Overview
The article explores the vital role of ethics committees in overseeing clinical trials in Latin America, emphasizing their responsibilities in protecting participant rights, ensuring informed consent, and maintaining research integrity. It highlights the necessity for these committees to adapt to diverse regulatory environments and cultural contexts while advocating for participant welfare, ultimately aiming to enhance the ethical standards of clinical research in the region.
Introduction
In the realm of clinical research, ethics committees play a pivotal role in safeguarding the rights and welfare of participants, ensuring that studies are conducted with the utmost integrity. These committees, often known as Institutional Review Boards (IRBs), are tasked with a comprehensive review of research proposals, assessing everything from informed consent processes to the balance of risks and benefits.
As clinical trials increasingly extend their reach into diverse regions, such as Latin America, the complexities of ethical oversight become even more pronounced. Factors such as varying regulatory frameworks, cultural perceptions, and the evolving landscape of medical research present both challenges and opportunities for these committees.
This article delves into the essential functions of ethics committees, the hurdles they face, and the future directions that promise to enhance ethical standards in clinical trials, ultimately reinforcing public trust in the research process.
The Essential Role of Ethics Committees in Clinical Trials
Ethics groups, commonly referred to as Institutional Review Boards (IRBs), are essential to the oversight of clinical trials, demonstrating The Role of Ethics Committees in Latin American Clinical Trials by ensuring that the rights, safety, and well-being of participants are paramount. Their responsibilities encompass a comprehensive review of research proposals, focusing on the scientific validity of the study, the adequacy of informed consent processes, and the risk-benefit ratio. In regions such as Latin America, including Colombia, groups must navigate not only regulatory requirements set forth by INVIMA (the Colombia National Food and Drug Surveillance Institute) but also cultural factors that shape participant perceptions and experiences, highlighting The Role of Ethics Committees in Latin American Clinical Trials.
INVIMA, classified as a Level 4 health authority by the PAHO/WHO, plays a crucial role in overseeing medical devices, ensuring compliance with health standards, and suggesting technical standards for their manufacturing and marketing. This nuanced approach is essential, especially considering a recent review of 29 articles on Clinical Evaluation Committees (CECs) highlighted the variability in study quality based on design and data comprehensiveness, leading to the establishment of quality assessment criteria for high-caliber studies. Significantly, in the past five years, just five papers have been released on this subject, highlighting the necessity for more thorough evaluation of review board practices.
Glenn Begley, a recognized coauthor of a groundbreaking study discussing the irreproducibility of preclinical research, stresses the essential nature of moral supervision, stating,
These hypothesis generating trials should not, under any circumstances, initiate a clinical trial. I think there are thousands of patients who have been treated inappropriately based on publications about such studies in the top journals.
This emphasizes the necessity for strong oversight practices, such as The Role of Ethics Committees in Latin American Clinical Trials, to prevent unethical research conduct, which has raised questions about the sufficiency of current review processes, as highlighted in recent media coverage by Clinical Leader.
For instance, Clinical Leader reported on the challenges encountered by advisory groups in adapting to changing regulatory environments and highlighted the significance of independent examination in sustaining public trust. Proactive reforms in these practices are essential for enhancing oversight and establishing improved moral standards, highlighting The Role of Ethics Committees in Latin American Clinical Trials. By offering independent examination, ethics groups not only maintain public confidence in research but also guarantee compliance with international moral standards such as the Declaration of Helsinki and Good Clinical Practice (GCP).
Navigating Ethical Challenges in Latin American Clinical Trials
The role of ethics committees in Latin American clinical trials faces numerous challenges that complicate the review process. A significant factor is the diverse regulatory frameworks that differ across nations, emphasizing the role of ethics committees in Latin American clinical trials, which leads to inconsistencies in oversight. Cultural attitudes toward clinical research also play a crucial role in understanding the role of ethics committees in Latin American clinical trials, often influencing public awareness and comprehension of participant rights.
For instance, many communities may lack a clear grasp of informed consent, resulting in moral dilemmas that require careful navigation. Moreover, researchers frequently experience pressure to satisfy international sponsor demands while simultaneously adhering to the role of ethics committees in Latin American clinical trials. Significantly, participants in advisory groups who convene only half a day annually may find it challenging to engage thoroughly with these intricacies, affecting their supervisory abilities.
