Introduction
Informed consent stands as a fundamental pillar in the ethical and legal framework governing research involving human subjects. It is designed to uphold participants' autonomy by ensuring they are fully informed about the study they are engaging in, including potential risks and benefits. This process of transparency not only fosters trust between researchers and participants but also enhances the overall quality and integrity of the research.
Historical unethical research practices, such as those during World War II and the Tuskegee Syphilis Study, led to the establishment of regulatory safeguards, including Institutional Review Boards (IRBs), which protect the rights and dignity of participants. These bodies serve as an objective third party to ensure ethical research practices are adhered to in compliance with federal regulations.
The primary goal of informed consent documents is to aid potential subjects in deciding whether to participate in a research study by presenting necessary information clearly and comprehensibly. However, the increasing complexity of these documents has posed challenges to clinical trial enrollment, particularly among underserved minority populations. Despite these hurdles, informed consent remains crucial for maintaining public trust and ensuring ethical conduct in research.
The Importance of Informed Consent in Research
Informed consent serves as a cornerstone in both ethical and legal frameworks for studies involving human subjects. It upholds individual autonomy by ensuring they are fully informed about the study they are involved in, including the potential risks and benefits. This openness not only builds confidence between scholars and subjects but also improves the standard and honesty of the study.
Historical unethical practices, such as those seen during World War II and the Tuskegee Syphilis Study, led to the establishment of regulatory and ethical safeguards. These safeguards, including Institutional Review Boards (IRBs), are in place to protect the rights and dignity of participants. IRBs act as an impartial third party to guarantee that studies are carried out ethically and in compliance with federal regulations.
The main purpose of explanatory documents is to help prospective participants determine if they wish to engage in a study by clearly providing essential information in an understandable way. However, these documents have become increasingly complex, often presenting obstacles to clinical trial enrollment, particularly among underserved minority populations. Regardless of these obstacles, knowledgeable agreement is crucial for sustaining public confidence and guaranteeing ethical behavior in studies.
Basic Elements of Informed Consent
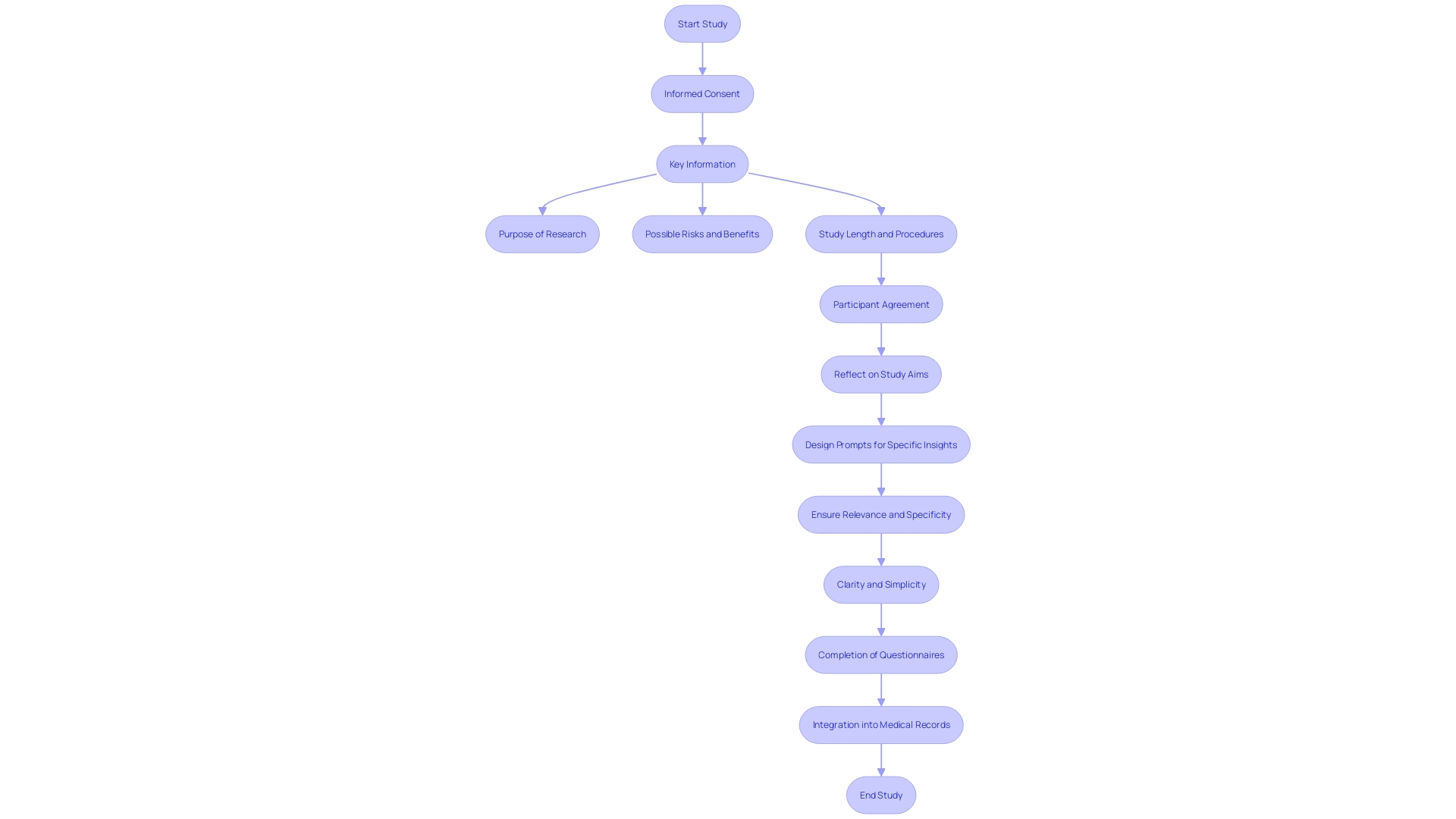
