Introduction
The Pre-Market Approval (PMA) process is a crucial step for medical device manufacturers seeking to market their high-risk class III devices in the United States. Regulated by the Food and Drug Administration (FDA), this meticulous review ensures that devices meet the highest standards of safety and efficacy. Navigating the PMA pathway involves understanding the FDA's classification system, which categorizes devices based on the level of risk they pose.
Class III devices, such as pacemakers, undergo the most rigorous review process due to their critical role in life-sustaining functions. Streamlining the approval pathway has become a recent focus, aiming to expedite the launch of innovative devices that address unmet medical needs. Success in this regulatory journey requires project managers to blend project management skills with a deep understanding of regulatory nuances, always prioritizing patient safety and product quality.
What is a PMA Submission?
The Pre-Market Approval (PMA) process is crucial for medical equipment manufacturers aiming to market their high-risk class III products in the United States. The Food and Drug Administration (FDA), a key player in safeguarding public health, mandates this stringent and meticulous review to ensure products meet the highest standard of safety and efficacy.
Understanding the FDA's classification system is necessary to navigate the PMA pathway, as it categorizes instruments into three classes according to the level of risk they pose to patients. Class III instruments, because of their important role in vital or life-supporting functions, are subjected to the PMA procedure, the most stringent of the FDA's instrument regulatory frameworks. To support the safety and effectiveness of the equipment, extensive data, including results from clinical trials, is required.
Actually, class III objects, such as crucial implants like pacemakers, make up about 10% of objects overseen by the FDA and encounter the longest approval timelines. The intricacy of these tools and the circumstances they handle are elements that contribute to the duration of the PMA procedure, reflecting the FDA's dedication to patient safety.
Simplifying the approval process has been a recent priority, with the goal of expediting the introduction of innovative products, especially those meeting unaddressed healthcare requirements. These efforts, accelerated by the demand for rapid solutions during the COVID-19 pandemic, highlight the importance of collaboration between regulatory bodies and industry entities.