A recent study titled 'Call to Action for Standards in Inquiry' revealed significant gaps in the application of moral guidelines across 22 nations, underscoring the need for collaboration among health authorities, scientific agencies, and academic institutions. To effectively navigate these complexities, the role of ethics committees in Latin American clinical trials must prioritize ongoing education and establish collaborative relationships with researchers. This method guarantees that local contexts are honored, standards of conduct are upheld, and integrity in inquiry is preserved.
Strengthening ties with community stakeholders further fosters trust and can significantly enhance participant recruitment and retention in clinical trials. As Elizabeth Heitman observes, 'The environment in scholarly integrity has transformed considerably in Latin America and the Caribbean over the last twenty years,' highlighting the changing nature of these challenges and the necessity for ongoing adjustment.
The Process of Ethics Review: Key Steps and Considerations
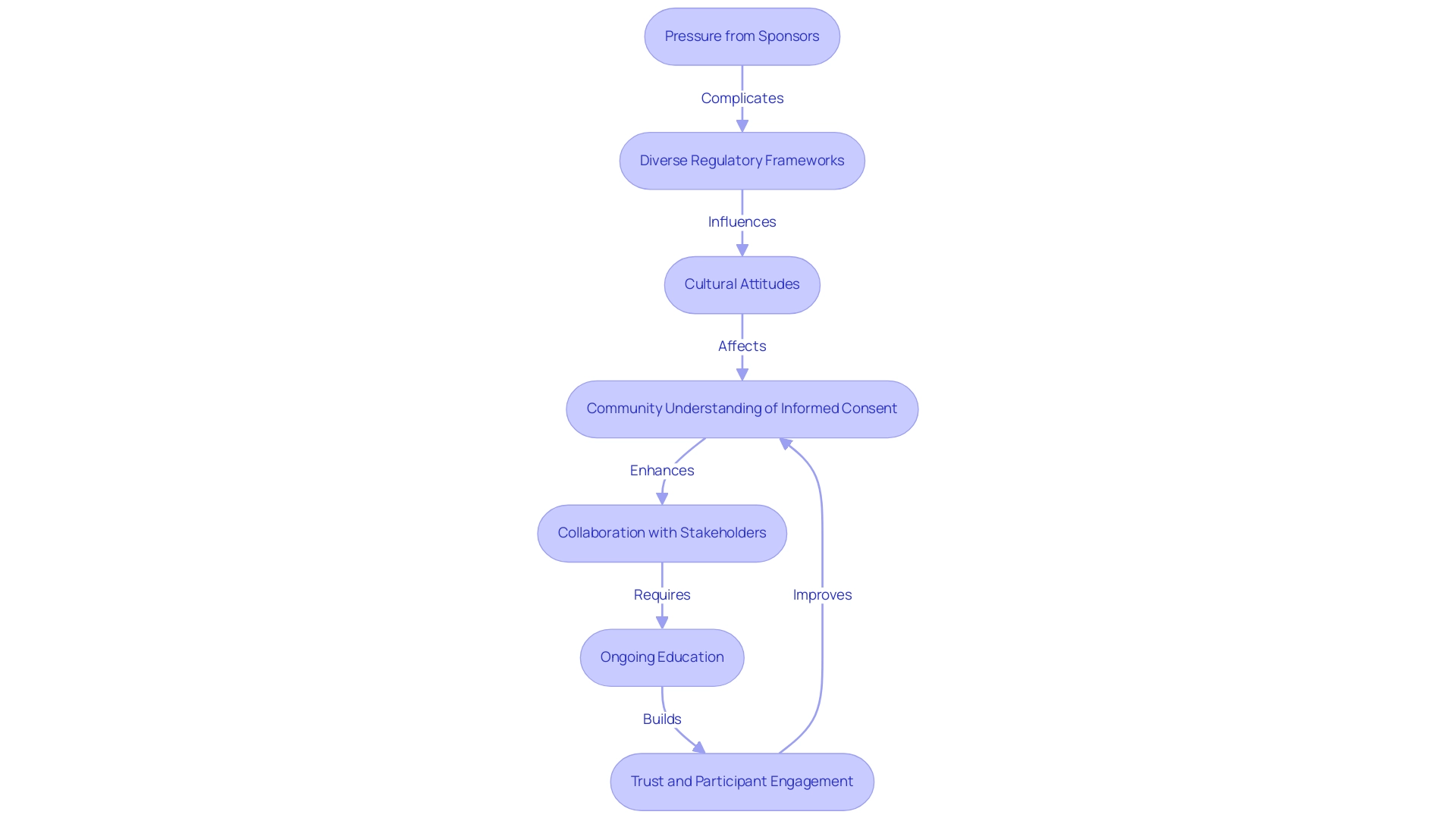
The review process for clinical research is vital for guaranteeing that studies uphold the highest moral standards, emphasizing The Role of Ethics Committees in Latin American Clinical Trials. It typically comprises several key steps:
- Submission of Protocol: Researchers start by submitting a thorough study protocol, which encompasses the objectives, methodology, and informed consent documents required for compliance.