Informed agreement includes several essential elements to ensure participants fully understand the study process. These elements include a clear statement indicating that the study involves investigation, an explanation of its purposes, the expected duration of participation, and a description of the procedures involved.
The primary goal of informed consent documents is to assist prospective subjects in making an informed decision about participation by presenting necessary information clearly and facilitating comprehension. Yet, as time has passed, these documents have grown more intricate, extensive, and challenging for all involved parties, including IRBs, physicians, clinical trial sponsors, research subjects, and regulators. The list of mandatory items can run to over 270 words, and documents have expanded from three to four pages to over twenty pages in many cases. They are often written at a reading level too high for many participants and have become more legalistic to comply with legislation, posing an obstacle to clinical trial enrollment, especially among underserved minority populations.
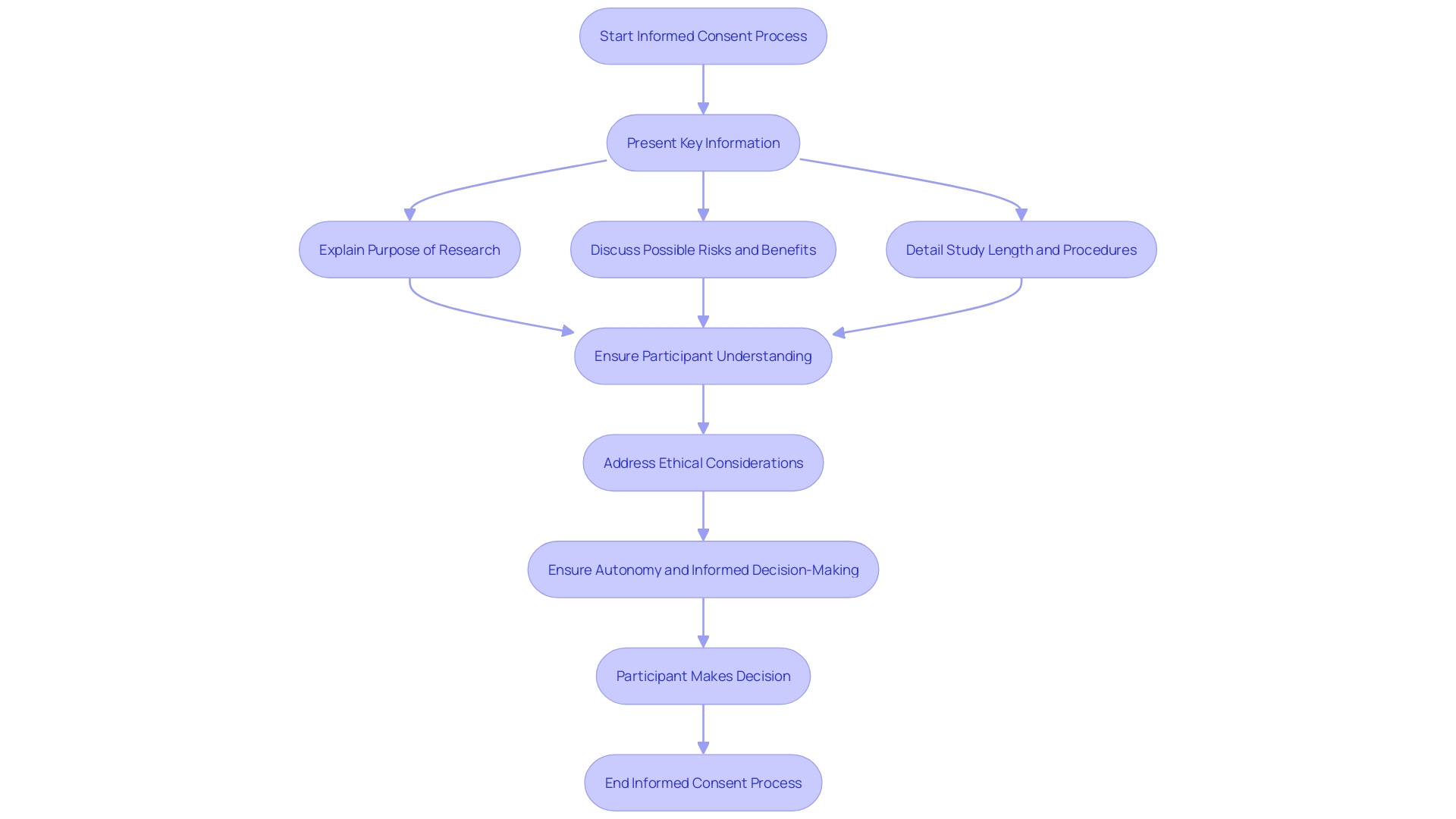
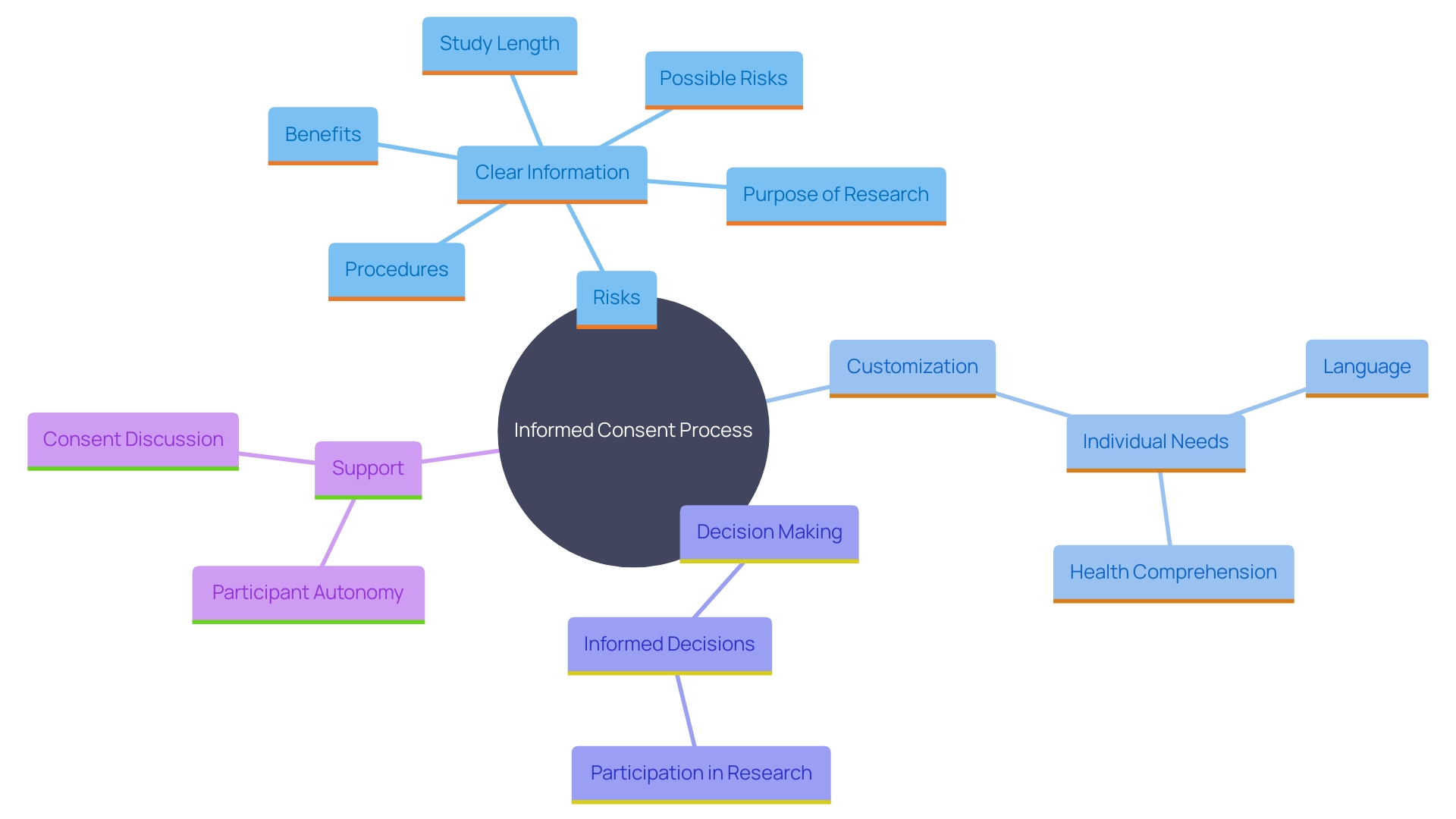
Essential information should be presented in a clear and concise manner at the beginning of the informed agreement document. This includes the purpose of the research, possible risks and benefits, and the study's length and procedures. The inclusion of key information can be a valuable resource for current study individuals and support the agreement discussion between the investigator and potential subjects.
