Introduction
In the realm of medical device regulation, Premarket Approval (PMA) stands as a critical gateway ensuring the safety and effectiveness of high-risk medical devices. Governed by the U.S. Food and Drug Administration (FDA), the PMA process mandates rigorous scrutiny and extensive data collection for devices classified under Class III, those posing significant risk to patients. Unlike the more expedited 510(k) pathway, PMA requires comprehensive scientific and clinical evidence to affirm that the benefits of a device outweigh its potential risks.
This article delves into the intricacies of the PMA process, outlining the steps involved in preparing and submitting an application, the critical components required for a complete PMA submission, and the exhaustive review mechanisms employed by the FDA. Additionally, it contrasts the PMA pathway with the 510(k) process, highlighting the heightened scrutiny and regulatory demands of the former. The essential nature of post-market surveillance (PMS) is also emphasized, underscoring its role in maintaining device safety and effectiveness over time.
By elucidating these elements, the article aims to provide a thorough understanding of the PMA process and its paramount importance in safeguarding public health.
What is Premarket Approval (PMA)?
Premarket Approval (PMA) is a rigorous regulatory procedure created by the U.S. Food and Drug Administration (FDA) to guarantee the safety and efficacy of high-risk medical equipment. Devices categorized as Class III, which present considerable danger to patients, must go through the PMA procedure before they can be legally sold. This entails a thorough review of scientific and clinical evidence demonstrating that the benefits of the apparatus outweigh its potential risks.
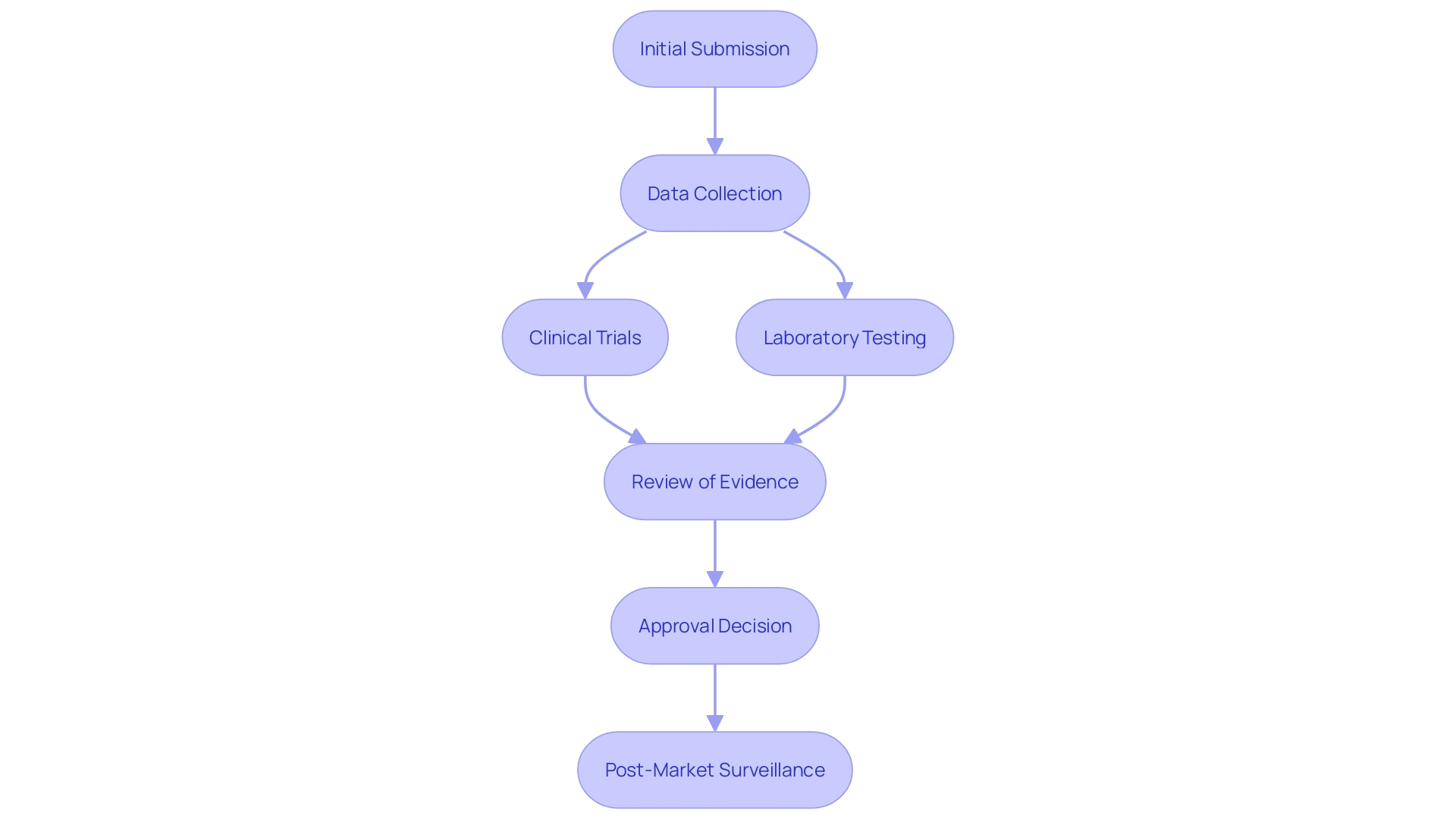
The PMA process is comprehensive, requiring extensive data collection and analysis. This encompasses clinical trials, laboratory testing, and thorough documentation to support the intended use of the product. According to recent FDA guidance, using real-world evidence is encouraged to support regulatory submissions. This means information gathered from real equipment usage in clinical practice can be used to demonstrate safety and effectiveness, provided the data is of high quality.