According to industry experts, success in this regulatory journey is not just about compliance but also about a profound commitment to patient outcomes. As such, project managers in this field must deftly blend project management acumen with a deep understanding of regulatory nuances, always prioritizing patient safety and product quality.
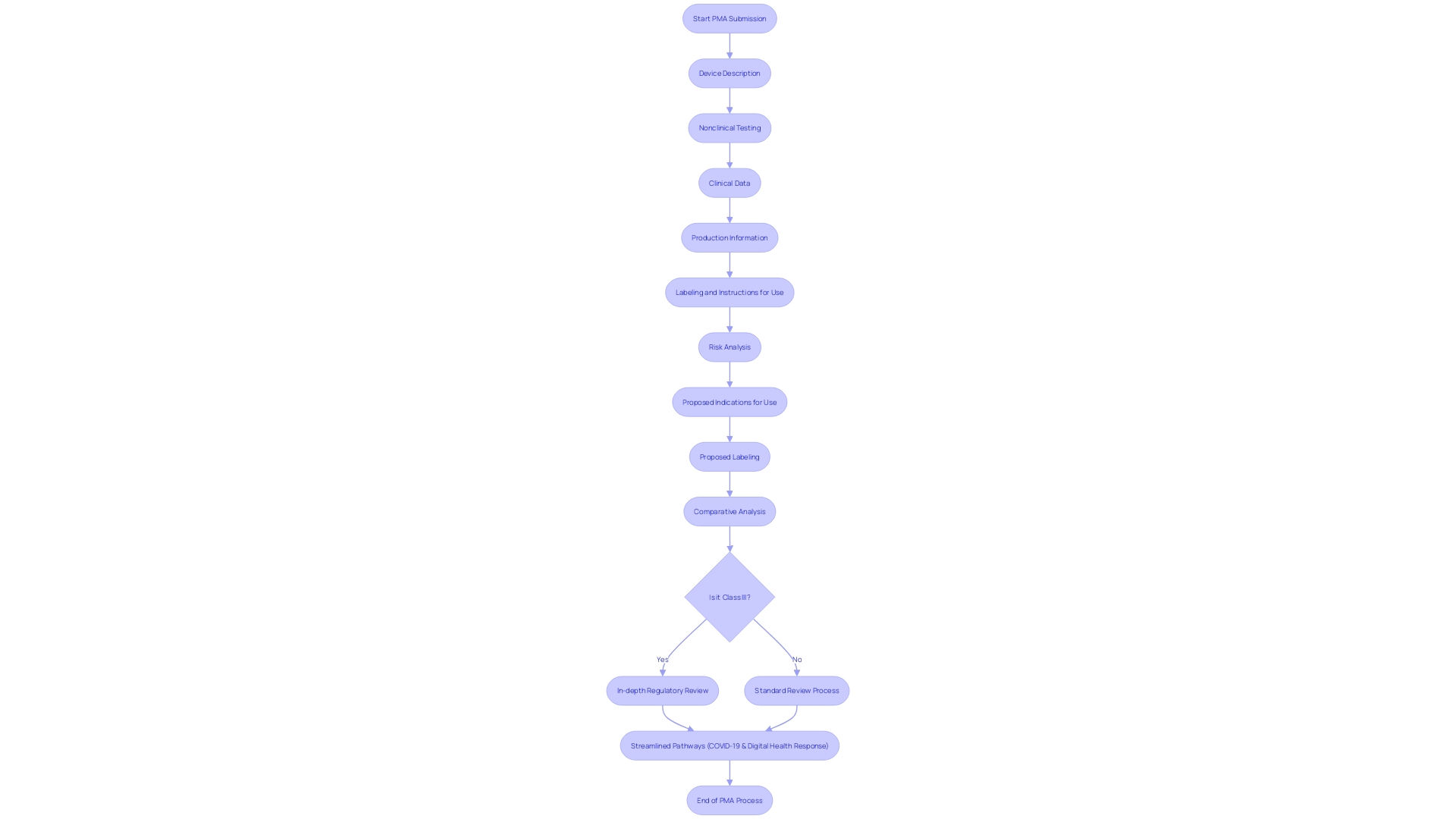
Key Components of a PMA Submission Package
The Premarket Approval (PMA) procedure for healthcare equipment is a detailed pathway that necessitates a thorough submission package to guarantee the equipment is safe and efficient for its intended utilization. The package must include a robust Device Description, detailing the design, specifications, and intended use, which is critical for both regulatory bodies and the medical community. Furthermore, Nonclinical Testing data, which includes biocompatibility, animal studies, and performance tests, are essential to prove the safety of the product prior to its use on any patient.
Clinical Data from well-designed trials are crucial, demonstrating the efficacy and safety of the equipment in real-world settings. Production Information is also necessary, describing the manufacturing processes, quality control procedures, and assurance systems to uphold integrity. Labeling and Instructions for Use must be clear, outlining the product's proper application, contraindications, and precautions. Additionally, a comprehensive Risk Analysis is required to recognize and minimize potential hazards linked to the use of the equipment.
The submission also includes Proposed Indications for Use, specifying the health conditions the equipment aims to address, and Proposed Labeling, which presents intended use and safety information. It is imperative to fully comprehend the context of the electronic gadget; this encompasses its users, the competitive landscape, and conducting a comparative analysis with similar devices. As the FDA categorizes medical equipment from low to high risk, class three instruments, such as pacemakers, require a more rigorous review, reflecting in longer approval times. These instruments, crucial for life support, constitute approximately 10% of FDA-regulated apparatus.
Recent years have seen efforts to streamline regulatory processes and enhance collaboration between stakeholders, catalyzed by the urgency of the COVID-19 pandemic. This is especially important in the progress of digital health and personalized medicine, with the goal of decreasing approval times for technologies meeting crucial healthcare requirements. In this ever-changing and cutthroat market, companies that effectively combine software and hardware provide a clear edge, contributing to the industry's top systems and solutions.
