Introduction
21 CFR 812 is a crucial regulation that governs the conduction of clinical investigations of medical devices. It plays a vital role in ensuring the safety and efficacy of devices before they can be approved for public use. This article provides an overview of 21 CFR 812, covering key topics such as restricted devices, classification names, product codes, representative sampling of advertisements, and the FDA's role in regulating medical devices.
It also explores the purpose and scope of 21 CFR 812, the different types of device studies, and the requirements for significant risk (SR) and nonsignificant risk (NSR) device studies. Furthermore, the article delves into exempt studies, IRB approval, sponsor responsibilities, labeling and consent requirements, monitoring and reporting obligations, and prohibitions against promotion and other practices. Understanding the nuanced regulations of 21 CFR 812 is essential for clinical research professionals to navigate the complex landscape of medical device trials and contribute to the delivery of innovative healthcare solutions.
Overview of 21 CFR 812
21 CFR 812 sets forth the regulations required for the conduction of clinical investigations of medical devices. Understanding these regulations is paramount for ensuring that devices meet safety and efficacy standards before they can be approved for public use. This includes clear definitions, such as 'restricted device', which is a device that has certain limitations on its sale and distribution due to regulatory restrictions, and 'classification name', which is the FDA’s term for categorizing devices into different classes based on their function and risk. Moreover, it is crucial for stakeholders to comprehend the significance of a 'product code', which is FDA’s identification method for the generic category of devices, and the implications of 'representative sampling of advertisements', which refers to the typical promotional claims made for the device.
The FDA’s role extends to a broad spectrum of public health responsibilities, including the regulation of medical devices to ensure they are safe and effective for use. The agency’s oversight includes a careful review process, exemplified by the Impella Connect System, a device that underwent scrutiny to ensure its software functions for remote monitoring meet the strict requirements of premarket authorization. Additionally, the FDA provides clear instructions for submitting comments on regulations, emphasizing the responsibility of commenters to avoid sharing confidential information publicly.
The agency also addresses the need for adaptability during public health emergencies, as highlighted in recent guidance changes that expanded to cover disasters more broadly. This update included practical considerations for investigational product shipment and site monitoring, reflecting the FDA’s ongoing effort to learn from past experiences, such as the COVID-19 pandemic, which significantly disrupted clinical trials.
Furthermore, the FDA’s orphan drug exclusivity is a critical component that grants market exclusivity for seven years to drugs targeting rare diseases, provided they meet specific criteria. This exclusivity encourages the development of treatments for conditions affecting smaller patient populations, ensuring that pharmaceutical advancements are inclusive of all patients’ needs. Understanding the nuanced regulations of 21 CFR 812 is essential for clinical research professionals to navigate the complex landscape of medical device trials and contribute to the delivery of innovative healthcare solutions.
Purpose and Scope of 21 CFR 812
21 CFR 812 plays a pivotal role in clinical trials by safeguarding human subjects and setting forth requirements for investigational device exemptions (IDEs). This regulation is integral to the FDA's mission to ensure public health protection. It mandates that comments submitted in regards to regulatory matters most exclude confidential information, such as personal health details or sensitive business data. In line with recent FDA guidance, the use of patient-level historical data in clinical trials is emphasized, rather than solely relying on summary estimates. This approach is particularly valuable in the context of rare diseases, where patient scarcity necessitates innovative trial designs to minimize size and duration. Moreover, the FDA's recent final rule on direct-to-consumer drug advertisements underscores the importance of clear and neutral presentation of a drug's major side effects. As regulatory landscapes evolve, the FDA continues to address logistical concerns and risk-based evaluations for clinical trial monitoring, informed by experiences from public health emergencies like COVID-19. Such guidance is crucial for maintaining the integrity of clinical trials and ensuring that the results are robust and reliable. For orphan drugs, exclusivity is granted post-approval to prevent subsequent sponsors from gaining approval for the same drug and indication for a seven-year period, with certain exceptions for clinically superior drugs. This exclusivity is critical for encouraging the development of treatments for rare diseases, which often lack sufficient market incentives due to the small patient populations.
