibility and selection of study sites
- Site management
- Patient recruitment
Their deep understanding of local regulations and patient demographics allows for the development of more effective study designs and execution strategies. This localized expertise not only mitigates risks but also enhances the overall efficiency of assessments.
Collaborating with CROs enables researchers to navigate complex regulatory landscapes more smoothly, as these organizations often maintain established relationships with key opinion leaders and ethics committees, facilitating more effective interactions with regulatory bodies. As emphasized by Bill Andrews, Ph.D., President & CEO of Sierra Sciences, 'The collaborative efforts with LATAM CRO Experts have significantly streamlined our processes, enhancing our ability to enroll and retain subjects.' Furthermore, the elevated wages in the pharmaceutical sector enhance quality of life and spending in the economy, which can further promote patient involvement in research studies.
For example, Paraguay presently carries out one cancer study, demonstrating the emerging status of such exploration in the area. Given the challenges faced by the region, such as regulatory timelines, political instability, and limited financial resources, the role of CROs has never been more crucial. They can utilize the strong eagerness of patients to engage in studies and tackle local health concerns through customized initiatives.
Insights from the case study titled 'Challenges and Opportunities for Clinical Research in Latin America' emphasize that despite these challenges, CROs are crucial in overcoming barriers and revealing the potential for progress within the Latin America clinical research market. Their services, including setup, project management, and reporting, are essential in tackling these challenges effectively.
Best Practices for Successful Clinical Trials in Latin America
To ensure the success of research trials in the Latin America clinical research market, researchers should adopt several best practices that leverage the region's unique strengths:
-
Engage Local Expertise: Collaborating with local investigators and Contract Research Organizations (CROs) is crucial for effectively navigating the regulatory landscape. With an average of 7 years of overall experience in medical studies, local experts provide invaluable insights that improve compliance and operational efficiency.
-
Culturally Tailored Recruitment: Developing recruitment strategies that reflect cultural nuances and preferences is essential for improving participant engagement. Tailoring outreach efforts can lead to better recruitment outcomes, particularly in diverse populations.
-
Robust Training Programs: Comprehensive training programs for research teams are necessary to ensure adherence to protocols and ethical standards. This training should not only cover clinical procedures but also local cultural considerations that may affect execution.
-
Flexible Protocol Designs: Flexibility in trial designs allows for adaptation to local practices and patient needs, thereby enhancing participant retention. This adaptability is vital, especially given that dropout rates in Latin America are one-third of those in the U.S. and EU, reflecting a more favorable environment for participant engagement.
-
Continuous Monitoring: Establishing real-time monitoring systems is essential for promptly identifying and resolving issues, thus ensuring the integrity and quality of the research. With 53% of studies in the region overseen by a data monitoring committee, maintaining oversight is a critical component of successful management.
Moreover, it is essential to take into account the role of INVIMA, Colombia's National Food and Drug Surveillance Institute, which supervises regulatory compliance and medical device classification, guaranteeing that assessments meet necessary standards. As Julio G. Martinez-Clark, CEO of bioaccess™, notes,
Colombia has recognized these benefits and has an ambitious science, technology, and innovation plan for 2022–2031 to become a knowledge economy.
Furthermore, the economic impact of medtech research studies cannot be overlooked; they contribute to job creation, economic growth, and healthcare enhancement in local communities.
By adopting these optimal methods and leveraging the cooperative initiatives between entities such as bioaccess™ and Caribbean Health Group, clinical investigators can greatly improve the efficiency of their trials throughout the Latin America clinical research market, establishing Barranquilla as a premier location for studies.