Types of PMA Submissions
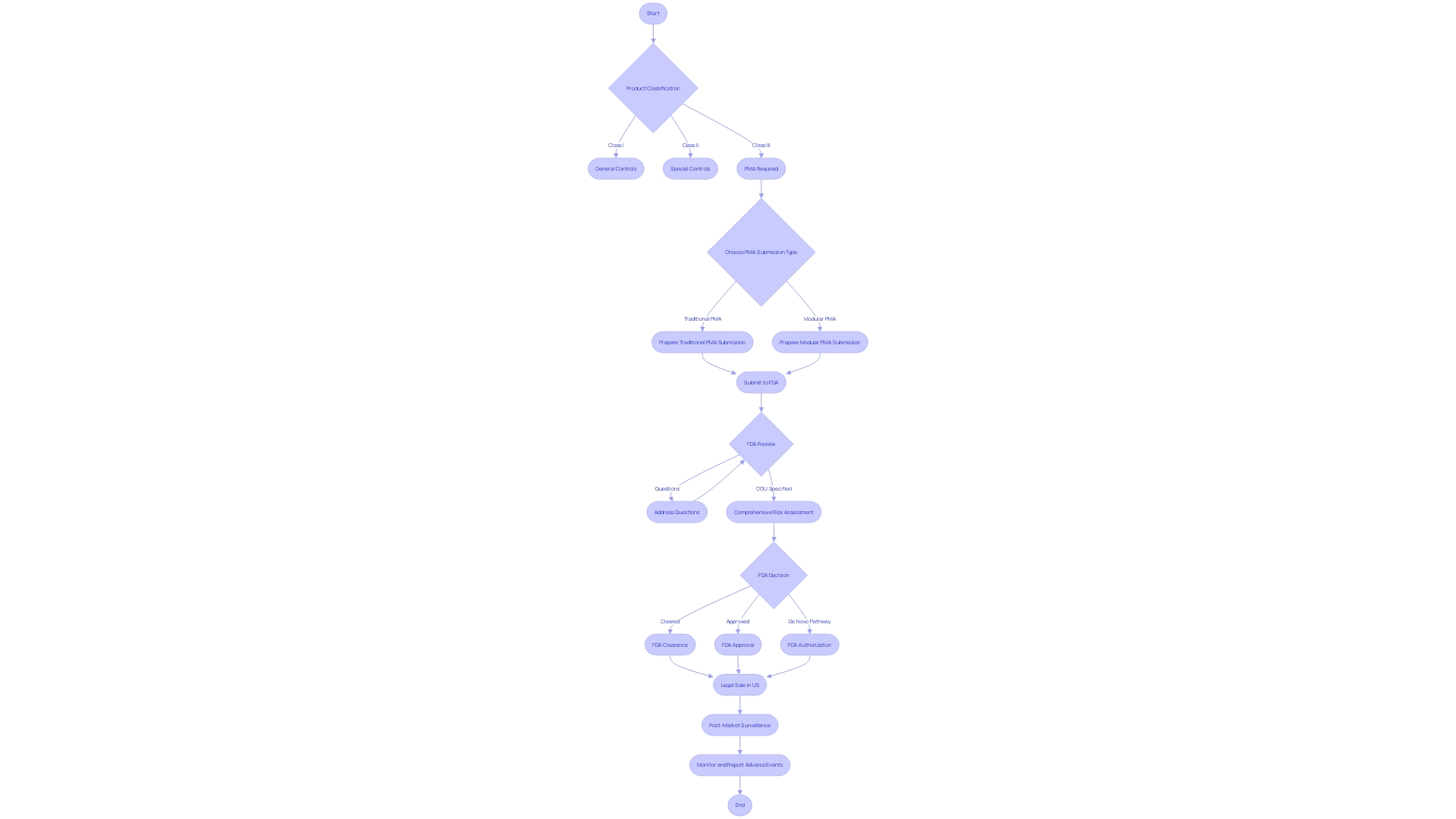
The Pre-Market Approval (PMA) procedure for healthcare equipment in the United States, supervised by the Food and Drug Administration (FDA), is a comprehensive regulatory mechanism that guarantees the safety and efficacy of high-risk class three instruments. These instruments, which make up approximately 10% of all FDA-regulated medical equipment, often have a crucial function in maintaining or supporting life, such as implantable pacemakers. Given their significance, they are subject to a stringent approval process to mitigate potential risks associated with their use.
There are principally two types of PMA submissions that manufacturers can consider, each serving a different strategic purpose. The Traditional PMA is a comprehensive submission that's indispensable for novel instruments or those that have been significantly modified in ways that might impact their safety or effectiveness. It requires substantial clinical data to confirm the safety and effectiveness of the equipment. On the other hand, the Modular PMA allows for a segmented submission procedure. Here, manufacturers can submit portions of the PMA application as individual modules. This is especially beneficial for apparatus made up of multiple components or intricate technologies. Each module is reviewed independently by the FDA, and the PMA is deemed complete when all modules meet approval criteria.