- Preliminary Review: The ethics board conducts an initial review to verify completeness and adherence to regulatory requirements, ensuring that all essential elements are present.
- Full Review: A thorough evaluation takes place during a meeting, where members critically analyze the moral implications of the study and engage in discussions regarding any potential concerns.
- Feedback and Revisions: The group often offers constructive feedback, requesting modifications to address identified ethical issues before granting approval.
- Final Approval: Upon satisfactory resolution of all concerns, the committee issues an approval letter, officially allowing the study to proceed.
This organized method not only promotes accountable inquiry practices but also corresponds with current best standards in ethical evaluation, such as those endorsed by independent review panels that oversee studies to reduce conflicts of interest and safeguard volunteer well-being.
As emphasized by the Indian Council of Medical Research, moral principles for biomedical studies were initially introduced in 2000 and revised in 2006, stressing the significance of adjusting review processes to address modern challenges. Additionally, the CIOMS guidelines, established in 2002, provide a foundational framework that has influenced global ethics review practices. A pertinent case study demonstrates The Role of Ethics Committees in Latin American Clinical Trials as essential in assessing proposals to ensure moral standards are upheld, thereby safeguarding the welfare of study participants.
Informed Consent: Best Practices and Cultural Considerations
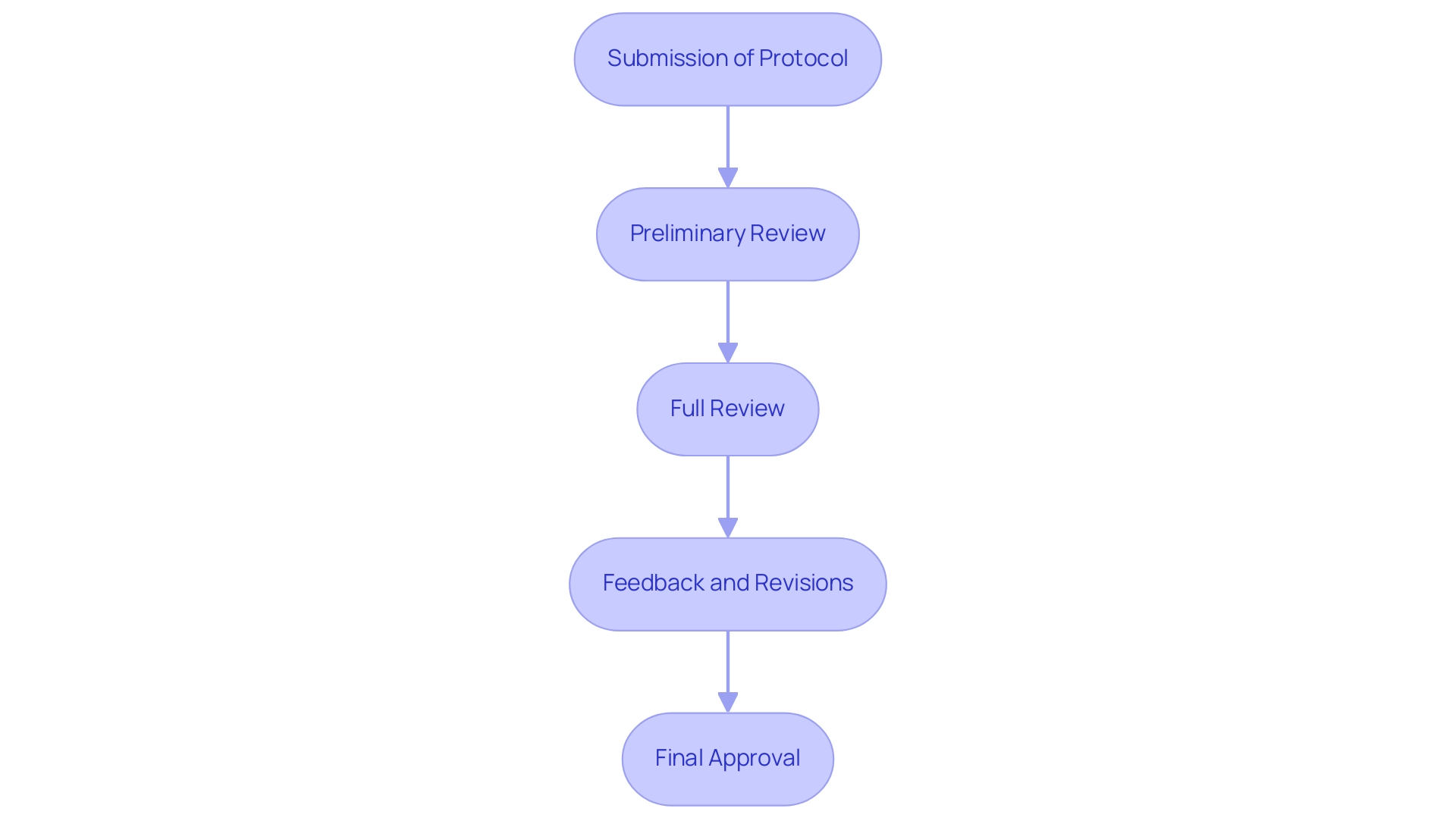