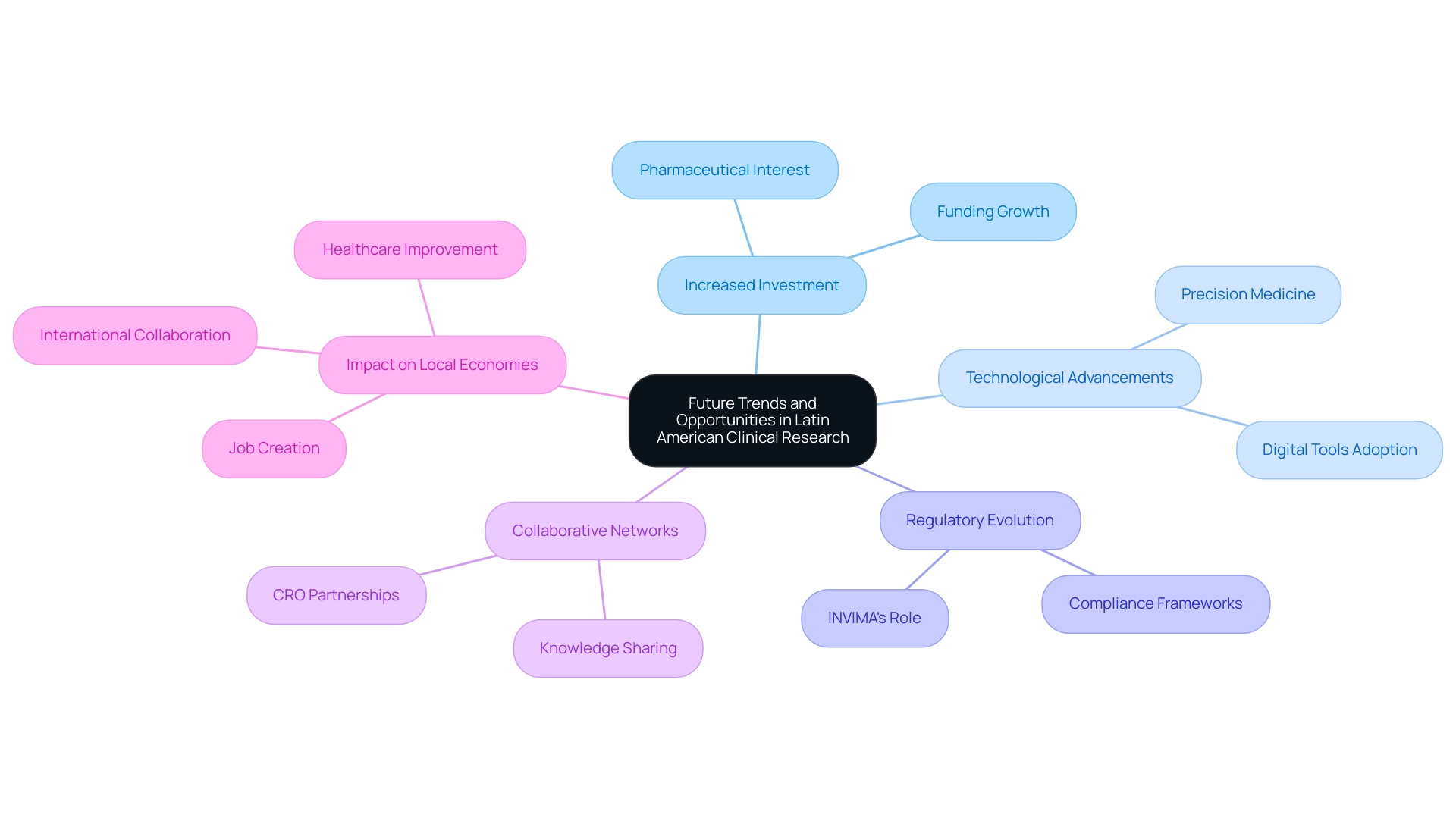
Future Trends and Opportunities in Latin American Clinical Research
The perspective for medical studies in the Latin America clinical research market is remarkably encouraging, characterized by various important trends ready to change the environment. First, there is a notable increase in investment in the Latin America clinical research market; the annual funding in the Andean Region has surged from $3-4 million to over $50 million, signaling strong interest from global pharmaceutical companies and creating more research opportunities. For example, in 2012, PAREXEL International Corporation broadened its activities in the Latin America clinical research market by opening a new depot in Santiago, Chile, which is anticipated to enhance the efficiency of research supply and logistics in this market, directly aiding the increasing demand for studies.
Second, technological advancements are playing a crucial role, with the adoption of digital tools such as telemedicine platforms and electronic data capture systems streamlining processes and enhancing patient engagement. Furthermore, the emphasis on precision medicine is growing, as researchers are progressively customizing treatments for specific populations, requiring varied and inclusive studies. Regulatory evolution is another critical aspect; organizations must navigate the refined frameworks set by INVIMA, Colombia's National Food and Drug Surveillance Institute, which plays a significant role in the Latin America clinical research market as a Level 4 health authority by PAHO/WHO.
This regulatory oversight enhances compliance and ensures the safety and effectiveness of research studies. Moreover, the thorough administration of research studies encompasses vital procedures such as study setup, obtaining import permits for investigational devices, and careful project management and oversight. These processes ensure that examinations are conducted efficiently and in compliance with local regulations.
Reporting on study status, inventory, and adverse events is also crucial for maintaining transparency and accountability. Lastly, the formation of collaborative networks among researchers, Contract Research Organizations (CROs), and health authorities is promoting knowledge sharing and improving the overall quality of health studies in the Latin America clinical research market. This collaborative approach, coupled with the impact of Medtech clinical studies on local economies—such as job creation, economic growth, healthcare improvement, and international collaboration—will be vital in navigating the complexities of clinical research and ensuring that Latin America remains an attractive destination for clinical trials.
Conclusion
Latin America stands at the forefront of global clinical research, driven by its diverse patient demographics, cost-effective solutions, and improving regulatory frameworks. The region's significant investment surge—from a mere $3-4 million to over $50 million annually—highlights the growing interest from global pharmaceutical companies and the establishment of Clinical Research Organizations (CROs) that are adept at navigating local landscapes. Countries like Colombia, Brazil, and Mexico showcase robust healthcare infrastructures that not only facilitate rapid trial execution but also enhance the quality and applicability of research findings.
Despite the promising landscape, stakeholders must remain vigilant regarding the challenges that accompany clinical trials in Latin America, including:
- Regulatory complexities
- Language barriers
- Cultural nuances
Employing best practices such as:
- Engaging local expertise
- Tailoring recruitment strategies
- Maintaining continuous monitoring
can significantly mitigate these challenges. The collaboration between CROs and local researchers is essential, as it fosters a deeper understanding of the regulatory environment and patient engagement, ultimately leading to more effective and efficient trials.
Looking ahead, the future of clinical research in Latin America appears bright, bolstered by technological advancements and a commitment to precision medicine. As the region continues to evolve, strategic partnerships and a focus on overcoming inherent challenges will be crucial in unlocking its full potential. By leveraging its unique strengths and addressing obstacles with innovative solutions, Latin America is poised to emerge as a leading destination for clinical research, contributing to improved healthcare outcomes and addressing global health disparities.