Types of Device Studies Under 21 CFR 812
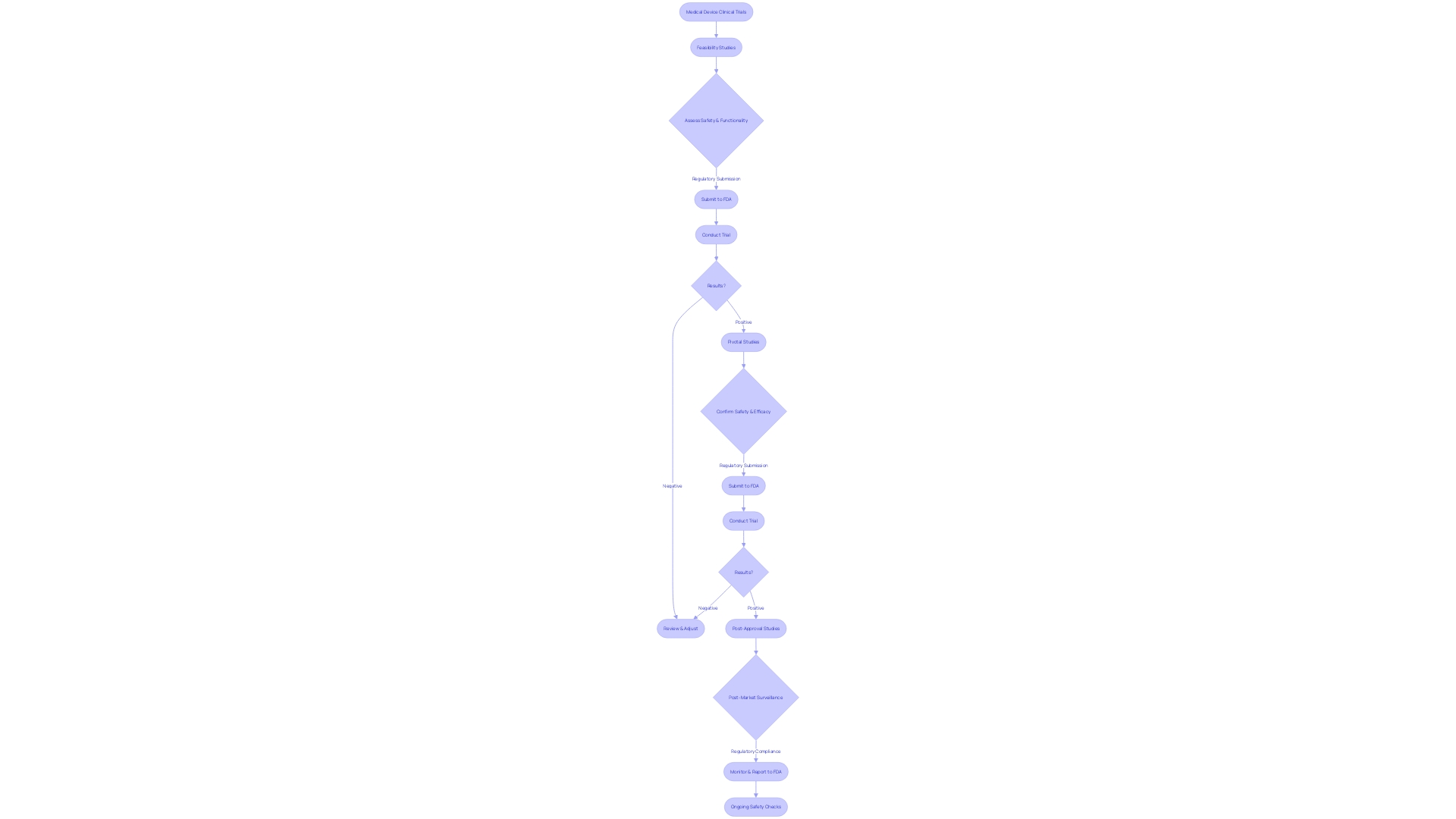
Within the scope of 21 CFR 812, clinical trials involving medical devices are subject to stringent regulations and oversight. Clinical trials can be segmented into several categories based on their objectives and the stage of device development. Notably, feasibility studies are designed to assess the preliminary safety and functionality of a device, often in a small cohort of participants. Pivotal studies, on the other hand, are more extensive and aim to establish the safety and efficacy of a device, typically forming the basis for FDA premarket submissions.
Post-approval studies may be mandated by the FDA after a device enters the market, to monitor long-term effects and ensure ongoing compliance with safety standards. Each study type is governed by specific regulatory requirements that may affect the sale, distribution, or use of the device, as well as the promotional claims made about the device in advertising and labeling. These requirements are crucial for maintaining the integrity and reliability of medical devices on the market.
The FDA's regulations also define key terms related to device marketing and distribution. For instance, 'Initial importer' refers to an entity that furthers the marketing of a foreign manufacturer's device within the United States without altering its packaging or labeling. Moreover, 'Restricted device' signifies a device that is subject to additional regulatory constraints due to its nature or intended use. 'Classification name' and 'Product code' are utilized by the FDA to categorize devices systematically, and any material change in labeling or advertisements must be scrutinized for its impact on the device's identity and effectiveness.
The FDA's commitment to safeguarding public health is reflected in their thorough review of advertising material and labeling for clarity, accuracy, and compliance with established standards. Advertisements must present information in a consumer-friendly manner, ensuring that the major side effects and contraindications of drugs are communicated clearly and neutrally. This regulatory environment underscores the importance of transparency and vigilance in the promotion and monitoring of medical devices to protect end-users and uphold public health standards.
