Introduction
The Premarket Approval (PMA) process is a cornerstone of the FDA's regulatory framework for high-risk medical devices. Designed for Class III devices, which include life-sustaining or life-supporting equipment like implantable pacemakers and heart valves, PMA ensures these products undergo rigorous scrutiny. This process requires extensive clinical data to validate both safety and effectiveness, underscoring its critical role in safeguarding public health.
Medical device classification is pivotal in determining the necessity for PMA. Devices are categorized into Class I, II, and III based on risk levels, with Class III requiring the most stringent review. Manufacturers must carefully evaluate their device's intended use and potential risks to navigate the appropriate regulatory pathway.
A successful PMA application hinges on several key components, including detailed device descriptions, comprehensive clinical data, and a robust post-marketing surveillance plan. The FDA review process is thorough, involving completeness checks, substantive reviews, and possibly public advisory committee consultations, ensuring only the safest and most effective devices reach the market.
The FDA's decision on a PMA application can result in an Approval Letter, an Approvable Letter, or a Not Approvable Letter, each with specific implications for the manufacturer. Common challenges in the PMA process include inadequate clinical data and miscommunication with the FDA, highlighting the importance of a well-planned regulatory strategy. Understanding these elements is essential for manufacturers aiming to bring high-risk medical devices to market successfully.
What is PMA and When is it Required?
'The Premarket Approval (PMA) process is a crucial regulatory route established by the FDA for high-risk medical equipment.'. Necessary for items classified as Class III, PMA ensures that these products, which pose the highest risk to patients, undergo stringent evaluation. Devices such as implantable pacemakers, heart valves, and certain orthopedic implants fall into this category. The PMA process demands extensive clinical data to demonstrate both safety and effectiveness, reflecting its rigorous nature.