Even with the demanding nature of the PMA procedure, merely around 1% of medical instruments are approved via this route. Most products are approved through the 510(k) pathway, which is quicker and relies on substantial equivalence to a legally marketed item. Nevertheless, for high-risk products, the PMA procedure remains essential in guaranteeing that only the safest and most effective instruments reach patients.
The necessity of the PMA process is underscored by the importance of post-market surveillance (PMS). PMS involves continuous monitoring of equipment in real-world settings to detect and mitigate potential risks. This continuous attentiveness is crucial for ensuring patient safety and enhancing equipment performance over time. By adhering to these stringent regulatory requirements, the FDA aims to protect public health and ensure that medical instruments provide significant benefits to patients.
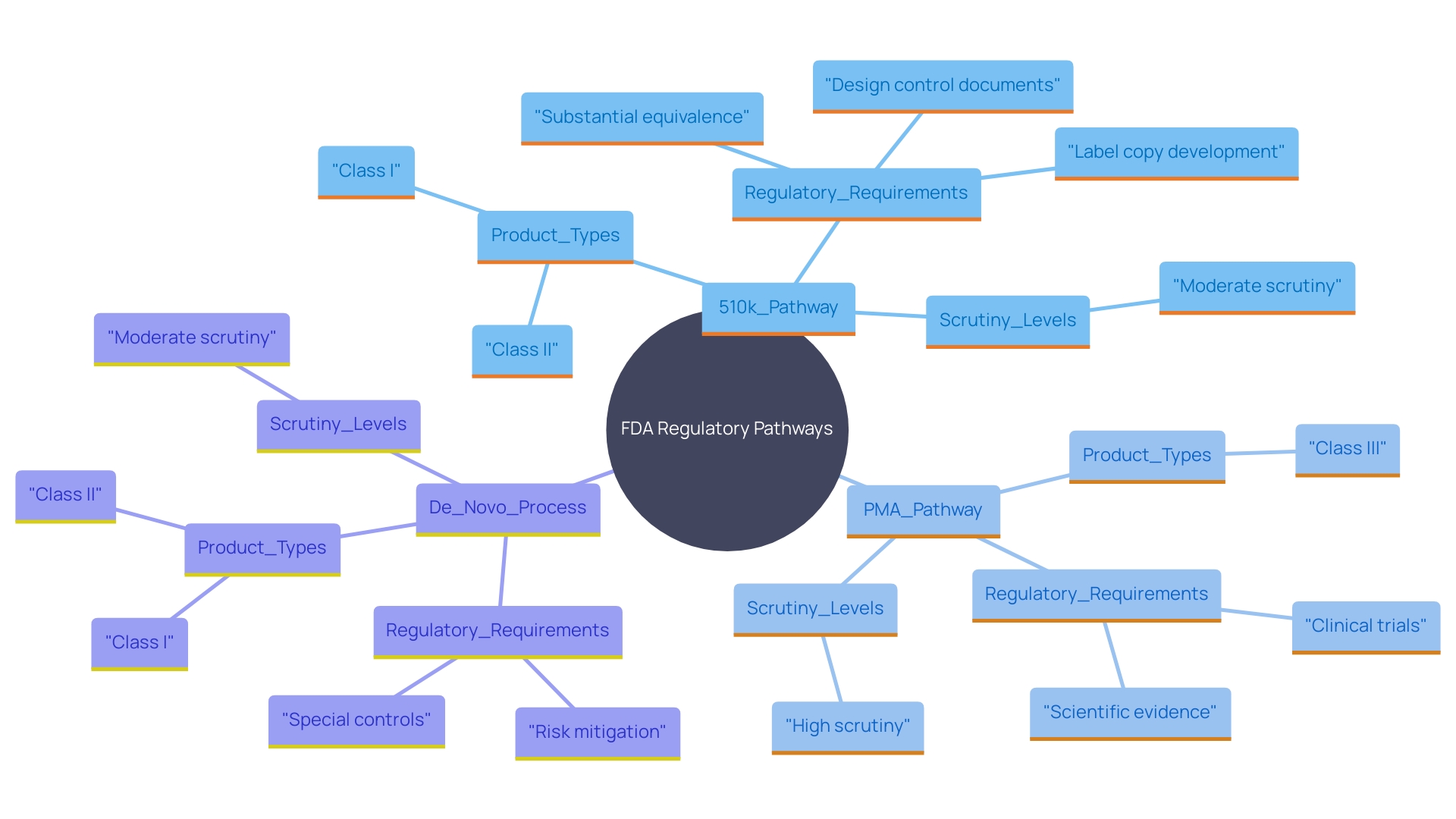