Significant Risk (SR) Device Studies
Clinical trials involving significant risk (SR) devices are subject to stringent oversight under 21 CFR 812, given their potential impact on patient health and safety. These trials necessitate a thorough Investigational Device Exemption (IDE) application, which outlines comprehensive details about the device, including its intended use, specifications, functional components, and properties relevant to its therapeutic or diagnostic function. The IDE application must also include a general description of the condition the device is designed to address, the patient population targeted, and the FDA's assigned reference numbers for any associated legally marketed medical devices.
The submission of an IDE application is a critical step in the regulation of SR device studies, ensuring that the device's safety and effectiveness are rigorously evaluated before it enters the market. The FDA also requires a representative sampling of advertisements and other promotional materials to review the claims made about the device. This process is integral to protecting public health by preventing misleading information from reaching consumers and healthcare professionals.
In addition, conducting SR device studies involves strict adherence to current good manufacturing practice (CGMP) requirements, which govern the design, manufacture, packaging, labeling, storage, installation, and servicing of all finished devices intended for human use. These guidelines are crucial in ensuring that devices are safe, effective, and in compliance with the Federal Food, Drug, and Cosmetic Act.
Monitoring obligations are also a key component of SR device studies, ensuring ongoing compliance and the ability to respond to any adverse effects promptly. The FDA's oversight extends to all facets of the trial, including informed consent procedures to guarantee that participants are fully aware of the potential risks and benefits associated with the study.
The evolving landscape of medical device regulation often presents challenges, including the need for cross-sectoral governance and consideration of ethical, legal, and social issues. Case studies highlight these complexities and underscore the importance of a clear understanding of the trajectory and impact of technology over time.
Moreover, statistics indicate a profound need for effective regulation, with more than 1.7 million injuries and 83,000 deaths in the United States over a 10-year period potentially linked to medical devices. This stark data drives the FDA's commitment to enhancing the safety monitoring of medical products.
Overall, SR device studies represent a critical intersection of innovation, regulation, and patient safety, necessitating a comprehensive and multidimensional approach to governance and oversight.
Nonsignificant Risk (NSR) Device Studies
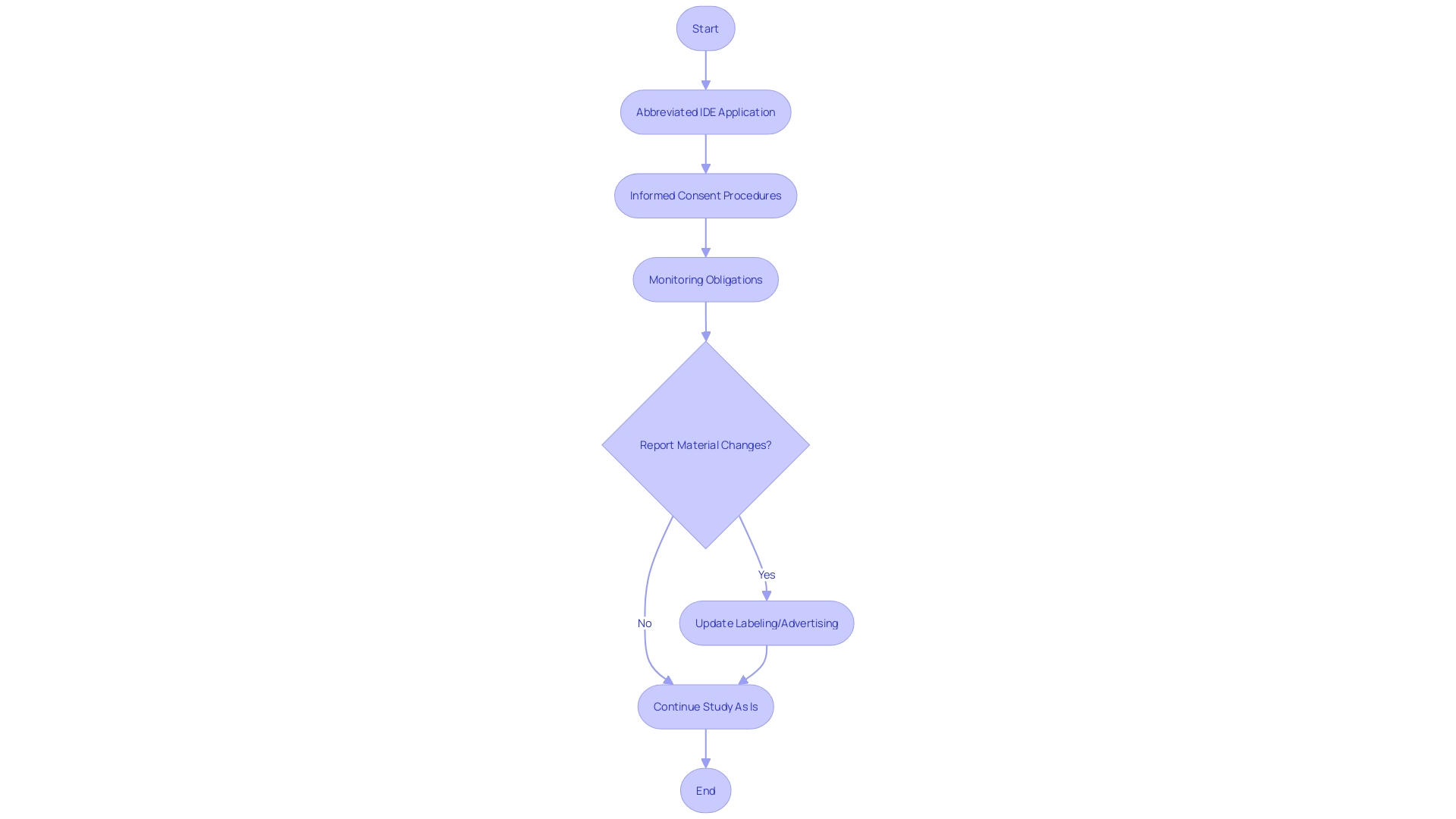