Understanding the necessity of PMA is vital for manufacturers, as it dictates the level of scrutiny and evidence needed for approval. Compared to other FDA pathways like 510(k) or De Novo, PMA involves a more comprehensive review. The FDA's role in this process emphasizes its dedication to safeguarding public health by ensuring the safety, effectiveness, and security of medical products.
Classifying Medical Devices: Determining the Need for PMA
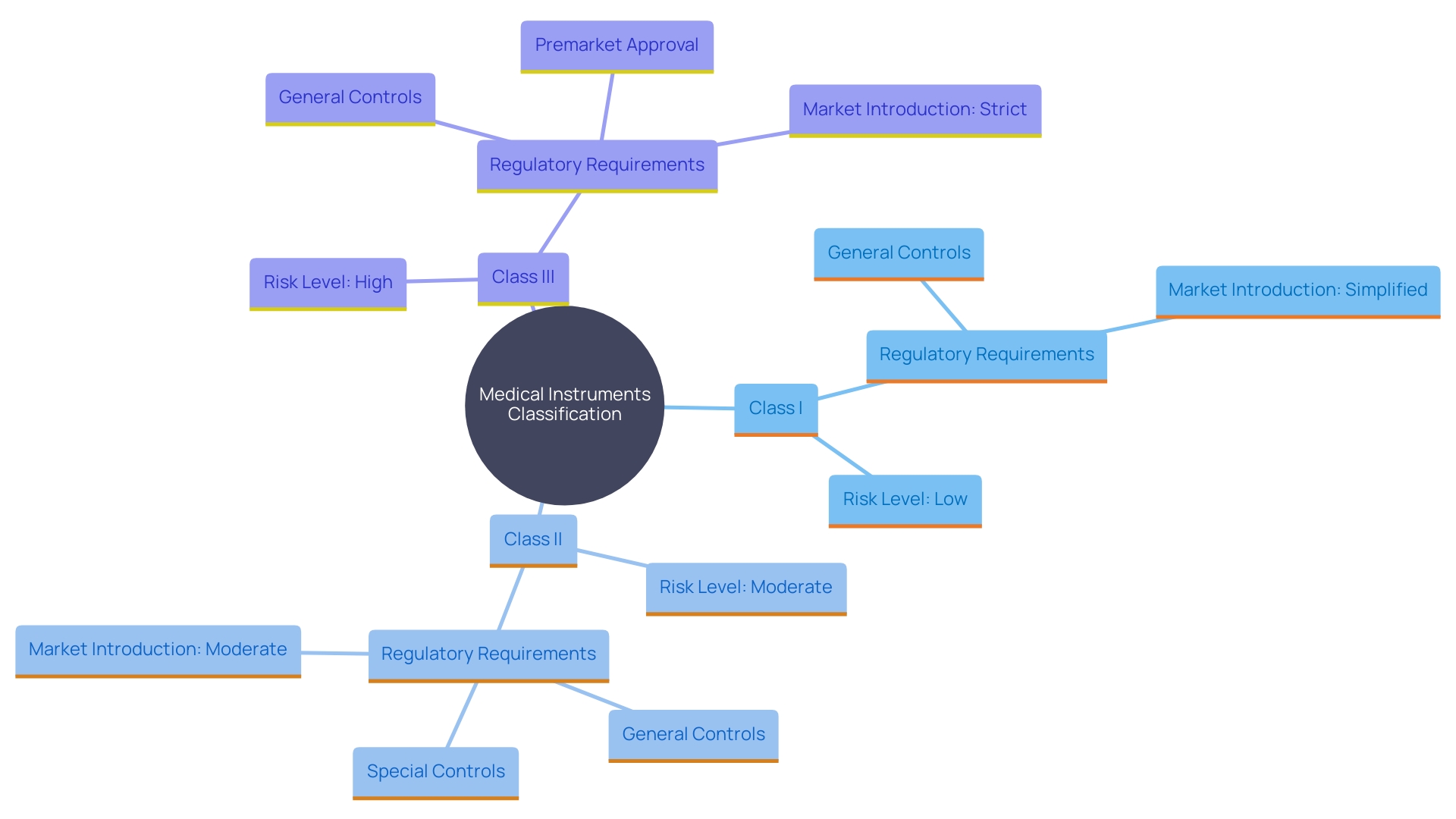
'Medical instruments are categorized into three classes—Class I, Class II, and Class III—based on the level of risk they present to patients and users.'. 'Class I items pose the lowest risk and are subject to general controls, including provisions concerning adulteration, misbranding, registration, and quality system regulation.'. Class II items, which are regarded as moderate risk, require both general and special controls. These often necessitate the 510(k) premarket notification submission to demonstrate substantial equivalence to a legally marketed product. Class III products, representing the highest risk, must undergo the rigorous Premarket Approval (PMA) process. This involves providing valid scientific evidence to ensure the safety and effectiveness of the product.
Manufacturers must carefully assess their product's intended use, technological characteristics, and potential risks to determine the appropriate classification. 'This classification not only determines the compliance requirements but also affects the strategic approach and timelines for introducing the product to market.'. For instance, the FDA has outlined the regulatory and procedural guidance for integral drug-device combinations, such as pre-filled syringes, which are regulated depending on their primary mode of action. This guarantees that all medical instruments, regardless of their complexity, adhere to stringent safety and efficacy standards, thus safeguarding public health.