Informed consent is crucial in ensuring that participants are thoroughly informed about the study's purpose, procedures, risks, and potential benefits. Recent updates from the FDA in 2023 have highlighted the importance of using varied media and simplified language in the informed consent process, reinforcing the need for best practices such as:
- Clear Language: Employ layman's terms to enhance understanding, steering clear of jargon and technical terminology.
- Cultural Sensitivity: Adapt the consent process to honor cultural norms and values. Engaging community leaders or representatives can significantly enhance the relevance and acceptance of the consent materials.
- Ongoing Consent: Reinforce that consent is not a one-time event. Participants should feel empowered to ask questions throughout the study and have the right to withdraw without facing any penalties.
- Documentation: Keep meticulous records of the consent process, ensuring all participants receive a copy of their consent form for their records.
Given the paucity of clear directions around best practices in the preparation of patient-facing documents, a literature review was conducted to identify existing guidance, revealing a significant gap in quantitative data and relevant practices.
This prompted the creation of 44 actionable guidelines for the development of accessible Patient Information Leaflets (PILs) and Informed Consent Forms (ICFs). By following these best practices, researchers can not only improve participant understanding but also maintain the highest moral standards in clinical trials, highlighting The Role of Ethics Committees in Latin American Clinical Trials and the ongoing dialogue around cultural considerations in the consent process.