In addition to the fundamental types of submission, it is essential for manufacturers to grasp the classification of the product, as this determines the suitable regulatory pathway—whether it's a 510(k) clearance for lower-risk items, PMA for those with higher risks, or the De Novo approach for certain innovative items without existing references. It's important to mention that before a product can be legally sold in the US, it must receive FDA Clearance, Approval, or Authorization through the De Novo pathway.
The procedure of PMA submission is supported by a structure created to specify the intended use of the apparatus and conduct a comprehensive risk assessment. This involves delineating the questions of interest the instrument aims to address, and specifying the Context of Use (COU), which encompasses the model's role and the manner in which its outputs will be utilized to respond to those questions.
In recent times, there have been concerted efforts to streamline regulatory pathways and foster cooperation between regulatory bodies and industry participants. This project, expedited by the pressing demands of the COVID-19 pandemic, aims to speed up the authorization procedure, especially for technologies that meet previously unfulfilled healthcare needs in emerging fields such as digital well-being and individualized treatment. The duration required for a healthcare equipment to obtain regulatory authorization depends on different aspects, such as the intricacy of the equipment, the health condition it treats, and the effectiveness of the regulatory processes in various regions.
The PMA Review Process
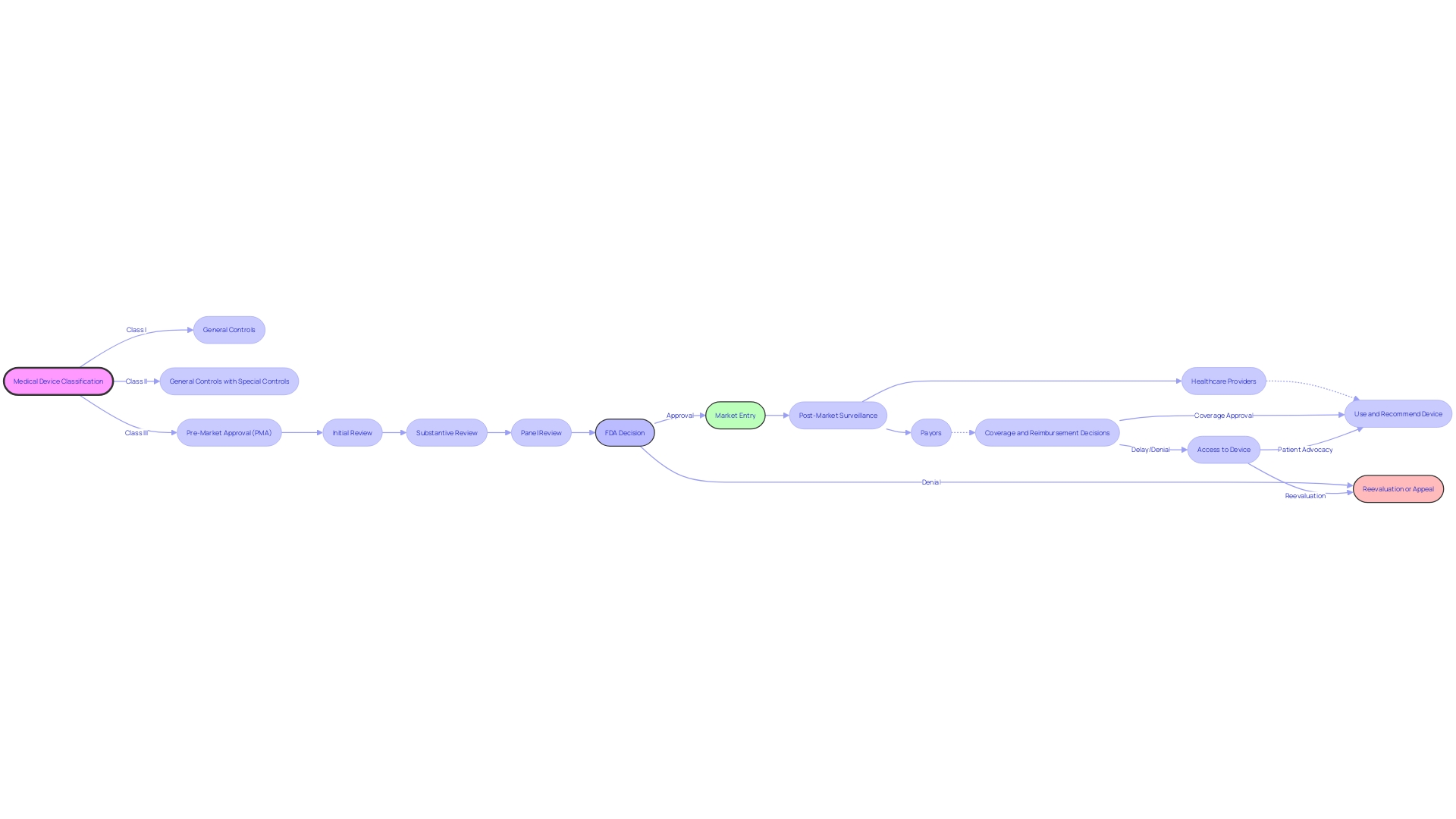
Having a thorough comprehension of the stages involved is crucial when navigating the Pre-Market Approval (PMA) pathway for medical devices with the FDA. The process begins with an Initial Review, where both administrative and limited scientific assessments are conducted to ensure all necessary data and documentation are complete. This is followed by a Substantive Review, a comprehensive examination of the scientific data, regulatory compliance, and quality system protocols. For equipment that may have a significant impact on public health, a Panel Review is convened, in which an advisory committee examines the equipment and provides recommendations. The last step is the Decision Announcement, where the FDA finishes its deliberations and issues a formal notification regarding the approval status of the product.