Key Components of the FDA PMA Application
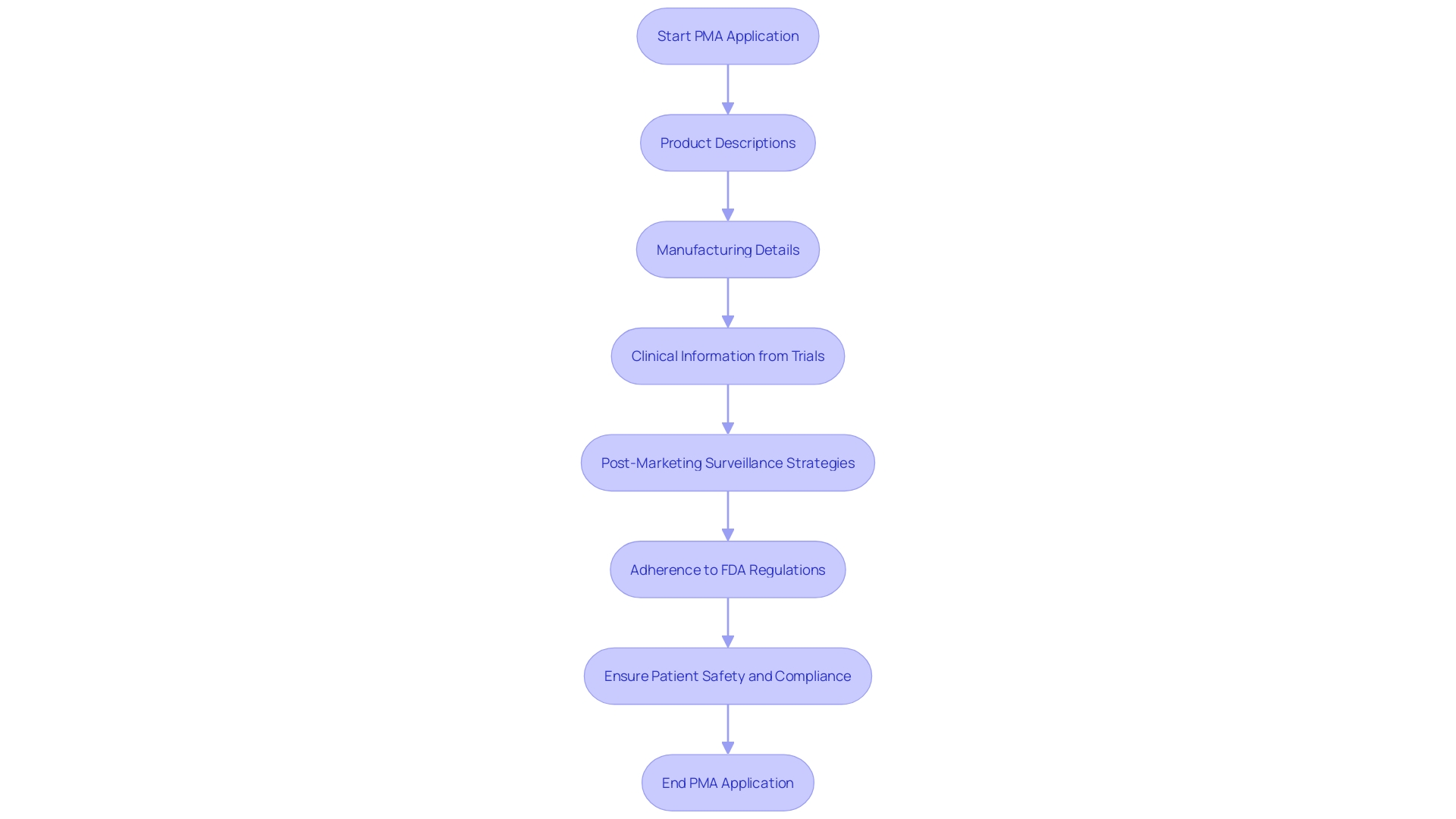
A PMA application must encompass several vital components, including comprehensive product descriptions, manufacturing details, and proposed labeling. Importantly, clinical information demonstrating the device's safety and effectiveness is essential. This information typically originates from clinical trials adhering to Good Clinical Practice (GCP). Furthermore, the application must encompass information on the suggested post-marketing surveillance strategy and any relevant details from prior studies or applications.
To enhance the robustness of clinical information, registries and natural history studies can play an integral role, particularly in the realm of rare disease treatments. These registries, organized systems collecting clinical and other information in standardized formats, are invaluable for understanding disease progression, which is crucial for developing effective treatments. For instance, in rare disease drug development, natural history studies allow researchers to track illness progression over time, informing clinical trial design and decision-making by authorities.
'The FDA's expectations for In Vitro Diagnostics (IVDs) emphasize the necessity of clinical information in submissions, underscoring the importance of precise and trustworthy information.'. As Carmen Brown notes, “With IVDs, you're looking at it from the perspective of what's the risk of a false result or an inaccurate result. 'So you're looking at who's interpreting the results and the type of condition.' This viewpoint emphasizes the essential requirement for accurate and trustworthy information to ensure patient safety and adherence to regulations.