The National Organization for Rare Disorders (NORD) has praised draft guidance that permits creative awareness methods, such as videos, to make the process more accessible. It is essential to customize consent to individuals' unique needs, considering factors like language barriers, hearing or vision impairments, developmental delays, and health literacy competencies. By utilizing simple phrases, plain language principles, and organizational tools like the bubble format, researchers can significantly enhance participants' comprehension of the information.
Statement that the Study Involves Research
Participants must be clearly informed that their involvement is part of a study. This distinction is crucial for ethical compliance, as it differentiates investigative activities from standard medical treatments. According to the updated International Council for Harmonization (ICH) E6 GCP guidelines, it is vital that individuals understand the purpose of the research, the potential risks and benefits, and the procedures involved. This clarity ensures that participants can make a knowledgeable decision about their participation.
The significance of knowledgeable agreement is profoundly embedded in ethical structures like the Declaration of Helsinki and the Belmont Report, which highlight regard for people and their right to make choices for themselves. These documents have been essential in forming human study ethics, highlighting the importance of understanding agreement at both the beginning and during the study process.
Recent advancements highlight the need for presenting key information in a concise and understandable manner. The draft guidance suggests incorporating subjects like the aim of the research, anticipated length, and possible risks and advantages right at the start of the approval document. This method promotes a clearer comprehension for those involved, assisting them in considering their choice to engage in the study.
Furthermore, creative methods for informed consent, including the use of videos and other easily accessible formats, are promoted to address the varied requirements of individuals. This flexibility is crucial for making sure that all potential contributors, regardless of language obstacles or sensory challenges, can fully understand the study they are being invited to join.
By following these principles and guidelines, scholars can maintain ethical standards, safeguard individuals' rights, and improve the validity and reliability of their findings.
Explanation of the Purposes of the Research
A comprehensive explanation of the study objectives is crucial for ensuring participants understand the significance of the investigation and how their involvement may contribute to broader scientific advancements. Effective communication of these objectives fosters an environment of trust and transparency, which is foundational in clinical research. Rooted in frameworks like the Declaration of Helsinki and the Belmont Report, informed consent emphasizes respect for individuals and their right to self-determination. This method corresponds with the moral standards that direct clinical trials, where individuals are not simply subjects but active contributors whose involvement can lead to significant results.
Patient and public involvement (PPI) in studies underscores the importance of collaborative partnerships between researchers and participants. These partnerships are vital in designing, conducting, and disseminating studies that address real-world issues. For instance, the Multi-Regional Clinical Trials Center at Brigham and Women’s Hospital and Harvard highlights the evolving nature of these partnerships, ensuring that investigations are both relevant and ethically sound.
Statistics indicate that clinical trials greatly affect patient care and results, making it essential that those involved are fully informed about the study's objectives. This openness not only improves the ethical behavior of studies but also guarantees that contributors understand how their involvement can result in progress in medical understanding and patient results. Engaging participants through clear communication and shared decision-making reinforces their role in the research process, ultimately leading to more effective and impactful scientific discoveries.
Expected Duration of Participation
Participants must be thoroughly informed about the study's duration, encompassing the length of individual sessions and any follow-up periods. Informed consent, as highlighted by the Declaration of Helsinki and the Belmont Report, necessitates clarity and transparency from the onset. The recently updated Guidance by the Multi-Regional Clinical Trials Center emphasizes presenting key information in a manner that enhances comprehension. This encompasses information regarding the study's length, which is essential for individuals to make knowledgeable choices about their engagement. 'Ensuring individuals are aware of the duration not only respects their autonomy but also aligns with ethical standards that underscore the importance of informed consent throughout the research process.'.