FDA Medical Device Classification
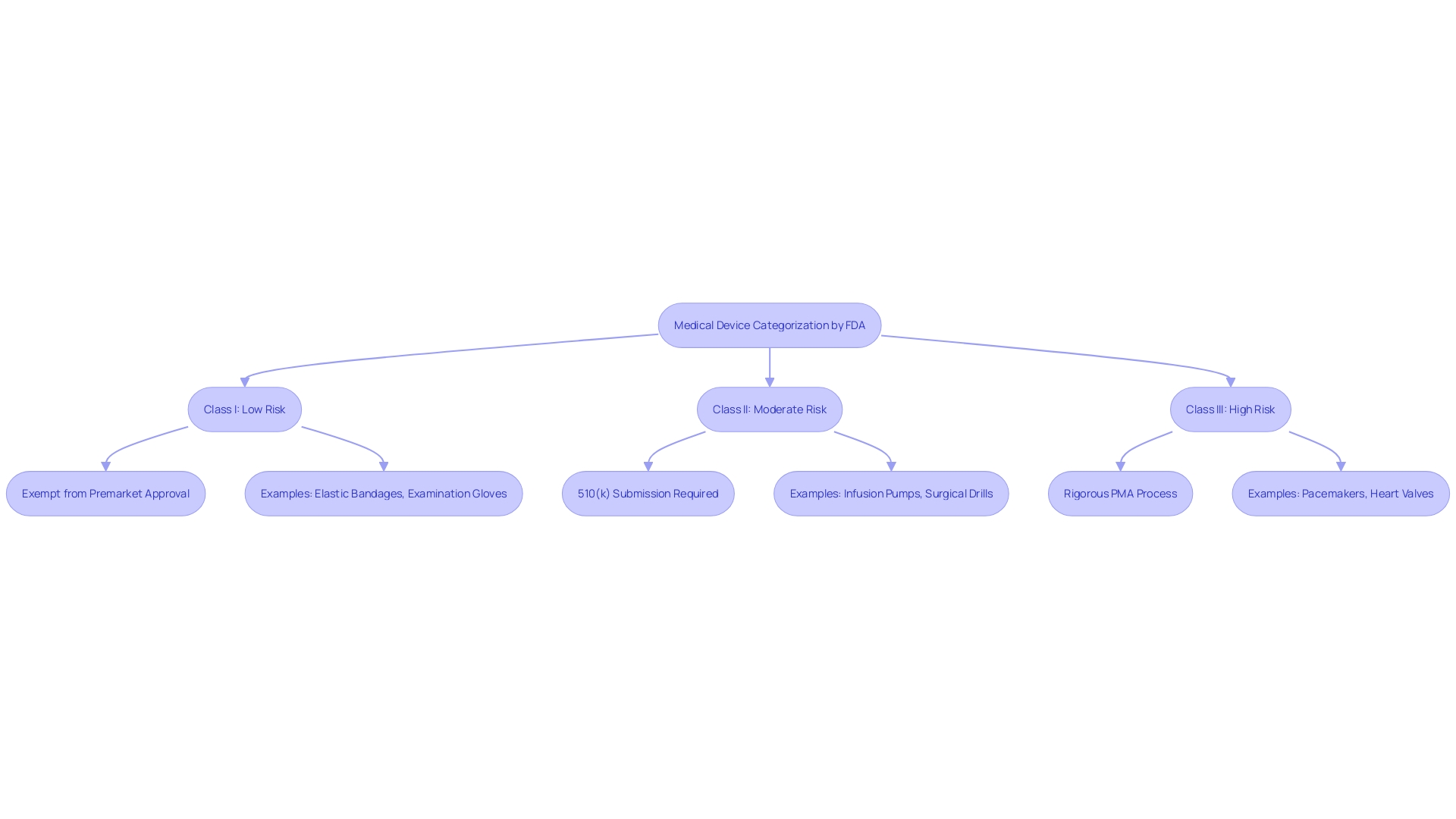
The FDA categorizes medical instruments into three groups based on risk: Class I, Class II, and Class III. Class I items are considered low-risk and are frequently exempt from Premarket Approval (PMA) requirements. Class II products, which carry a moderate risk, usually require a 510(k) submission to demonstrate substantial equivalence to existing products. This route is crucial for making sure that new equipment meets current standards for protection and effectiveness. Class III devices, including those that support or sustain human life, undergo the rigorous PMA procedure due to their higher risk profile. For instance, a catheter balloon repair kit falls under Class III and requires extensive clinical data to prove its safety and effectiveness. Understanding these classifications and the corresponding regulatory requirements is crucial for companies entering the market for the first time.