Additionally, efficient information standards are crucial for adherence to regulations and uniformity across research. Guaranteeing the integrity and reliability of clinical trial information through standardized formats is not only a compliance requirement but also a cornerstone of high-quality research. 'The FDA's dependence on information, including large-scale information, highlights the significance of presenting evidence-based arguments to support compliance submissions, as emphasized by industry experts.'.
In summary, a PMA application must be meticulously detailed, supported by rigorous clinical data, and aligned with regulatory expectations to ensure the successful approval and post-market surveillance of medical products.
PMA Application Review Process
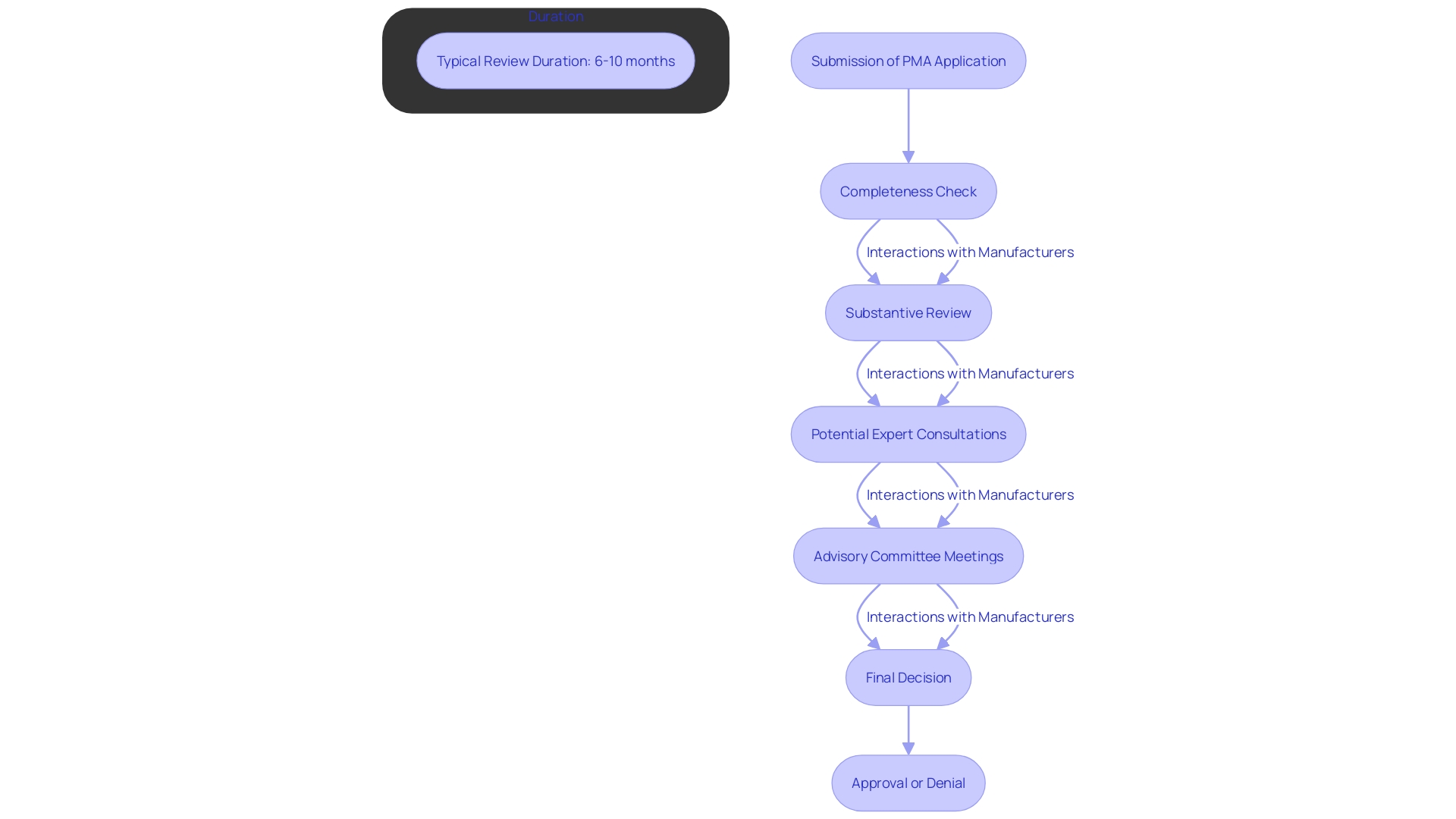
Once a PMA application is submitted, it enters a comprehensive multi-stage review process by the FDA. Initially, the application undergoes a completeness check, ensuring all required elements are present. If deemed complete, the FDA proceeds with a substantive review, which may involve expert consultations and public advisory committee meetings. This rigorous review phase typically spans around 180 days, although it can extend if the product is particularly complex or if additional information is required. During this critical period, manufacturers should anticipate potential interactions with the FDA, aimed at clarifying and addressing any concerns or queries that may arise. This meticulous process ensures that only safe and effective medical products reach the market, aligning with the FDA's commitment to safeguarding public health.