Description of Procedures to be Followed
A detailed account of the procedures individuals will undergo is crucial. This encompasses any interventions, assessments, or tests required during the study. For instance, individuals might need to complete specific questionnaires at various stages, such as baseline urinary and erectile function assessments, which are directly added to their medical records. 'The clarity in defining these steps ensures that individuals are well-informed about the specific aspects of the study, which is important for maintaining ethical standards and compliance with guidelines.'. As highlighted in recent updates to the International Council for Harmonization (ICH) E6 Good Clinical Practice (GCP) guidelines, the reliability of trial results depends on the safeguarding of subject data and the integrity of procedures such as randomization and dosing escalation.
Description of Any Reasonably Foreseeable Risks or Discomforts
It is crucial for researchers to provide individuals with clear, concise information about any potential risks or discomforts associated with the study. This openness is essential for knowledgeable agreement, enabling individuals to make choices based on a comprehensive understanding of the possible effects. As stated by the National Organization for Rare Disorders (NORD), the consent process should be available and customized to address the specific requirements of individuals, taking into account elements like language obstacles and health comprehension. By doing so, researchers can ensure that individuals are fully aware of the risks and benefits, thereby supporting their autonomy and right to make educated decisions.
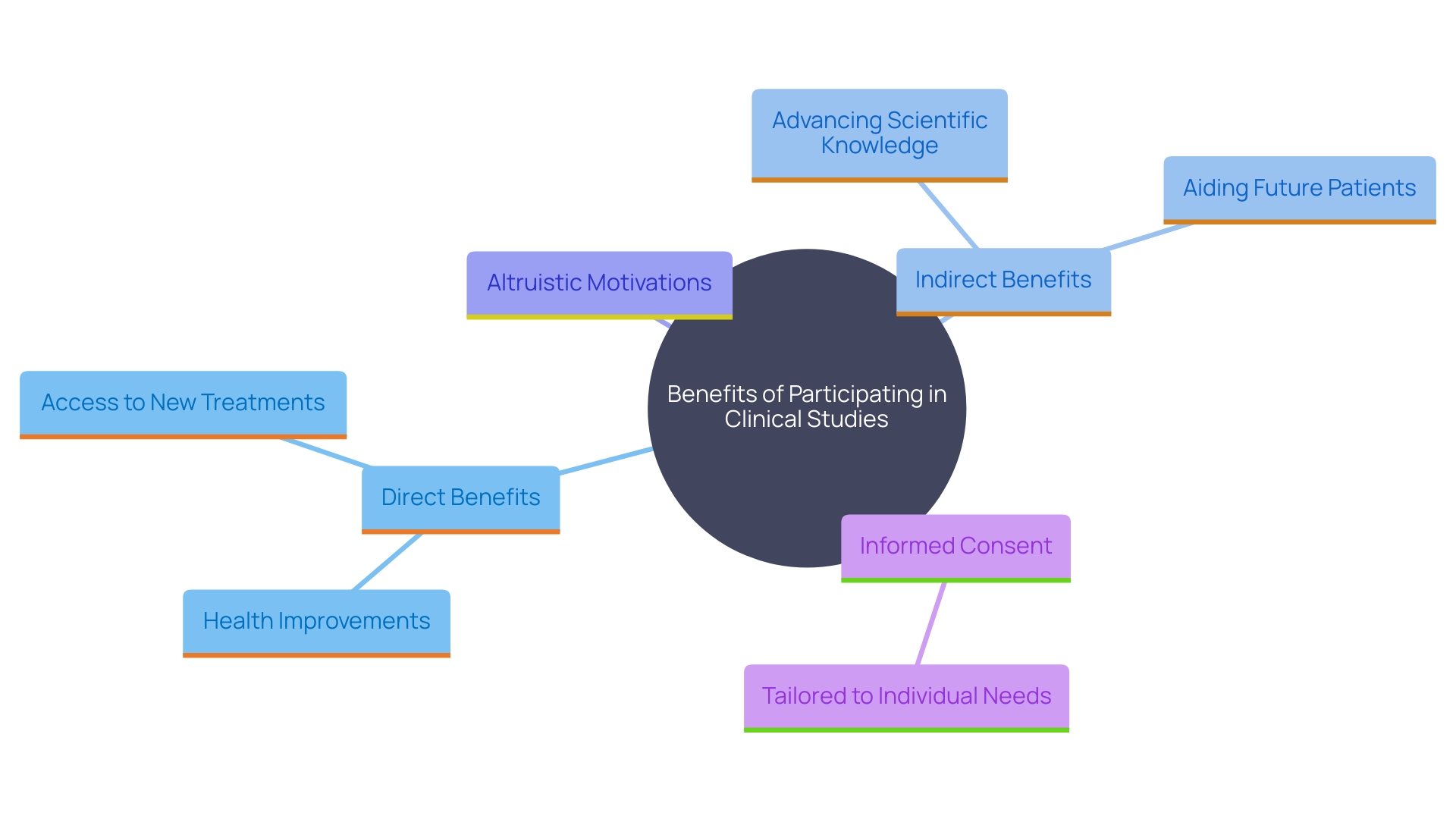
Description of Any Benefits to the Subject or Others
Participants should be completely aware of both direct and indirect advantages they may receive from their participation in clinical studies. Direct benefits often include health improvements or access to new treatments. Indirect benefits encompass the broader impact their participation can have on advancing scientific knowledge and potentially aiding future patients. Based on the 2023 Perceptions and Insights Study by the Center for Information and Study on Clinical Research Participation (CISCRP), a considerable number of individuals take part in studies mainly to assist in saving or enhancing the lives of others. This altruistic motivation underscores the importance of clearly communicating the potential societal benefits of clinical trials. As highlighted by the National Organization for Rare Disorders (NORD), knowledgeable agreement must be provided in accessible formats customized to individuals' specific needs, ensuring they completely comprehend the risks and benefits of involvement. This thorough method for informed agreement not only honors individuals' independence but also promotes a clear and principled setting for inquiry.
