Overview
The article explores the transformative role of AI and big data in medical device trials, emphasizing their ability to enhance trial design, patient recruitment, data management, and predictive analytics. This is supported by examples of improved outcomes, such as increased breast cancer detection rates and reduced recruitment times, demonstrating how these technologies streamline processes and improve research efficacy in the healthcare sector.
Introduction
The integration of artificial intelligence and big data is reshaping the landscape of medical device trials, heralding a new era of innovation and efficiency. As researchers harness these cutting-edge technologies, they unlock vast datasets that facilitate deeper insights into patient responses and trial outcomes. This transformation not only accelerates the research timeline but also enhances the overall quality of data collected, ultimately leading to improved patient outcomes.
From optimizing patient recruitment to ensuring compliance and data security, the applications of AI and big data are extensive and impactful. As the healthcare sector increasingly embraces these advancements, it becomes essential to explore their implications for the future of clinical research and the potential challenges that lie ahead.
The Transformative Impact of AI and Big Data on Medical Device Trials
The integration of AI and big data has transformed the design and execution of medical device studies, highlighting the role of AI and big data in medical device trials, including those managed by bioaccess®. These innovative technologies enable the analysis of extensive datasets, allowing researchers to extract insights that were once beyond reach. For instance, bioaccess® employs AI algorithms to detect patterns in individual responses, refine trial protocols, and enhance predictive accuracy concerning outcomes in Early-Feasibility Studies (EFS) and First-In-Human Studies (FIH).
Notably, AI-supported mammogram screening has been shown to increase breast cancer detection rates by 20 percent, illustrating the effectiveness of AI in clinical settings. Moreover, large information sets enable real-time tracking of health metrics, allowing prompt adjustments to enhance both safety and effectiveness. This transformative influence not only expedites the research timeline but also elevates the overall quality of the information gathered, leading to superior patient outcomes and more streamlined trials.
The suggested model, which incorporates aspects from the Technology Acceptance Model (TAM), diffusion of innovations theory, and resource-based view (RBV), provides a thorough framework for understanding the role of AI and big data in medical device trials and their influence on healthcare outcomes. Furthermore, based on a 2019 study by Allied Market Research, the market value of large information in North America is anticipated to rise to $34.16 billion by 2025, highlighting the increasing importance of analytics in the healthcare sector. The application of these technologies is now backed by recent legislative frameworks, such as the 21st Century Cures Act, which promotes the use of real-world evidence in clinical practice to evaluate therapies and expedite drug approvals, further enhancing the significance of RWE in medical device evaluations managed by bioaccess®.
Applications of AI and Big Data in Optimizing Medical Device Trials
The integration of AI and big data is transforming the landscape of medical device evaluations in several impactful ways:
- Patient Recruitment: AI algorithms are skilled at examining electronic health records, allowing researchers to pinpoint appropriate candidates for studies quickly. This capability not only reduces recruitment times—evidenced by GlobalCare Clinical Trials' partnership with bioaccess™, which achieved over a 50% reduction in recruitment time—but also enhances enrollment rates, addressing one of the critical bottlenecks in research. To fully realize AI's potential in clinical studies, collaboration among health care providers, researchers, and technology developers is essential.
- Data Management: Big data analytics play a crucial role in streamlining research data management. They facilitate real-time tracking of individual outcomes and adverse events, significantly improving the safety monitoring process. This proactive approach ensures that potential issues are addressed promptly, fostering a safer testing environment.
- Predictive Analytics: By utilizing historical information, AI can anticipate patient reactions to medical devices. This predictive capability informs trial design and allows for necessary protocol modifications, enhancing the overall efficiency and efficacy of the research process.
- Remote Monitoring: Wearable devices are increasingly utilized to collect continuous health information from participants. AI examines this information to deliver actionable insights concerning device performance and patient well-being, facilitating prompt interventions when needed.
Moreover, the recent partnerships in Latin America, such as IDx Technologies collaborating with bioaccess™ for information-licensing, highlight the dedication to advancing AI-driven disease detection in ophthalmology and the overall effect of Medtech research studies on local economies through job creation and healthcare enhancement. Bioaccess® specializes in managing various study types, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Follow-Up Studies (PMCF), utilizing their expertise and customized approach to navigate the complexities of research effectively. A recent case study titled 'Challenges in Retention Strategies for Medical Research' highlights the high dropout rates in medical studies and the absence of AI-driven retention strategies, underscoring the need for innovative solutions to enhance participant engagement and adherence, which are critical for successful study outcomes.