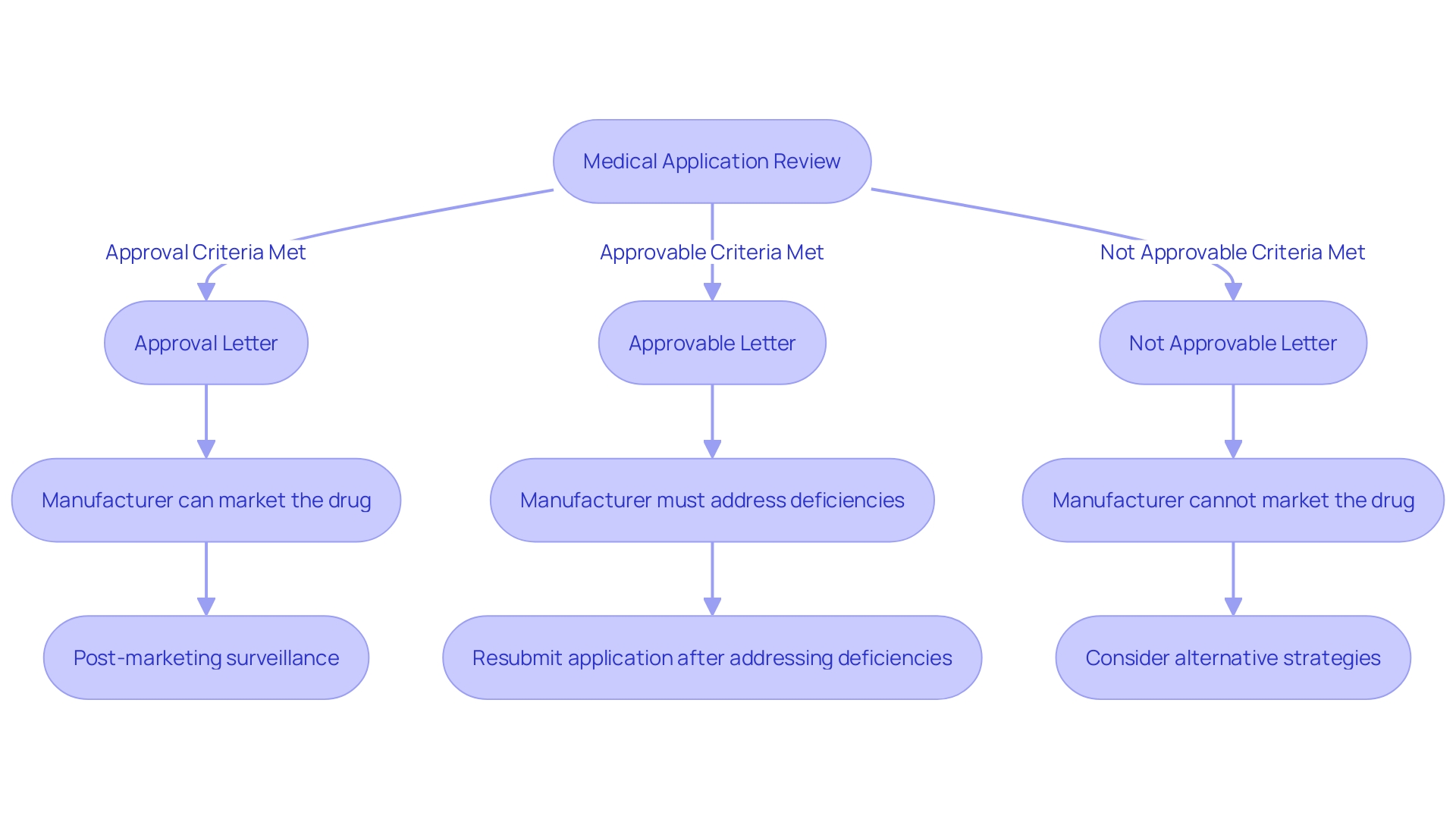
FDA Actions on a PMA Application: Approval, Approvable, and Not Approvable Letters
Upon completing the review process, the FDA will issue one of three possible decisions for a medical application: an Approval Letter, an Approvable Letter, or a Not Approvable Letter. An Approval Letter indicates that the product has met all necessary safety and effectiveness standards, allowing it to be marketed. An Approvable Letter signifies that the item could receive approval, but specific conditions or further information must be supplied beforehand. On the other hand, a Not Approvable Letter means the application does not meet the required standards, and the manufacturer needs to address specific deficiencies identified by the FDA. Each decision follows a rigorous, collaborative review process, underscoring the FDA's commitment to ensuring the safety and efficacy of medical devices for public health.
Common Mistakes and Challenges in the PMA Process
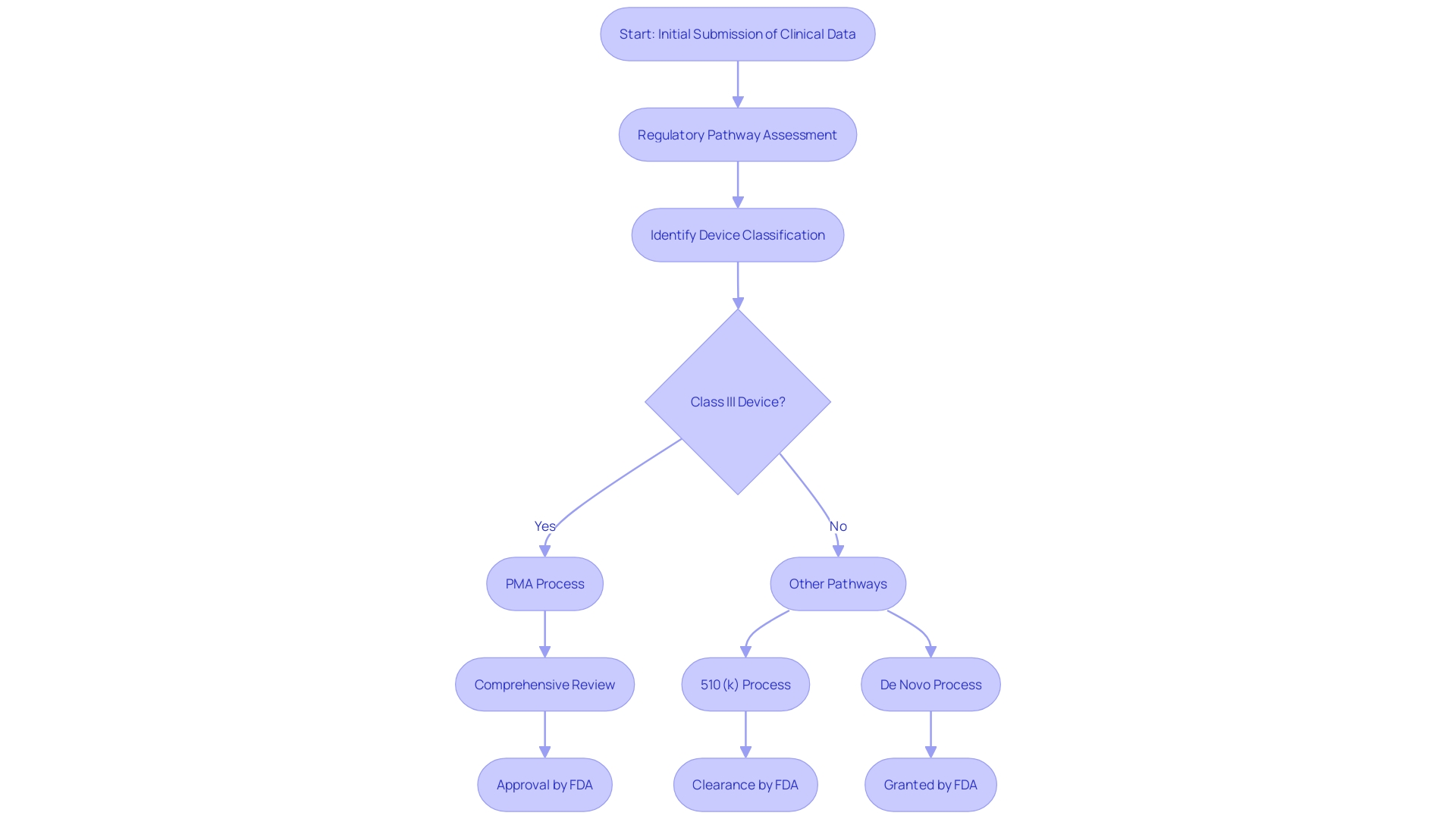
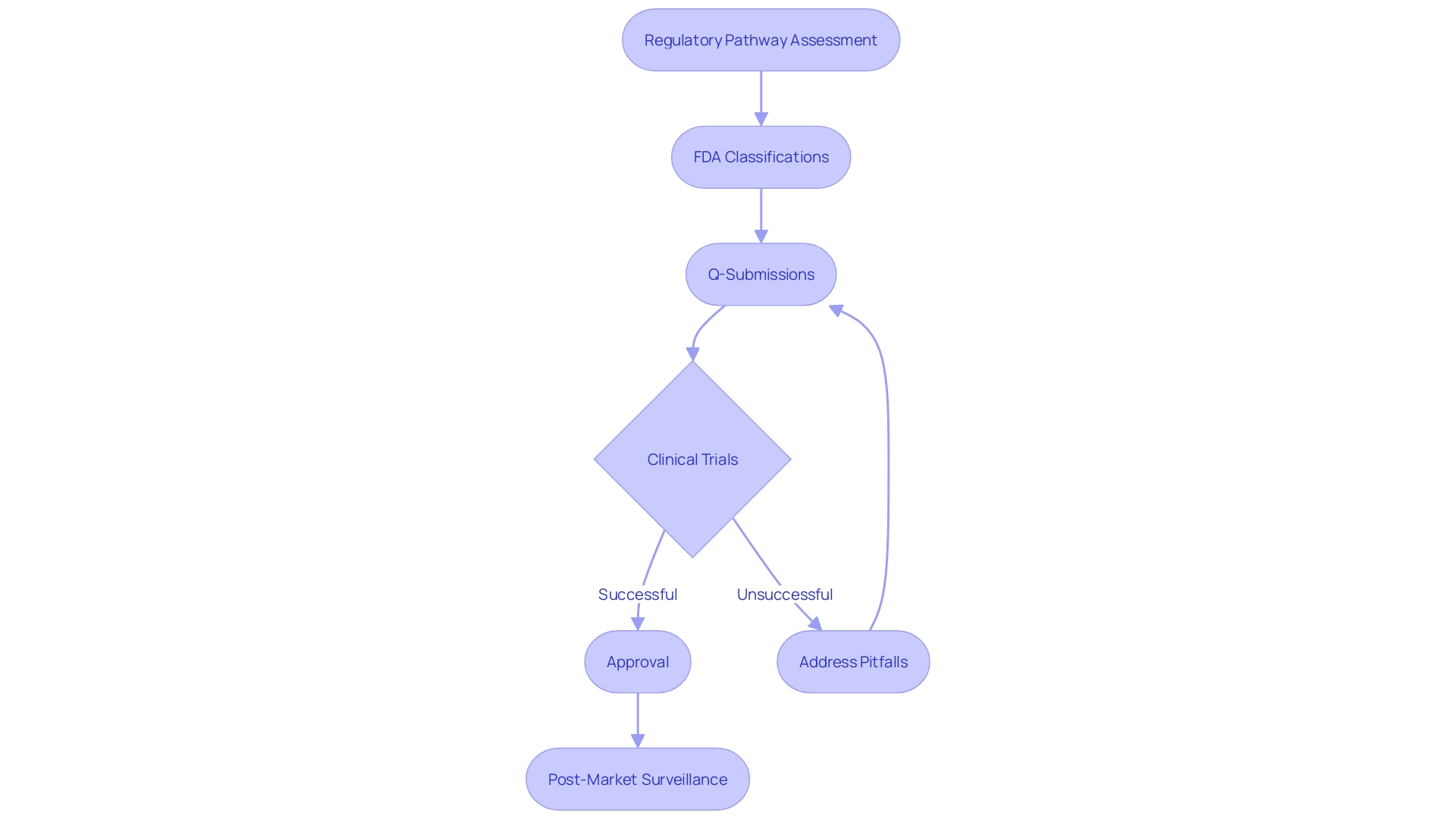