PMA Application Process
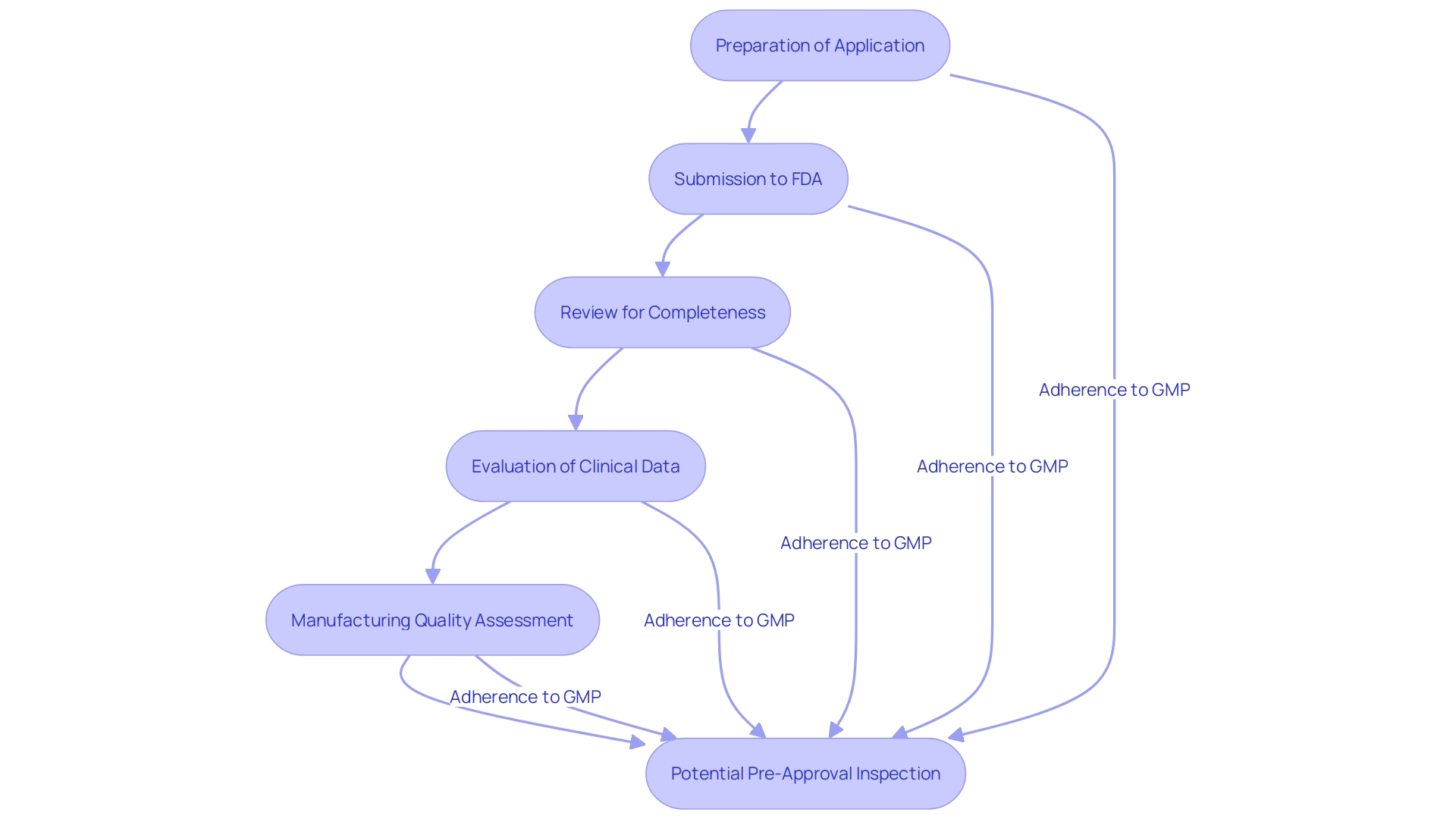
The PMA application procedure involves several critical steps, starting with the preparation of the application. This preparation includes comprehensive details about the equipment, manufacturing processes, and clinical studies. Once the PMA application is ready, applicants must submit it to the FDA, which first reviews it for completeness. Upon acceptance, the FDA conducts an exhaustive evaluation encompassing an assessment of clinical data, manufacturing quality, and labeling. Additionally, the FDA may perform a pre-approval inspection to ensure adherence to Good Manufacturing Practices (GMP).