Overall, these applications illustrate the role of AI and big data in medical device trials, demonstrating that their integration not only enhances operational efficiency but also contributes to more reliable and insightful research outcomes. As senior author LL notes, who played a pivotal role in formulating the study conception and drafting the protocol, this ongoing study, set to commence in March 2024 and conclude by May 2024, aims to further explore these innovations and their implications for future AI and clinical research.
Enhancing Patient Engagement Through Technology
Improvements in technology, especially through the role of AI and big data in medical device trials, are reshaping participant engagement strategies. Effective patient engagement can be categorized into several key areas:
- Personalized Communication: AI-powered platforms facilitate the tailoring of reminders and updates, ensuring that individuals stay informed and feel actively engaged in the process. This tailored approach not only strengthens the patient-provider relationship but also aligns with the insights of Sima Marzban, PhD, who emphasized that patient engagement (PE) aims to empower individuals in self-care and health management.
- Mobile Applications: The integration of mobile apps has become more common in clinical studies, enabling participants to report health information, access educational resources, and maintain direct communication with study coordinators. This fosters a supportive community that enhances participant commitment and satisfaction.
- Feedback Loops: Utilizing data analytics enables researchers to gather participant feedback efficiently, promoting ongoing enhancements in the experience. This iterative approach addresses participant concerns promptly and enhances overall satisfaction, ultimately leading to better retention rates.
- Gamification: The inclusion of gamified components within study activities helps to inspire individuals, making compliance with study protocols more engaging. By introducing game-like features, such as rewards for completing tasks, participants are more likely to remain committed to the trial's requirements.
The significance of involvement from individuals receiving care is further emphasized by Liang's scoping review, which indicated that engaging veteran parents in program design can enhance their impact on hospital services. This emphasizes the potential for enhanced health results, compliance, and self-efficacy as direct outcomes of effective engagement strategies. Furthermore, a case study titled "Engagement in hospital health service planning and improvement" illustrates that soliciting insights from individuals can lead to significant enhancements in hospital services.
In summary, utilizing these technology-driven strategies, including The Role of AI and Big Data in Medical Device Trials, not only boosts patient engagement but also aids in better retention rates and the overall success of research initiatives, as shown by recent studies supporting patient insights in healthcare service planning.
Ensuring Compliance and Data Security in Trials
As artificial intelligence and large-scale information continue to transform the environment of medical device assessments, The Role of AI and Big Data in Medical Device Trials has become crucial for ensuring adherence to compliance regulations and protecting information security. Our comprehensive clinical trial management services encompass feasibility studies, site selection, compliance reviews, trial setup, import permits, project management, and detailed reporting, all designed to enhance the efficiency and effectiveness of your research initiatives. Recent findings reveal that 53% of businesses leave over 1,000 files containing sensitive information accessible to all employees, highlighting a critical vulnerability that must be addressed.
Moreover, 70% of venture capitalists favor investing in companies with SOC 2 compliance, highlighting the significance of compliance not only for regulatory adherence but also for attracting investment.
Key strategies for enhancing information security include:
- Information Anonymization: Implementing information anonymization techniques is vital for protecting patient privacy. By ensuring that information gathered during trials is de-identified, researchers can prevent any possibility of tracing details back to individual participants, thus maintaining confidentiality.
- Regular Audits: Conducting systematic audits of management practices not only ensures compliance with regulatory standards but also aids in identifying potential vulnerabilities before they can be exploited. A case study on the primary causes of breaches indicates that weak passwords, social engineering, and insider threats are significant contributors to vulnerabilities, reinforcing the need for regular assessments to foster a proactive approach to security.
- Training and Awareness: Comprehensive training programs for all team members on information protection regulations—such as HIPAA—are essential. This ensures that everyone involved is well-informed about their responsibilities in safeguarding participant information and adhering to compliance standards.
- Selecting Secure Platforms: The choice of AI and large information platforms should prioritize security features to mitigate risks associated with breaches. By choosing secure technologies, organizations can greatly lessen their vulnerability to cyber threats.