Disclosure of Appropriate Alternative Procedures or Courses of Treatment
Informed agreement must include information about any alternative treatments or procedures available outside the research study. This transparency ensures individuals can make well-informed decisions about their involvement. As highlighted by the National Organization for Rare Disorders (NORD), presenting informed consent in accessible manners—such as through videos—can significantly enhance individuals' understanding of their options. Furthermore, the draft guidance released by the FDA and OHRP highlights the significance of conveying essential information clearly and succinctly, including the aim of the study, potential risks and advantages, as well as the duration and methods of the investigation. Incorporating this practice not only aligns with ethical standards but also facilitates better comprehension, empowering individuals to make choices that best suit their needs.
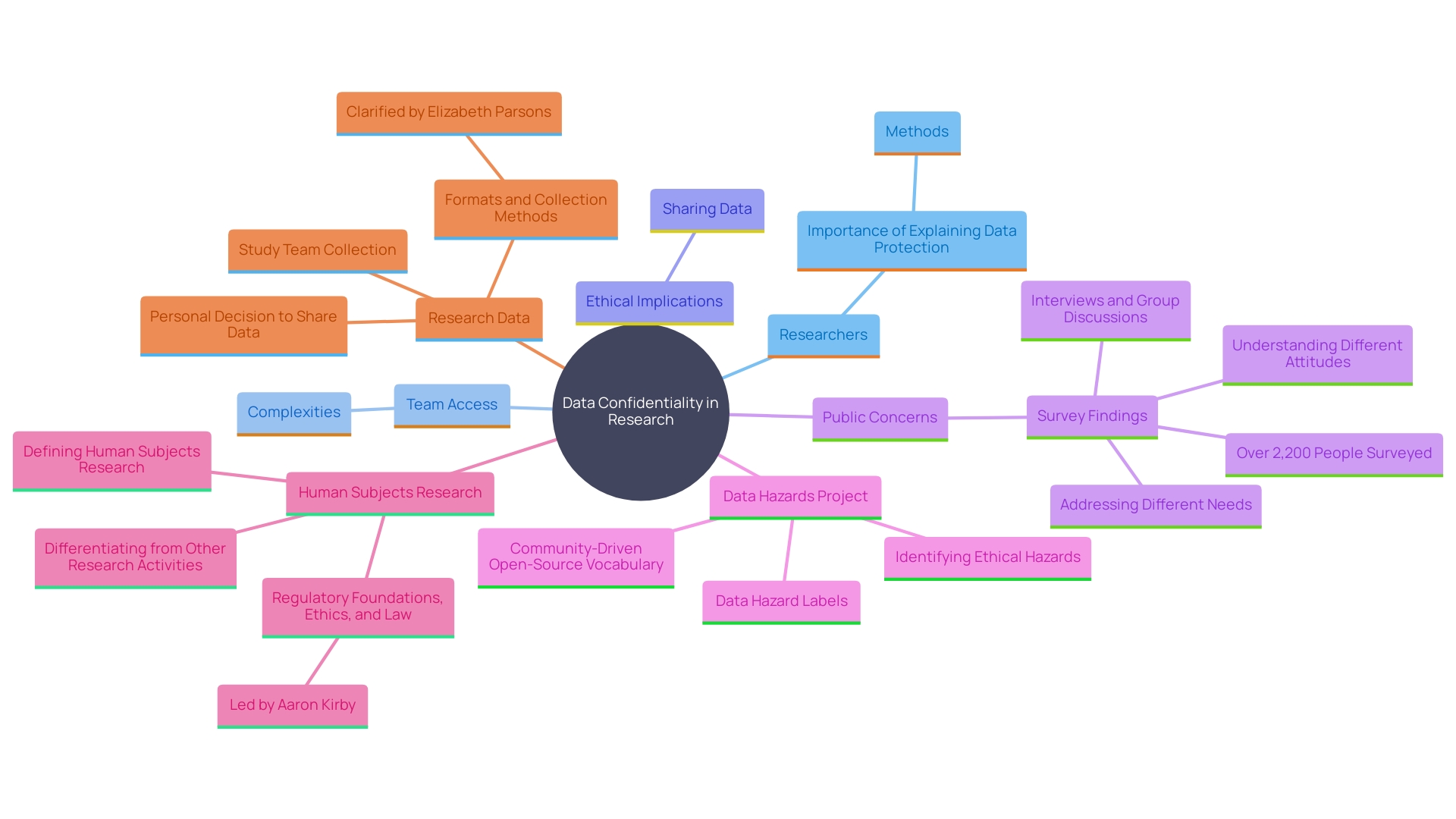
Statement Describing the Extent of Confidentiality of Records
Participants require assurance about the confidentiality of their personal information and data collected during the study. Researchers must clearly explain the methods for data storage, access limitations, and protection measures. As stated by Joe Zurba, head of security and regulatory adherence at Harvard Medical School, a study includes various team members, which can complicate it for individuals to grasp who will have access to their information. Therefore, it’s crucial to outline the protocols for data handling. 'Elizabeth Parsons, IRB administrator for the Harvard University Area IRB, emphasizes that sharing data can enhance public health but deciding to share private information is a personal choice.'. Making sure that individuals are aware of how their information will be protected can help foster trust and encourage ethical research methods. This is particularly important given the growing concerns among Americans about data privacy, as highlighted by a recent Pew Research Center survey indicating increased unease about how personal data is used by companies and the government.
Additional Elements of Informed Consent
Beyond the basic elements of informed consent, there are additional considerations that may be relevant depending on the study's context. These include compensation for involvement, which acknowledges the time and effort of those taking part. Medical treatment for research-related injuries is another critical aspect, ensuring that individuals receive necessary care if they experience any adverse effects. Moreover, the return of clinically relevant results to those involved is gaining attention, emphasizing the importance of transparency and respect for the individuals concerned. The Declaration of Helsinki and the Belmont Report have long emphasized these ethical considerations, and recent updates by the Multi-Regional Clinical Trials Center provide further guidance on these matters. The National Organization for Rare Disorders (NORD) advocates for creative methods to secure consent, such as employing videos to enhance the process's accessibility, emphasizing the necessity to customize consent documents to the distinct requirements of participants.