Studies involving devices deemed to present a non significant risk (NSR) to participants are a critical component of clinical research, as outlined in 21 CFR 812. These NSR device studies require a streamlined regulatory approach through an abbreviated Investigational Device Exemption (IDE) application. To conduct these studies, researchers must adhere to mandated informed consent procedures to ethically and transparently enroll participants. Additionally, the monitoring obligations ensure the ongoing safety and compliance throughout the study duration. It is essential that all communications and documentation, including advertising and labeling material, align with FDA regulations to maintain the integrity and safety of the research, particularly as they pertain to the identity and effectiveness of the device under investigation. The FDA stipulates that any material change in labeling or advertising that impacts the device's safety or effectiveness must be reported. This regulatory framework not only safeguards public health by ensuring device safety and efficacy but also supports the scientific community in advancing medical innovation through well-regulated clinical trials.
Exempt Studies
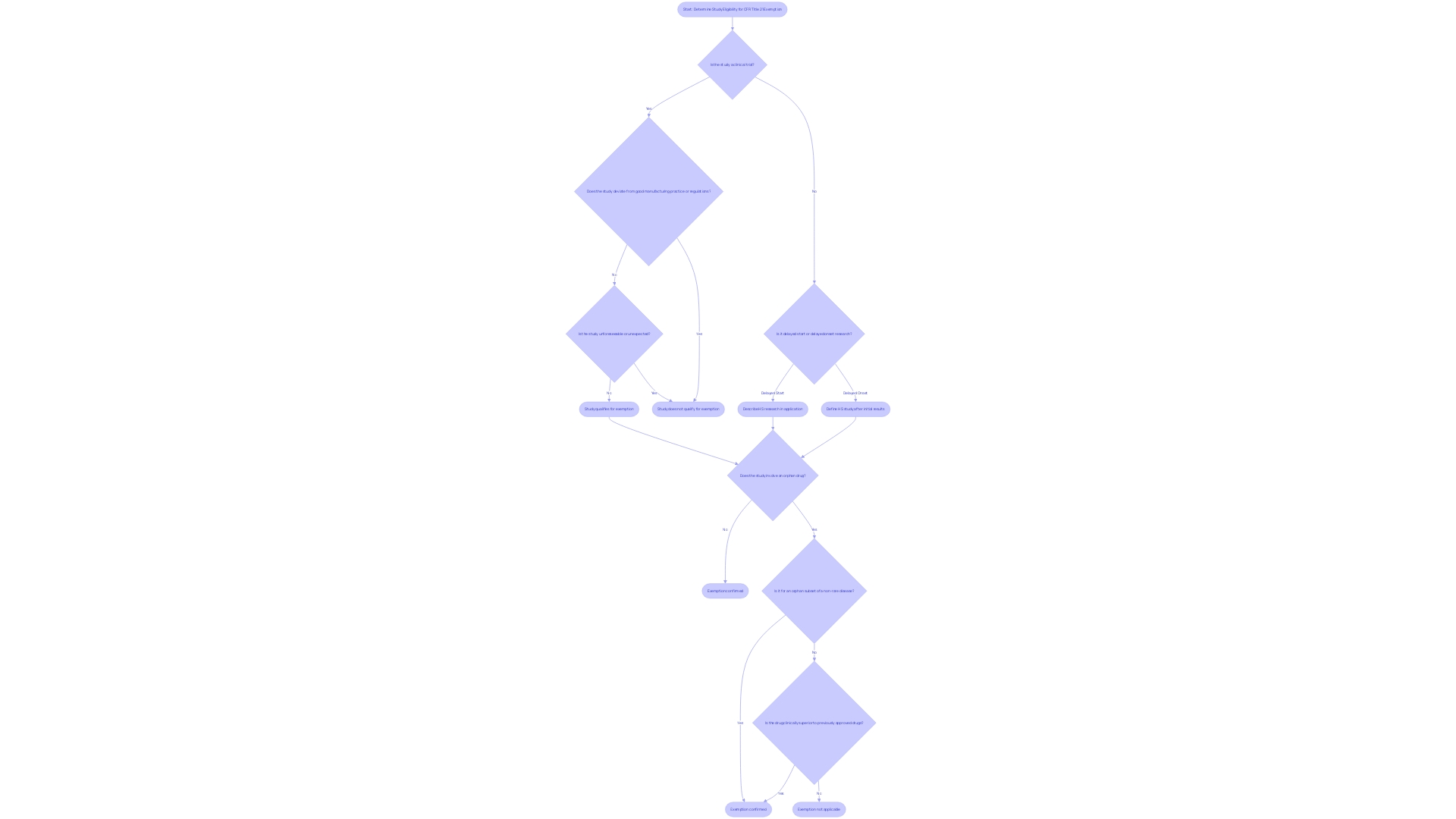
The intricacies of the Code of Federal Regulations (CFR) Title 21 lay out the foundational guidelines for clinical trials and FDA regulations. Within this framework, certain studies may be exempt from the requirements stipulated in 21 CFR 812. Identifying whether a study qualifies for exemption hinges on understanding specific criteria, which necessitates a thorough examination of the research approach and the nature of the human subjects involved. It becomes essential to distinguish between types of delays in research commencement—such as delayed start versus delayed onset—as these affect how a study may comply with or be exempt from regulatory requirements.
For instance, studies that may qualify for exemption include those designated as involving orphan drugs. An orphan drug, as defined by FDA, is intended for use in a rare disease or condition. Under section 526 of the act, an orphan-drug designation signifies that the FDA grants a request for designation, which carries implications for approval exclusivity. This means that for seven years following the approval date stated in the FDA's approval letter, no subsequent sponsor may receive approval for the same drug for the same use or indication, except under specific legal provisions or parts of the CFR.
Moreover, the eCFR, displayed with paragraphs split and indented to follow the document's hierarchy, is an invaluable resource for researchers to understand the regulatory environment. This automated process is designed for user convenience and aids in navigating the regulatory text, which can be dense and complex. This is particularly useful when documenting and justifying exemptions, as the eCFR provides a clear structure to the regulations that researchers must adhere to.
Ultimately, when considering exemptions, it is crucial to document and justify the rationale behind the exemption, drawing upon the case of orphan drugs and the distinctions between delayed start and delayed onset research terms. The eCFR serves as an essential tool in this process, ensuring that researchers can clearly align their studies with the regulatory expectations set forth by the FDA.