Adherence to GMP is crucial as it embodies the essential elements of a quality system without prescribing specific methods. 'Manufacturers must ensure their quality systems meet FDA's QS regulation, which applies to finished product manufacturers intending to distribute commercially.'. Notably, the actual work can be delegated, but the responsibility for meeting these requirements and having objective evidence of compliance cannot be.
The FDA's emphasis on enhancing the quality, safety, and effectiveness of medical products aligns with its regulatory efforts to harmonize with other global authorities, promoting consistency and timely introduction of high-quality products. This harmonization is part of FDA's broader strategy to improve patient outcomes by ensuring medical products adhere to stringent regulatory standards.
Key Components of the FDA PMA Application
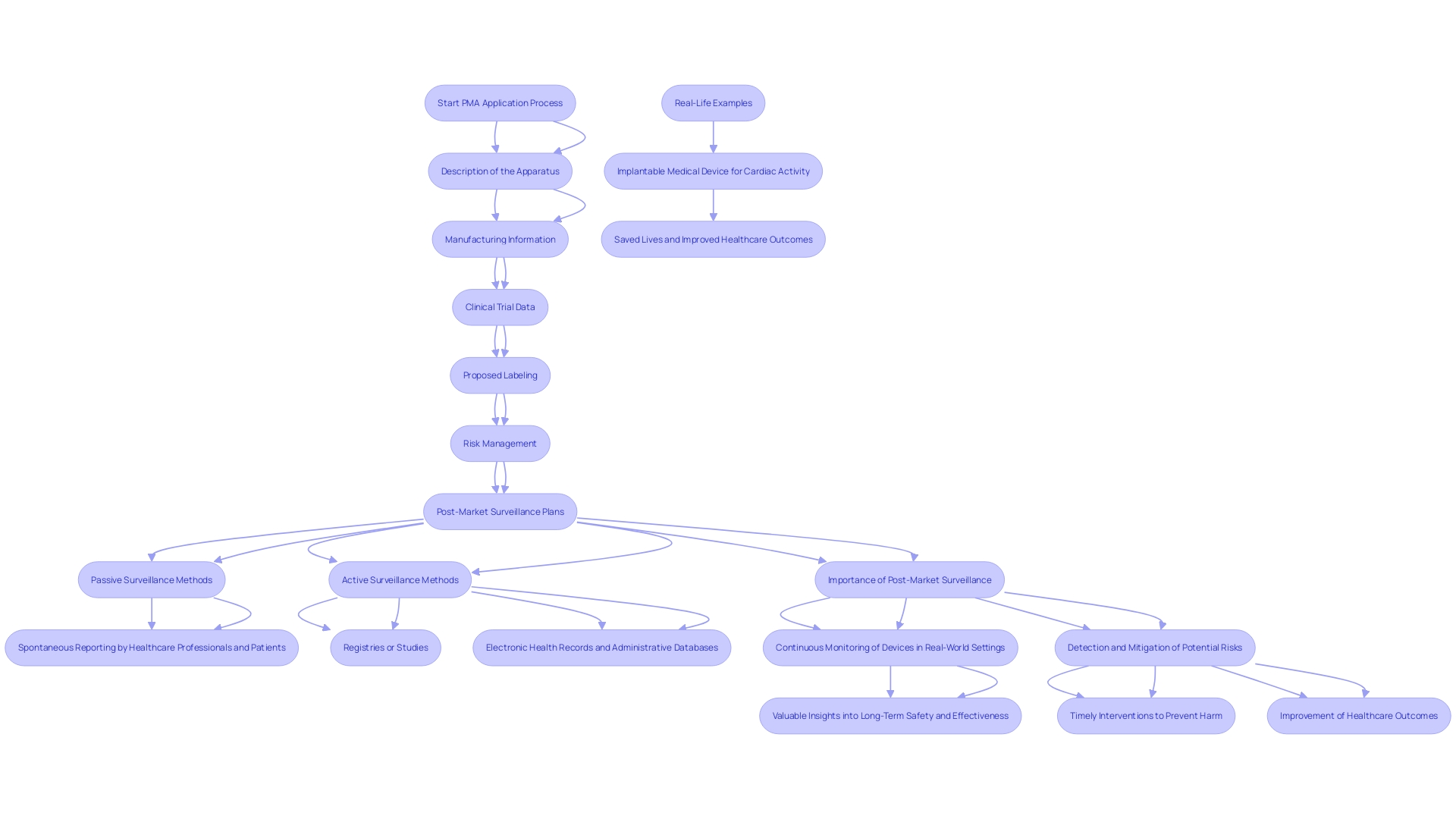
A complete PMA application includes several elements essential for the comprehensive assessment of a medical instrument. These components include a detailed description of the apparatus and its intended application, manufacturing information, and data from clinical trials demonstrating reliability and effectiveness. The application must also include proposed labeling and any relevant literature. Critical to the PMA application is the inclusion of information on the apparatus's risk management and post-market surveillance (PMS) plans.