It is crucial to recognize that the PMA process is just one part of a broader ecosystem involving various stakeholders. For example, following FDA approval, healthcare providers and payors, such as CMS and private health plans, play a crucial role in determining whether an item will be covered and reimbursed. This is a critical consideration since the data submitted for FDA approval may differ from what these organizations require for coverage decisions, potentially leading to delays or denials even after FDA clearance.
The FDA categorizes medical equipment into three classes based on risk, with Class III apparatus, like pacemakers, undergoing the most rigorous PMA due to their significant role in sustaining life. These high-risk products represent a small portion of FDA-regulated items but necessitate a thorough examination due to their intricacy and potential influence on patient health. Recent regulatory updates from agencies such as the UK's MHRA reflect a global trend towards patient safety and international harmonization, highlighting the significance of navigating the PMA within a dynamic regulatory landscape.
Pre-Submission Preparation and Communication with FDA
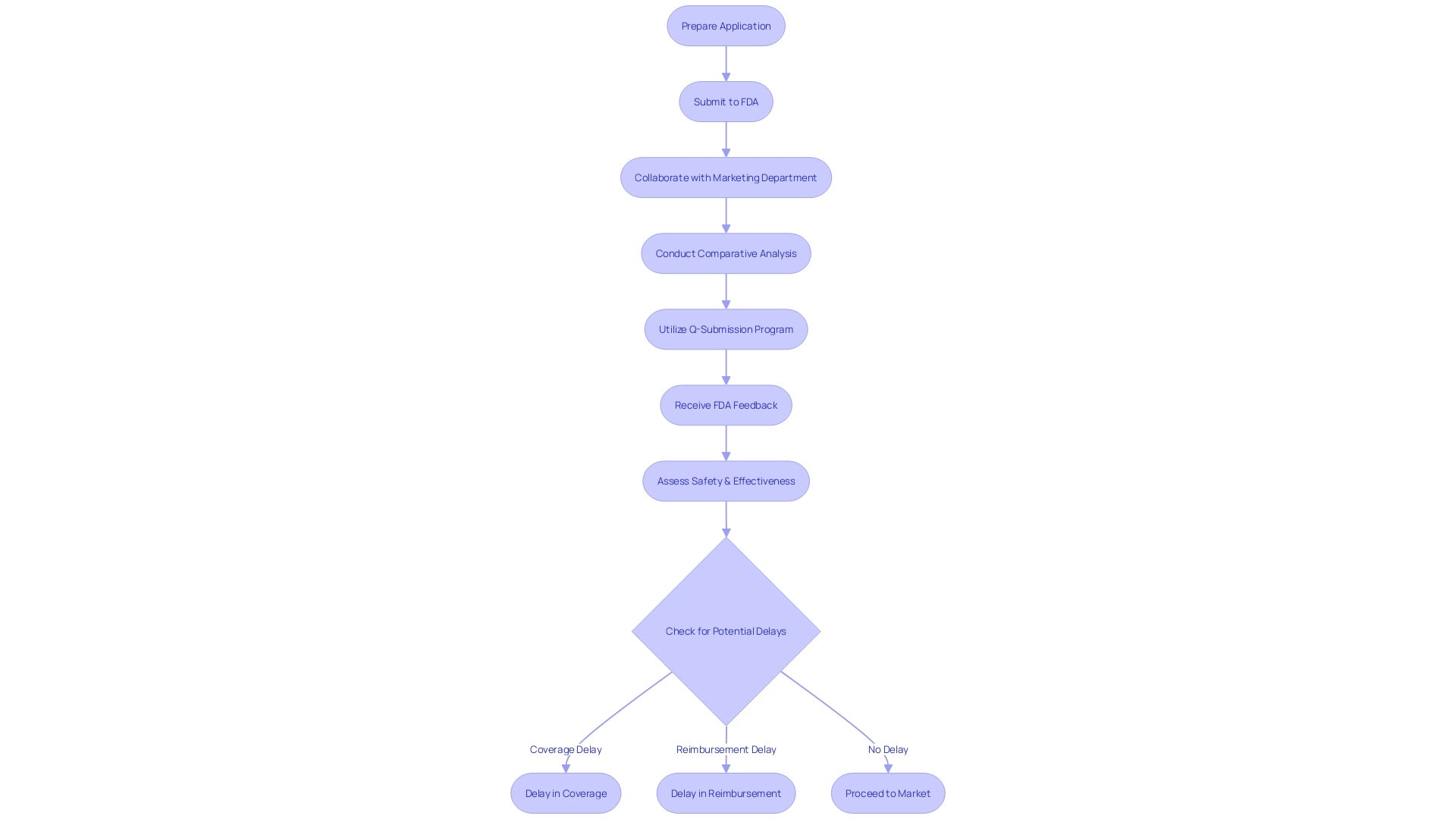
To navigate the intricacies of the PMA (Pre-Market Approval) process for healthcare products, manufacturers must dedicate significant effort to thorough preparation before submitting an application to the FDA. This meticulous approach involves not only a profound understanding of the medical instrument itself but also an in-depth analysis of its intended users, such as clinicians and patients. Warnings, cautions, and detailed instructions for use must be thoroughly delineated. Collaborating closely with the marketing department, manufacturers should scrutinize the competitive landscape, examining research literature, clinical studies, and competitor marketing materials. Recognizing analogous gadgets (predicate instruments) is a crucial phase, encompassing the development of a comparative chart to emphasize the technological attributes and intended purpose that are similar to the fresh apparatus.