Steering through the PMA process is naturally difficult, with frequent traps such as insufficient clinical data, inability to satisfy compliance needs, and miscommunication with the FDA. It is crucial for manufacturers to conduct a comprehensive regulatory pathway assessment to build a solid regulatory strategy. This involves scavenging the FDA databases to understand the product code, optional submission paths, and exemptions. Additionally, engaging with the FDA through the Q-submission process to address pertinent questions can provide critical guidance.
A key aspect of the PMA process is understanding the classification of the medical instrument. The FDA classifies instruments into three tiers, each linked to varying patient risk values. Determining the correct classification is essential before choosing the appropriate registration pathway, whether it be Premarket Notification (510(k)), Premarket Approval (PMA), or the De Novo process. It is vital to recognize that only FDA Cleared, Approved, or Granted devices can be legally marketed in the US.
Moreover, manufacturers often underestimate the time and resources required for comprehensive clinical trials and data collection. Optimistic timelines frequently face delays due to necessary approvals from ethics committees or institutional review boards (IRBs). Proactively addressing these potential pitfalls, such as by obtaining and thoroughly understanding relevant standards and increasing organizational competence, can significantly enhance the likelihood of a successful PMA application.
Conclusion
The Premarket Approval (PMA) process is a critical regulatory mechanism designed to ensure the safety and effectiveness of high-risk medical devices classified as Class III. By requiring extensive clinical data and a rigorous review process, PMA plays a vital role in protecting public health. Manufacturers must grasp the importance of PMA, as it dictates the level of scrutiny their devices will face and establishes the necessary evidence for approval.
Medical device classification significantly influences the regulatory pathway. Class I and II devices face less stringent requirements, while Class III devices necessitate the comprehensive PMA process. Understanding this classification is essential for manufacturers to navigate the regulatory landscape effectively and align their strategies with FDA expectations.
A successful PMA application hinges on meticulous preparation, including detailed device descriptions, robust clinical data, and a well-structured post-marketing surveillance plan. The review process itself is thorough, ensuring that only devices meeting safety and efficacy standards receive FDA approval. This multi-stage review not only addresses the completeness of the application but also includes consultations and potential public advisory committee meetings.
Ultimately, the outcomes of the PMA review—Approval, Approvable, or Not Approvable Letters—have significant implications for manufacturers. Awareness of common challenges, such as inadequate clinical data and miscommunication with the FDA, can aid in developing a solid regulatory strategy. By proactively addressing these potential pitfalls, manufacturers can enhance their chances of successfully bringing high-risk medical devices to market, thereby contributing to advancements in healthcare and patient safety.