Post-market surveillance is crucial for tracking the product's performance after it is available to consumers, recognizing possible risk concerns, and enhancing product effectiveness over time. Different techniques are used to gather essential information about the protection and functionality of medical instruments. These methods include passive surveillance systems, such as spontaneous reporting by healthcare professionals and patients, active surveillance through registries or studies, and the utilization of electronic health records and administrative databases. These methods allow for the ongoing observation of equipment in practical environments, offering useful information regarding their long-term reliability and effectiveness.
The significance of PMS cannot be overstated. It serves a crucial function in patient well-being by assisting in identifying and reducing possible hazards related to medical equipment. Timely interventions based on PMS findings can prevent harm and contribute to the long-term well-being of patients. Real-life examples abound, demonstrating how PMS has saved lives and improved healthcare outcomes. For instance, consider an implantable medical instrument designed to regulate cardiac activity. Ongoing observation and information gathering via PMS have resulted in prompt modifications and enhancements, guaranteeing the equipment's reliability and effectiveness for patients.
PMA Review Process
The PMA assessment is a thorough and demanding evaluation that can extend over several months to years, depending on the item's complexity and the quality of the submitted information. The FDA's scientific review meticulously assesses the safety and efficacy of the product, leveraging clinical evidence provided by the applicant. To strengthen the review system, advisory committees consisting of experts may be convened to provide specialized opinions.
This thorough analysis is essential, particularly given that over 1.7 million injuries and 83,000 fatalities during a recent decade in the U.S. may be possibly associated with defective medical equipment. Such statistics underscore the critical need for robust and thorough assessments. The review process concludes with a decisive approval or denial, accompanied by detailed feedback from the FDA. As part of its dedication to equipment security, the FDA is also constructing a monitoring system to oversee post-market security concerns, despite encountering obstacles in financing and patient recognition. This initiative aims to mitigate adverse outcomes and ensure the continuous safety of medical equipment.