Compensation and Medical Treatment for Research-Related Injury
Participants must be fully informed about any compensation they will receive for their participation, as well as the medical treatments available in case of research-related injuries. This is vital not only for ethical reasons but also to adhere to legal standards such as those outlined in the Declaration of Helsinki, which has been a cornerstone of medical ethics for decades. Recent surveys indicate that public trust in clinical studies is significantly influenced by transparency regarding compensation and injury protocols. For example, a national survey commissioned by Research!America and ACRO found that 77% of respondents prefer receiving information about clinical trials from their healthcare providers, emphasizing the need for clear and thorough communication. Furthermore, real-life accounts like that of Barbara, who discovered an undiagnosed heart condition through participation in a clinical trial, highlight the importance of informing participants about available medical follow-ups. These measures are essential for maintaining ethical standards and fostering trust in the investigation process.
Contact Information for Questions or Concerns
Supplying contact information for scholars or ethics committee members is a crucial element of consent in healthcare and studies. This transparency ensures that participants can ask questions or voice concerns about the study before and during their participation. According to the Declaration of Helsinki, established by the World Medical Association, respect for the individual and their right to self-determination are paramount. This approach is supported by the Belmont Report's principles of respect for persons, beneficence, and justice, which are foundational to ethical considerations in clinical trials.
In practice, including contact information facilitates patient and public involvement and engagement, which are critical for the success and ethical integrity of clinical trials. It enables collaborative partnerships and shared decision-making, ensuring that the study is pertinent, well-executed, and that results are communicated effectively to those who will use them to make informed choices about treatments. For example, in the United Kingdom, patient involvement in research often includes prioritizing research questions, design, delivery, oversight, analysis, and dissemination.
A case study from the Bangladesh Hypertension Control Initiative (BHCI) emphasizes the significance of sustaining dialogue with individuals involved. Despite the initiative's aim to control hypertension, it faced significant challenges with follow-up, as 44% of registered patients had not visited the clinic for three months or more by the end of 2021. Offering clear and accessible contact information could assist in resolving such issues, ensuring individuals remain engaged and well-informed throughout the study.