By implementing these strategies, researchers can ensure adherence and safeguard sensitive patient information, thereby fostering trust among participants and stakeholders. As John Minnix aptly puts it, 'Our mission is to protect you from cybersecurity threats,' which aligns with these strategies aimed at securing data and maintaining participant confidentiality. Furthermore, our services aid local economies by generating employment, fostering economic development, and enhancing healthcare results, with studies indicating that research can lead to a 15% rise in local job opportunities and a substantial increase in healthcare service availability.
This strengthens the importance of investing in medical research.
The Future of AI and Big Data in Medical Device Trials
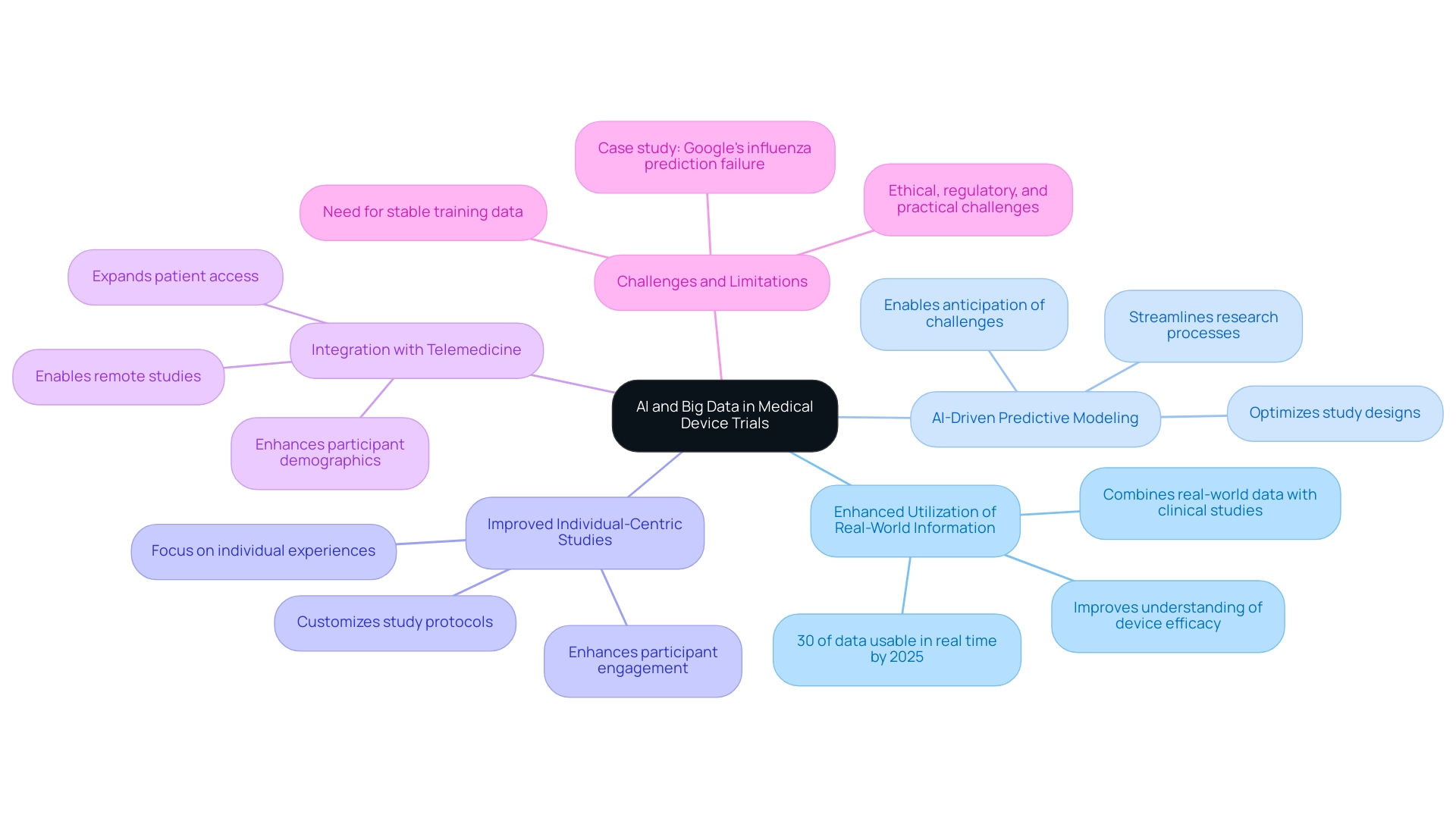
The expected path for AI and extensive research in medical device evaluations highlights the role of AI and Big Data in medical device trials, suggesting an environment ready for innovation and expansion, especially within the thorough management services offered by bioaccess® in Latin America, supported by more than 20 years of expertise in Medtech. Key advancements on the horizon include:
-
Enhanced Utilization of Real-World Information: The combination of real-world information with conventional clinical studies improves understanding of device efficacy and patient results beyond regulated environments.