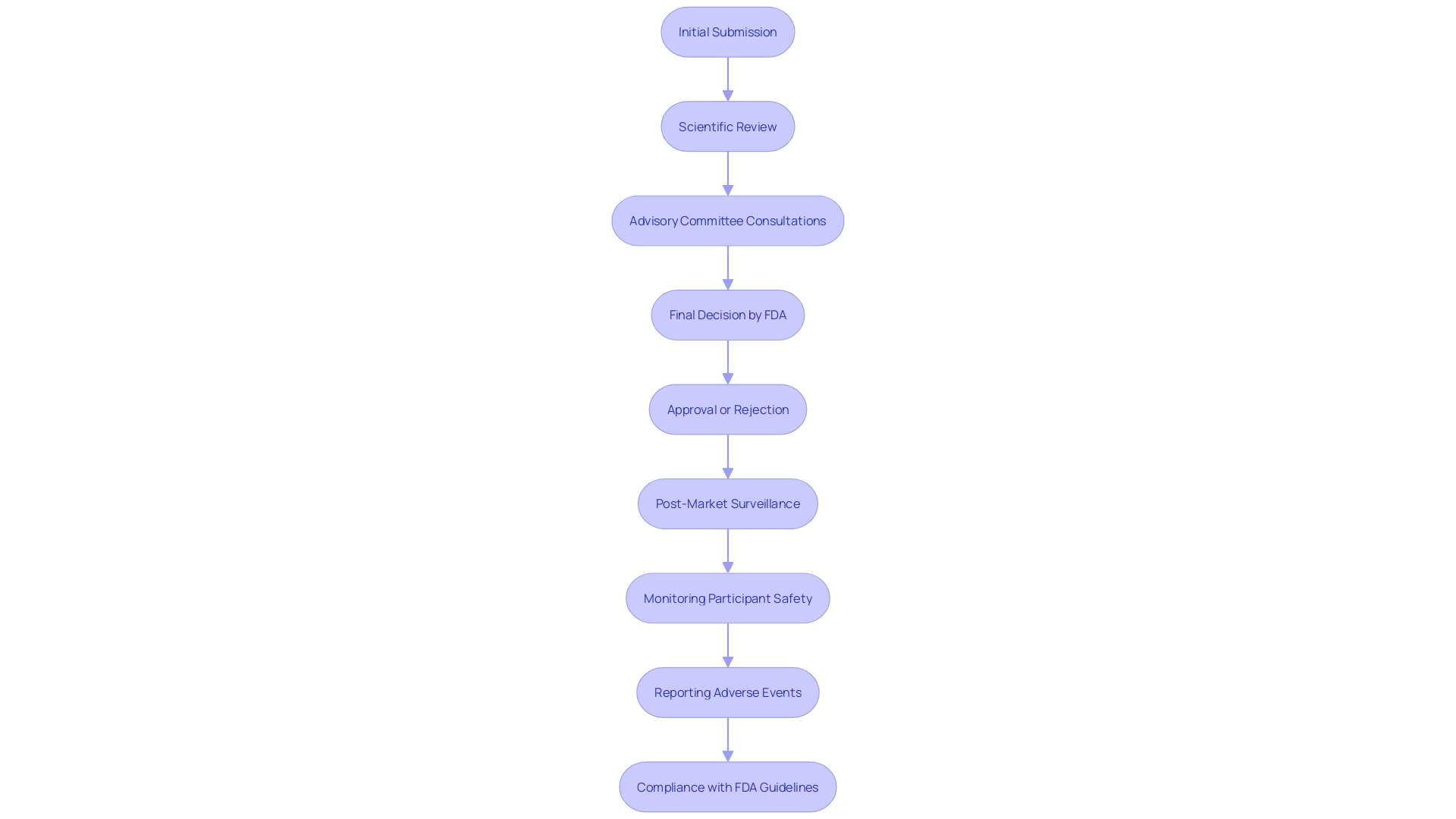
Differences Between 510(k) and PMA Pathways
The 510(k) pathway and the PMA pathway differ significantly in terms of regulatory requirements and scrutiny levels. The 510(k) procedure enables products to show substantial equivalence to currently authorized items, simplifying the approval procedure for Class I and Class II items. This pathway, frequently swifter and requiring fewer resources, offers a more rapid route to market, as shown by the fact that only 1% of medical instruments are approved through the PMA method.
In contrast, the PMA pathway requires extensive clinical data to establish the safety and effectiveness of Class III products. This thorough procedure reflects the greater risk linked to these instruments, necessitating comprehensive evaluations and extended approval periods. As noted by industry experts, meeting the standards in the 510(k) procedure doesn't always guarantee a safe and effective product, underscoring the importance of thorough scrutiny in the PMA pathway. The De Novo process also provides an alternative path for novel innovations without a substantial equivalent, further illustrating the complexities of aligning medical product development with FDA regulations.
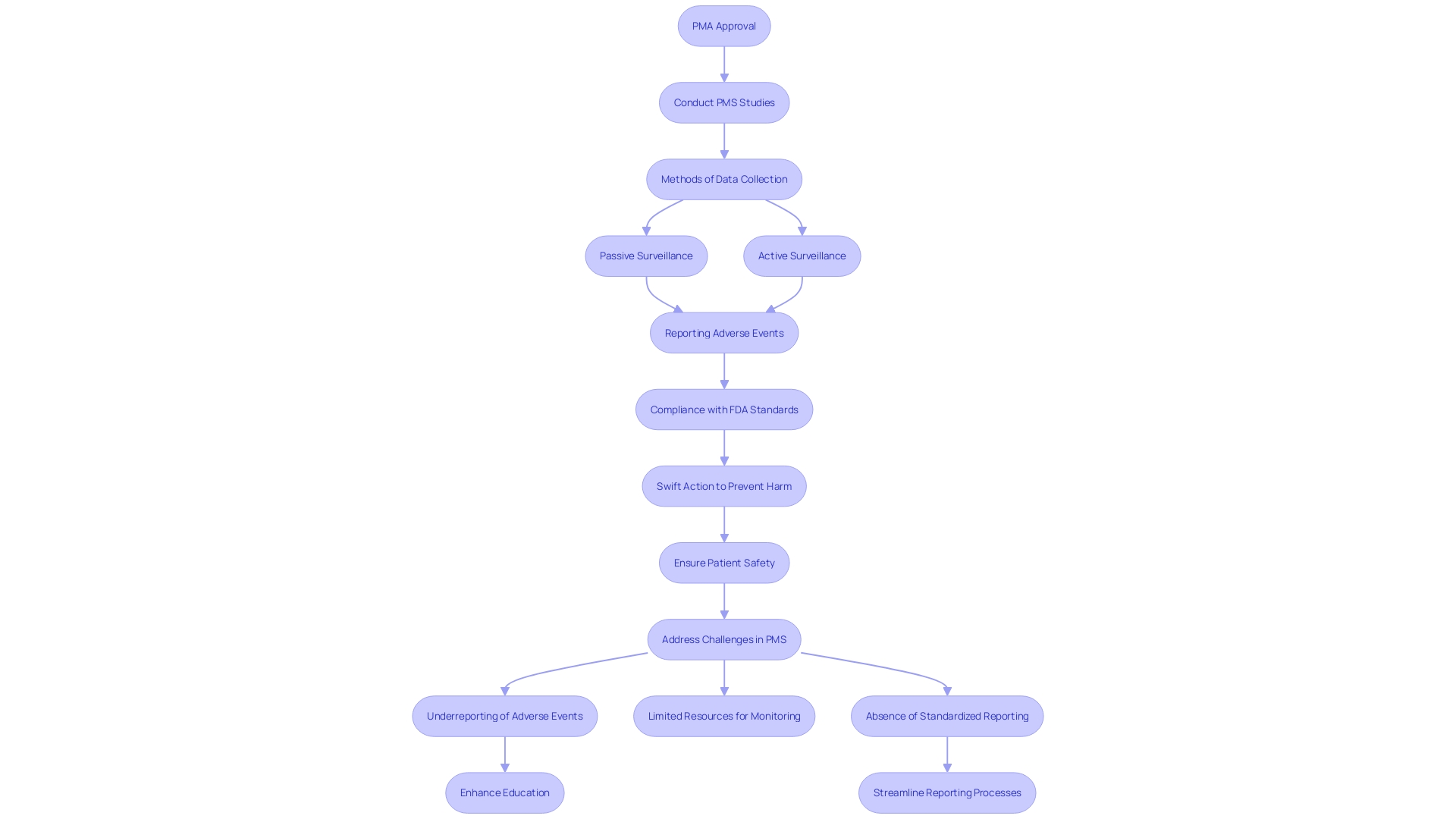
Post-Approval Requirements
Once a medical instrument receives PMA approval, manufacturers must adhere to stringent post-approval requirements to ensure ongoing safety and effectiveness. This involves carrying out post-market surveillance (PMS) studies, which are crucial for observing the long-term performance of the equipment. Various methods such as passive surveillance through spontaneous reporting by healthcare professionals and patients, active surveillance via registries or studies, and the use of electronic health records help collect critical data.
Swift action based on PMS data can prevent harm to countless patients and save lives. 'However, effective PMS encounters challenges, such as underreporting of adverse events, limited resources for monitoring, and the absence of standardized reporting methods.'. Tackling these challenges is essential for the continuous safety of medical instruments in practical environments. For instance, healthcare professionals may sometimes be unaware of reporting requirements or hesitant to report incidents due to potential repercussions.