IRB Approval and Sponsor Responsibilities
The Institutional Review Board (IRB) is essential in the clinical trial ecosystem, tasked with the ethical oversight of research involving human subjects. Critical to this role is its responsibility to review and approve study protocols and informed consent documents, as mandated under 21 CFR 812. Sponsors must navigate these procedures meticulously to gain IRB approval, which is pivotal in ensuring compliance with ethical standards and regulatory requirements.
An IRB's ethical review is fundamental to safeguarding participant rights and endorsing the integrity of the research. As highlighted by the FDA's efforts to align human subject protection regulations with the HHS Common Rule, efficient and ethically conducted research underpins the FDA’s mission to advance medical product development. The harmonization aims to streamline clinical research while maintaining rigorous participant protections.
The rigorous review process conducted by IRBs is reflective of a legacy shaped by historical ethical breaches in research. For instance, the Tuskegee Syphilis Study's legacy underscores the necessity for stringent ethical oversight. Today's IRBs embody this commitment to ethics, scrutinizing study proposals to ensure that the risks and benefits are appropriately balanced and that informed consent is thoroughly addressed.
In the context of advancing public health, the sponsor's responsibilities extend beyond obtaining IRB approval. They must also ensure adherence to the guidelines set forth by the FDA, which are designed to confirm the safety and efficacy of medical products. This involves a comprehensive understanding of regulations, such as those pertaining to delayed onset research, orphan-drug designation, and the presentation of information in direct-to-consumer advertisements.
Moreover, the importance of timely and efficient research cannot be overstated. With clinical trials often experiencing delays, with up to 80 percent failing to finish on time, sponsors are under pressure to expedite development while upholding ethical standards. This urgency is further amplified by competitive market dynamics and legislative changes that prioritize rapid access to innovative treatments for patients with unmet medical needs.
Labeling, Consent, and Record-Keeping Requirements
Labeling investigational devices, obtaining informed consent, and maintaining accurate records are pivotal under 21 CFR 812 for clinical trials. Labeling provides critical information about the investigational product and its appropriate use, directly impacting the product's perceived effectiveness and safety. For instance, the label indication is derived from clinical trial designs, which define the contexts where the product is effective. Efficient and compliant record-keeping ensures that a trial's conduct can be reconstructed and audited, while informed consent is fundamental to participant rights, requiring clear communication of study purposes, risks, benefits, and procedures.