With projections indicating that by 2025, 30% of generated data will be usable in real time, The Role of AI and Big Data in Medical Device Trials becomes increasingly significant, particularly for Early-Feasibility Studies (EFS) and First-In-Human (FIH) evaluations.
-
AI-Driven Predictive Modeling: Future iterations of AI systems are expected to deliver sophisticated predictive modeling capabilities, enabling researchers to anticipate challenges and proactively optimize study designs.
This could significantly streamline research processes, particularly in Pilot and Pivotal Studies, and enhance study outcomes.
-
Improved Individual-Centric Studies: A growing emphasis on individual experiences and results is expected, with extensive information playing an essential role in customizing study protocols to address unique needs and preferences.
This shift towards patient-centricity is essential for enhancing participant engagement and improving data quality in Post-Market Clinical Follow-Up Studies (PMCF), highlighting The Role of AI and Big Data in Medical Device Trials.
-
Integration with Telemedicine: The combination of telemedicine and AI is likely to enable remote studies, expanding patient access and enhancing participant demographics, which is essential for the success of medical research.
However, despite its potential, the role of AI and Big Data in medical device trials encounters limitations that require collaborative efforts to navigate ethical, regulatory, and practical challenges. For instance, healthcare organizations are currently leveraging AI and machine learning for early disease detection and faster drug development, with over 100 applications submitted to the FDA in 2021. This highlights the urgency of addressing these challenges as the industry evolves.
Additionally, a case study titled 'Challenges in Machine Learning Model Development' illustrates the practical difficulties faced in AI modeling. It revealed that developing machine learning models requires stable, well-structured training sets; deviations can lead to over-fitting, as seen in Google's failed attempt to predict influenza prevalence based on search terms. The project was discontinued due to poor predictive performance, emphasizing the importance of stable data for effective AI modeling.
These developments not only promise to enhance the efficiency of medical device trials managed by bioaccess®, but also contribute to improved quality in clinical research overall, showcasing the role of AI and Big Data in medical device trials. As Bradley Merrill Thompson, a notable expert in regulatory strategy, aptly stated,
I frankly look forward to the future that involves many of these creative applications, but at the same time recognize the risks that need to be managed.
Striking a balance between innovation and risk management will be crucial as the industry navigates these advancements.
Conclusion
The integration of artificial intelligence and big data into medical device trials marks a significant turning point in clinical research, driving innovation and improving efficiency. By leveraging advanced analytics, researchers can:
- Optimize patient recruitment
- Enhance data management
- Implement predictive analytics that streamline trial processes
The ability to monitor patient health metrics in real-time and adapt protocols accordingly not only accelerates research timelines but also elevates the quality of data collected, ultimately leading to better patient outcomes.
Furthermore, the emphasis on patient engagement through technology—ranging from personalized communication to mobile applications—demonstrates the potential for enhancing participant involvement and satisfaction. As patient-centric approaches become more prevalent, the success of clinical trials hinges on effective engagement strategies that foster commitment and retention.
As the healthcare sector continues to embrace these advancements, it is crucial to address compliance and data security challenges. Implementing robust data protection measures and ensuring that all stakeholders are well-informed about regulatory responsibilities will help build trust among participants and safeguard sensitive information.
Looking ahead, the future of AI and big data in medical device trials appears promising, with anticipated innovations that will further enhance trial efficiency and patient outcomes. However, it is essential to navigate the ethical and regulatory complexities that accompany these advancements. A balanced approach to innovation and risk management will be vital as the industry evolves, ensuring that the benefits of these technologies are realized while maintaining the integrity of clinical research.