Manufacturers are also required to report adverse events to the FDA and comply with ongoing quality control standards. Any alterations to the equipment or its manufacturing process may require additional submissions to the FDA for review and approval. This continuous oversight ensures that devices remain safe and effective throughout their lifecycle.
Conclusion
The Premarket Approval (PMA) process plays a vital role in the regulation of high-risk medical devices, ensuring that only products demonstrating rigorous safety and effectiveness standards reach the market. Governed by the FDA, this comprehensive evaluation process requires extensive clinical evidence, detailed documentation, and adherence to strict manufacturing practices. The necessity of such a stringent pathway becomes evident when considering the significant risks associated with Class III devices, which can directly impact patient health and safety.
In comparing the PMA pathway to the 510(k) process, it is clear that the former embodies a more exhaustive level of scrutiny, reflecting the critical need for thorough assessments of devices that pose greater risks. While the quicker 510(k) process facilitates market entry for lower-risk devices, the PMA ensures that high-risk devices are subjected to the highest standards of regulatory oversight. This distinction underscores the importance of maintaining robust regulatory frameworks to protect public health.
Post-market surveillance (PMS) further enhances the PMA process by enabling ongoing monitoring of device performance in real-world settings. Through continuous data collection and analysis, PMS identifies potential safety issues and facilitates timely interventions to improve patient outcomes. The challenges associated with effective PMS highlight the need for collaborative efforts among manufacturers, healthcare professionals, and regulatory bodies to enhance reporting processes and ensure the safety of medical devices throughout their lifecycle.
In conclusion, the PMA process is crucial for safeguarding public health by ensuring that high-risk medical devices meet stringent safety and efficacy standards. The interplay between rigorous premarket evaluations and ongoing post-market surveillance creates a comprehensive framework designed to protect patients and improve healthcare outcomes.