When labeling investigational devices, it's imperative to include contraindications, or circumstances where the product should not be used, as well as directions for use and actions to take in the event of an overdose. These considerations are crucial for patient safety and the drug's efficacy. Moreover, the representation of the drug product, as well as the roles of licensed practitioners, manufacturers, and distributors, must be clearly defined and compliant with FDA regulations.
Informed consent documents should start with key information presented succinctly to aid understanding. This ensures potential participants can make an informed decision about their involvement. Recent FDA draft guidance underscores the importance of such clarity in consent documents, illustrating the agency's commitment to protecting participant rights and facilitating medical advances.
Accurate record-keeping is another cornerstone of clinical research under 21 CFR 812. It involves documenting the trial's conduct, ensuring data integrity, and maintaining transparency. This includes representative samples of advertisements and any other labeling that makes promotional claims about the device, highlighting any material changes that could affect the device's identity or its safety and effectiveness.
These components—labeling, informed consent, and record-keeping—form the bedrock of ethical and effective clinical trial management, helping to navigate the complexities of clinical research while safeguarding participant welfare and ensuring regulatory compliance.
Monitoring and Reporting Obligations
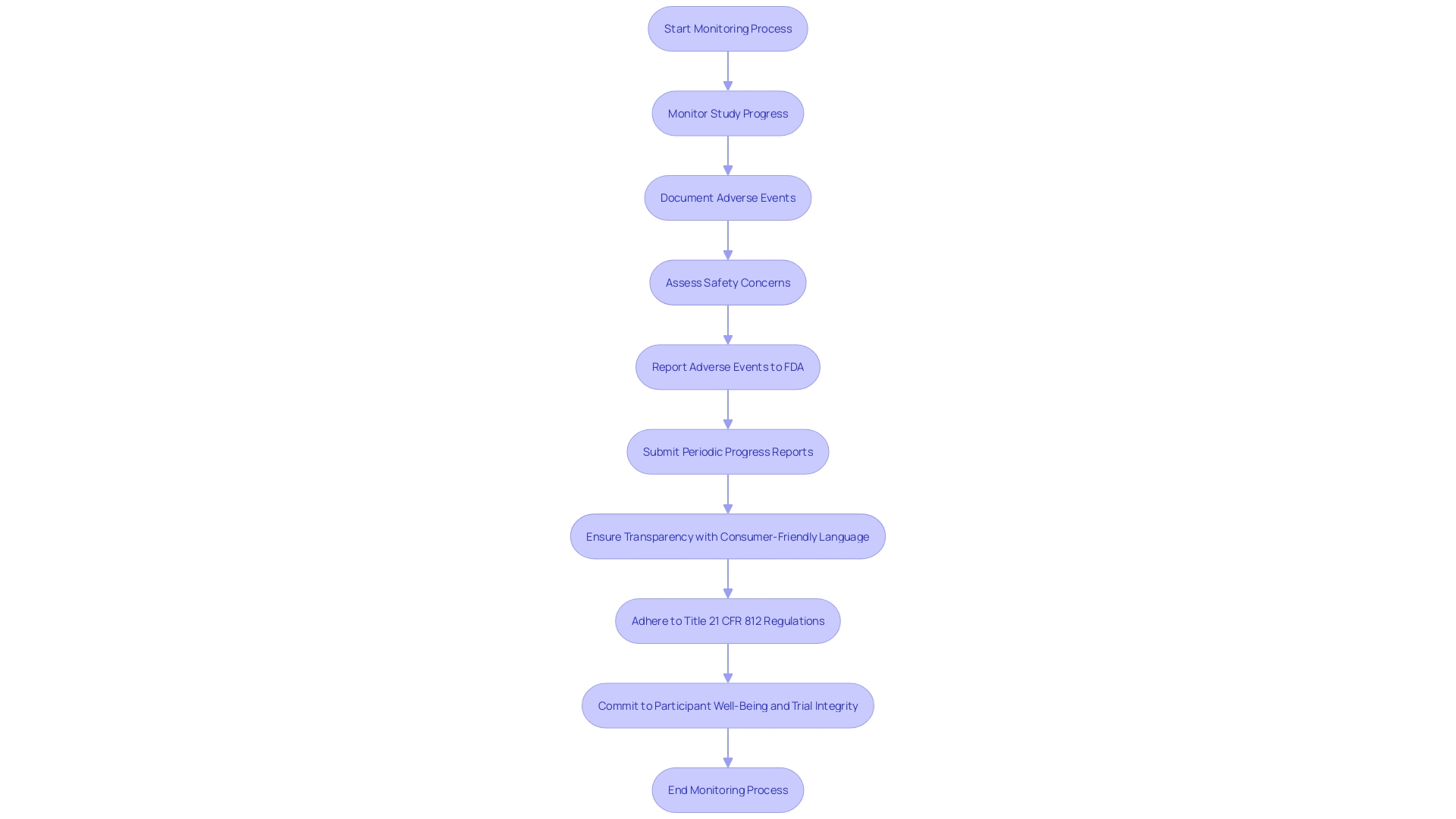
Ensuring the safety and integrity of clinical trials is a pivotal responsibility under Title 21 CFR 812. This involves meticulous monitoring of study progress, thorough documentation and reporting of adverse events, and the submission of regular progress reports to the FDA. These obligations serve as the foundation for protecting participant rights and facilitating medical advances.