The Q-Submission Program, as detailed in the FDA's draft guidance, offers a pathway for manufacturers to seek valuable pre-submission feedback. By utilizing this program, manufacturers can discuss their product, its use, and the planned testing protocols with the FDA. This proactive conversation aims to clarify uncertainties and align the submission with the FDA's regulatory expectations, potentially mitigating delays in the approval procedure.
The FDA's responsibility goes beyond the assessment of the safety and effectiveness of devices; it is a gatekeeper safeguarding public health. Although FDA approval is crucial, it does not guarantee immediate coverage or reimbursement from payors like CMS or private health plans. Manufacturers must recognize that the data required by the FDA might differ from the data payors need to make coverage decisions, which can lead to delays or denials even after FDA clearance.
To ensure that the Agency considers comments on the draft guidance, stakeholders are encouraged to submit their feedback before the stipulated deadline. This ongoing conversation between the FDA and industry experts demonstrates the ever-changing nature of regulatory requirements and highlights the significance of keeping up with FDA guidelines to facilitate the approval procedure and prompt patient access to innovations in healthcare.
Common Challenges and Pitfalls in PMA Submissions
Navigating the Pre-Market Approval (PMA) pathway for healthcare instruments requires accuracy, comprehensiveness, and a deep comprehension of the regulatory environment. A Project Manager must integrate traditional management skills with the specific requirements of the medical instrument industry, where patient safety and regulatory compliance are paramount. The PMA submission is indeed intricate, with common challenges such as providing sufficient clinical data to demonstrate the device's safety and effectiveness. This necessitates conducting clinical trials that are both well-controlled and thoughtfully designed to generate compelling evidence.