Moreover, the Multi-Regional Clinical Trials Center of Brigham and Women's Hospital and Harvard emphasizes the necessity of returning individual data and results to those involved, further underlining the importance of open lines of communication. This practice not only honors the participants' contributions but also improves the validity and generalizability of the findings by ensuring that participants stay engaged and informed throughout the study duration.
Voluntary Participation Statement
A clear affirmation that participation is voluntary is fundamental in any study. Participants must understand that they have the right to decline or withdraw from the study at any point, without suffering any penalties. This principle is deeply rooted in the ethical framework established by the Declaration of Helsinki, which emphasizes respect for individuals and their autonomy in medical studies. It is essential for participants to feel enabled to make choices about their involvement freely and without coercion, ensuring the integrity and ethical standards of the process.
Procedures for Withdrawal from the Study
Participants must be completely educated about the procedures for withdrawing from a study. This includes detailed steps they need to take and assurances that their decision will not impact their future care or treatment. The Declaration of Helsinki highlights the importance of knowledgeable agreement throughout the research process, emphasizing respect for the individual's right to self-determination. The Belmont Report further reinforces this by advocating for the principles of respect for persons, beneficence, and justice. Ensuring clear communication about withdrawal procedures is not only an ethical obligation but also a legal one, as highlighted by the Multi-Regional Clinical Trials Center's updated guidance. Given the complexity and demanding nature of clinical trials—where individuals in Phase 3 studies, for instance, may attend up to 20 visits and undergo numerous procedures—their right to discontinue involvement without any repercussions is paramount. Addressing this explicitly in the awareness agreement process supports the ethical framework and respects the substantial investment of time, energy, and resources made by participants.
Ensuring Understandability and Voluntariness
Making certain that the authorization procedure promotes true comprehension is essential. This involves presenting key information in a clear, concise manner, emphasizing the purpose of the research, its potential risks and benefits, and the procedures involved. Utilizing lay language, visual aids, and allowing ample time for questions are effective strategies to enhance comprehension. As stated by the National Organization for Rare Disorders (NORD), knowledgeable agreement must be available and customized to personal requirements, taking into account elements such as language obstacles and health understanding. This method not only promotes improved comprehension but also tackles issues raised by stakeholders concerning the complexity and legalistic character of current informed agreements, which often exceed twenty pages and obstruct clinical trial participation.
The Role of the Institutional Review Board (IRB)
The Institutional Review Board (IRB) is crucial in ensuring the ethical conduct of studies involving human subjects. To protect participants' rights and welfare, IRBs carefully examine study protocols, which include detailed descriptions of the investigation's purpose, procedures, risks, benefits, and approval documents. This rigorous process, codified in the National Research Act of 1974, aims to prevent unethical practices in studies that have marred history, such as the Tuskegee Syphilis Study. In this egregious case, Black men were denied treatment for syphilis for 40 years, leading to over 100 deaths.
IRB reviews can take weeks or even months, as proposals are often sent back for revisions to meet ethical standards. As Dr. Steven Kritz, a retired physician and current IRB Chair, emphasizes, the inclusion of consent is non-negotiable in any research involving pharmaceutical products. This steadfast commitment ensures that participants are fully informed about the study, including its purpose, potential risks, benefits, and duration. The IRB's role is not just about ticking boxes but about fostering an environment where ethical considerations are paramount, ultimately contributing to advancements in medical knowledge and patient care.
Conclusion
Informed consent is essential for ethical research involving human subjects, serving as a safeguard for participants' autonomy and ensuring they are well-informed about the studies they engage in. This process fosters trust between researchers and participants, while also enhancing the integrity of the research. The necessity of informed consent is underscored by historical unethical practices, which have led to the establishment of regulatory bodies like Institutional Review Boards (IRBs) that oversee compliance with ethical standards.
Key components of informed consent include clear communication regarding the study's purpose, expected duration, procedures, risks, and potential benefits. The growing complexity of consent documents poses challenges, particularly for underserved populations, highlighting the need for accessible and comprehensible materials. Innovations such as multimedia presentations can aid in bridging these gaps, ensuring participants have the information required to make informed decisions about their involvement.
Ultimately, informed consent is not merely a legal formality; it is a fundamental ethical obligation that respects the rights of individuals. By prioritizing transparency and understanding, researchers can uphold ethical standards, maintain public trust, and contribute to the advancement of medical knowledge. Ensuring that participants are fully informed encourages meaningful engagement, which is vital for the success of clinical trials and the broader research landscape.