Monitoring the study's progress demands a comprehensive understanding of the CFR's requirements. Clinical trials must adhere to stringent standards, with phase one focusing on safety through a small cohort of healthy volunteers, while phase two expands to more participants, including those with the targeted condition, to assess both efficacy and safety.
Adverse event reporting is another crucial element, ensuring any deviations from good manufacturing practice or unexpected events that may impact the safety, purity, or potency of a product are promptly communicated. This is not only a regulatory requirement but a commitment to participant well-being and the integrity of the trial.
Periodic progress reports are a window into the ongoing status of a trial, providing the FDA with critical updates. These reports must include representative samplings of advertisements and other labeling to ensure transparency and compliance with FDA standards, emphasizing the use of consumer-friendly language and dual modality in the presentation of information.
The FDA's recent final rule on direct-to-consumer prescription drug advertisements further underscores the importance of clear and neutral presentation of a drug's major side effects and contraindications, reflecting the agency's commitment to public health and informed decision-making.
By fulfilling these monitoring and reporting obligations, clinical trials can not only uphold the highest ethical standards, as outlined in the Declaration of Helsinki, but also pave the way for medical breakthroughs that could benefit future generations. As researchers like Gamertsfelder and policy advocates like Osipenko highlight, the human element at the heart of these trials is central to the pursuit of quality and integrity in clinical research.
Prohibitions Against Promotion and Other Practices
Title 21 CFR 812 delineates clear boundaries for medical device clinical trials, explicitly prohibiting promotional activities that could potentially skew study outcomes. A pivotal element of this regulation is the definition of a 'restricted device,' which refers to any device whose sale, distribution, or use has been limited by specific FDA regulations or conditions set during premarket approval. This is to ensure that the integrity of the clinical trial is maintained and the device is not commercialized in a manner that could bias the research.
The FDA also outlines precise terminology for classifying devices, such as the 'classification name' and 'product code.' These terms help in categorizing devices under section 513 of the act and ensure uniformity within the regulatory framework. Advertisements for devices, including a 'representative sampling of advertisements,' must reflect the promotional claims made for the device without overstating its efficacy or safety. Any 'material change' to the labeling or advertisements that could impact the device's identity or its safe and effective use must be carefully evaluated and reported.
Moreover, the FDA emphasizes the significance of informed consent, ensuring that participants receive essential information about the study's purpose, potential risks, benefits, and procedures. This process is not just a regulatory requirement but a cornerstone of ethical research, safeguarding participant rights while facilitating medical advancements.
The FDA's commitment to the integrity of clinical research is further demonstrated by its efforts to harmonize human subject protection regulations with the HHS Common Rule. This harmonization aims to streamline clinical research while preserving the welfare of research participants. The agency's actions, including the publication of guidelines for direct-to-consumer prescription drug advertisements, reinforce the principle that information should be presented in a consumer-friendly manner, enhancing the public's understanding of medical products.
In summary, strict adherence to 21 CFR 812's provisions is imperative for maintaining the ethical standards of clinical research and ensuring the generation of reliable data, which are fundamental to FDA's decision-making processes regarding medical products.