Moreover, the documentation accompanying a PMA submission must be meticulous. Any discrepancies, errors, or omissions can cause a significant delay in the review. Ensuring complete and precise documentation, in line with FDA guidelines, is non-negotiable. Additionally, maintaining open channels of communication with the FDA is essential. It allows for prompt resolution of any queries or issues that may arise, facilitating a smoother submission process.
A comprehensive risk analysis is another critical component of the PMA submission. It serves to identify and address potential risks associated with the equipment, underscoring the commitment to patient safety. An incomplete risk analysis can lead to concerns regarding the safety and effectiveness of the equipment. Considering that medical instruments span from basic tools such as tongue depressors to intricate life-supporting apparatus, the degree of examination in their authorization varies accordingly. Class III products, such as life-sustaining implants, undergo a more rigorous review process due to their high-risk nature.
Understanding the purpose of the equipment and its competitive landscape is also crucial. This includes not just getting to know the users and their needs but also conducting comparative analysis with similar equipment. This level of knowledge is instrumental in navigating the PMA process effectively. The FDA's categorization of medical apparatus into three classes grounded on risk elements directs the path for registration, be it a 510(k) clearance for lower-risk apparatus or PMA for those with higher risks, underscoring the significance of accurately determining the apparatus's categorization from the beginning.
Post-Approval Requirements and Compliance
Upon receiving Pre-Market Approval (PMA) from the FDA, manufacturers are mandated to adhere to several post-approval requirements to ensure ongoing compliance. These include the conduct of post-approval studies, which may be required by the FDA to gather additional data regarding the equipment's long-term safety and efficacy.
Furthermore, adherence to the FDA's Quality System Regulations is crucial to ensure the consistent and reliable production and management of the product. Furthermore, manufacturers have a duty to inform the FDA of any negative incidents or malfunctions of the product. This reporting must be both prompt and precise to uphold the safety profile of the equipment.
Labeling must also be kept current, incorporating any new information or changes in the equipment's indications, contraindications, warnings, or precautions. The importance of accurate and up-to-date labeling is emphasized by the FDA's recent publication of standards for presenting major side effects and contraindications in direct-to-consumer drug advertisements.
However, it is critical to note that even after FDA approval, there can be a gap between the data submitted to the FDA and the data required by payors for coverage decisions. This misalignment may result in delays or denials in coverage, affecting patient access to new healthcare equipment. As seen in the 2018 documentary 'The Bleeding Edge', the FDA's clearance process, including the 510(k) pathway, does not always require clinical trials, potentially leading to patient safety issues.
The FDA's categorization system for healthcare instruments, which encompasses three levels of hazard, determines the suitable regulatory route for approval or clearance. Decisions by payors and healthcare providers on whether to cover, pay for, or utilize an apparatus can be influenced by these classifications as well as by the specific coding and labeling associated with each apparatus.
The seriousness of these post-approval activities is emphasized by statistics that show over a 10-year period, more than 1.7 million injuries and 83,000 deaths in the United States were potentially linked to devices. This highlights the need for rigorous post-market surveillance and the FDA's commitment to patient safety through clear and neutral communication of medical product information.
Conclusion
In conclusion, the Pre-Market Approval (PMA) process is a crucial step for medical device manufacturers seeking to market their high-risk class III devices in the United States. Success in this regulatory journey requires project managers to blend project management skills with a deep understanding of regulatory nuances, always prioritizing patient safety and product quality.
Navigating the PMA review process involves several stages, such as an initial review, substantive review, panel review, and decision announcement. Manufacturers must also be mindful of the different types of PMA submissions available, including traditional PMA and modular PMA, each serving a different strategic purpose.
Preparation and communication with the FDA are key to a successful PMA submission. The Q-Submission Program offers a valuable opportunity for manufacturers to seek pre-submission feedback from the FDA, aligning their submission with regulatory expectations.
Common challenges and pitfalls in PMA submissions include providing sufficient clinical data, ensuring meticulous documentation, maintaining open communication with the FDA, conducting a comprehensive risk analysis, and understanding the device's purpose and competitive landscape.
Overall, the PMA process requires precision, thoroughness, and a profound understanding of the regulatory landscape. By navigating this process effectively and prioritizing patient safety, manufacturers can successfully bring their innovative medical devices to market, addressing unmet medical needs and improving patient outcomes.
Contact bioaccess™ today to ensure a successful PMA submission for your medical device.