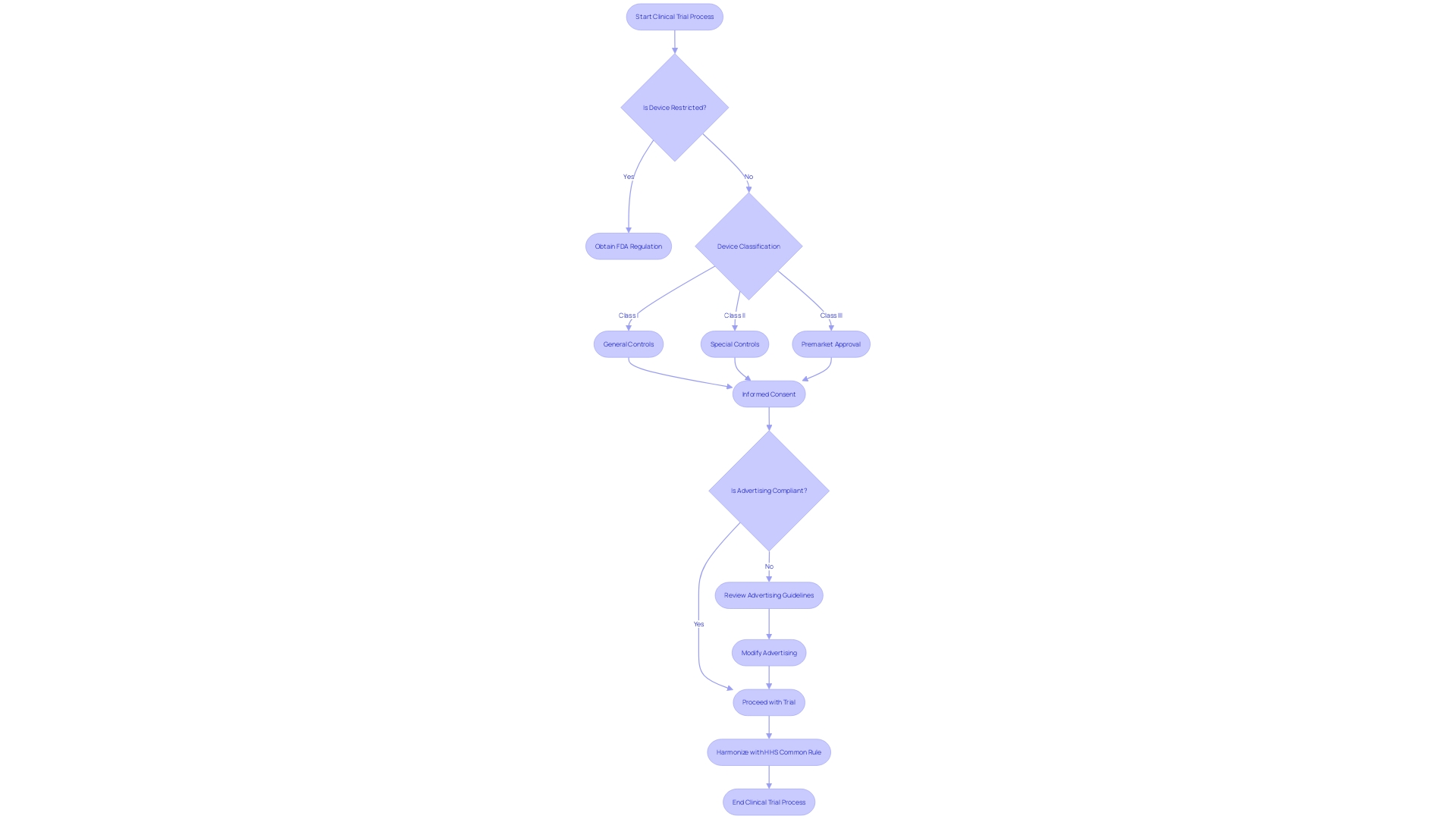
Conclusion
In conclusion, 21 CFR 812 plays a crucial role in ensuring the safety and efficacy of medical devices during clinical investigations. Compliance with these regulations is essential for clinical research professionals to navigate the complex landscape of medical device trials and contribute to innovative healthcare solutions.
The regulation covers various key aspects, including definitions of terms such as "restricted device" and "classification name," which categorize devices based on function and risk. The FDA's oversight extends to public health responsibilities, rigorous review processes, and clear instructions for submitting comments on regulations.
Different types of device studies, such as feasibility, pivotal, and post-approval studies, have specific regulatory requirements. Compliance with these requirements is vital for maintaining the integrity and reliability of medical devices on the market.
Significant risk (SR) device studies undergo stringent oversight, requiring thorough Investigational Device Exemption (IDE) applications, adherence to good manufacturing practice (CGMP) requirements, and monitoring obligations. Nonsignificant risk (NSR) device studies follow a streamlined approach, with abbreviated IDE applications and compliance with FDA regulations for labeling, advertising, and record-keeping.
Exempt studies, including orphan drugs, require careful examination of research criteria. The eCFR serves as a valuable resource for understanding the regulatory environment and justifying exemptions.
IRB approval and sponsor responsibilities are integral to ethical clinical trial management. Compliance with ethical standards and regulatory requirements is crucial for gaining IRB approval and ensuring participant safety.
Labeling, consent, and record-keeping requirements are fundamental in clinical trial management. Accurate labeling, informed consent documents, and compliant record-keeping ensure participant safety, understanding, and transparency.
Monitoring and reporting obligations are critical for participant safety and medical advances. Compliance with FDA standards in labeling and advertising supports transparency and informed decision-making.
Prohibitions against promotion and other practices maintain the integrity of medical device clinical trials. The FDA's emphasis on informed consent and harmonization of human subject protection regulations demonstrates a commitment to ethical research and public understanding of medical products.
In conclusion, adherence to 21 CFR 812 is essential for clinical research professionals to navigate medical device trials and contribute to innovative healthcare solutions while upholding ethical standards and generating reliable data for FDA decision-making processes.