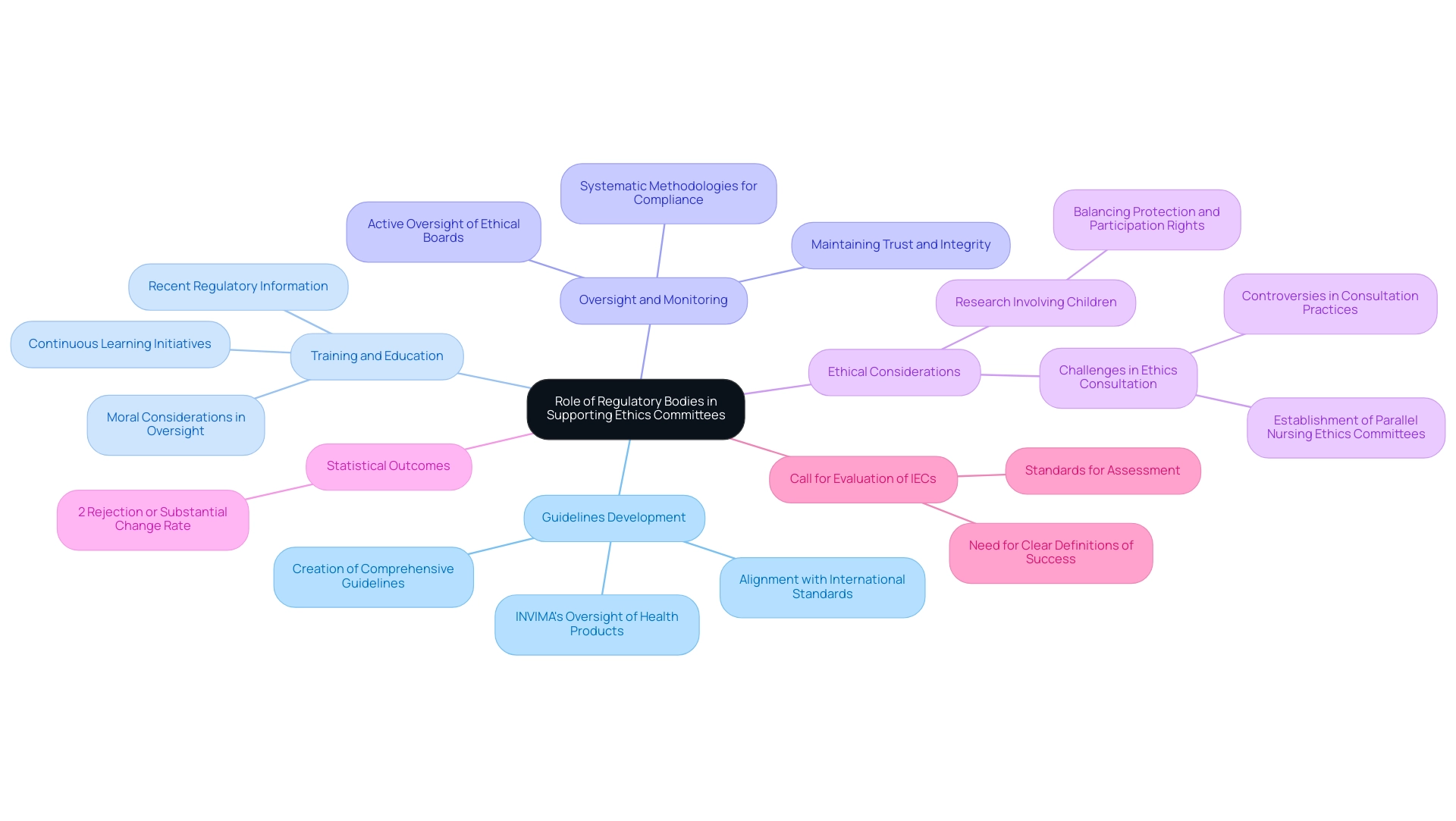
The Role of Regulatory Bodies in Supporting Ethics Committees
Regulatory bodies are essential in shaping the guidelines and standards that govern ethics committees in clinical studies. Their support encompasses several critical areas:
-
Guidelines Development: Regulatory bodies, such as INVIMA (Colombia National Food and Drug Surveillance Institute), are responsible for creating and disseminating comprehensive guidelines for review processes, which align with international standards.
This ensures that research practices not only comply with legal requirements but also maintain moral integrity. INVIMA's role includes inspecting and supervising the manufacturing and marketing of health products, as well as overseeing the import and export of these products, further reinforcing these standards. -
Training and Education: Continuous learning initiatives are essential for review board members, providing them with the most recent regulatory information and moral considerations.
This continuous professional development fosters an environment where ethical oversight is prioritized, supported by INVIMA's commitment to regulatory excellence in medical device oversight. -
Oversight and Monitoring: By actively overseeing the functions of ethical boards, regulatory bodies ensure adherence to established protocols and guidelines.
This oversight is crucial in maintaining the trust of participants and the integrity of clinical trials.
INVIMA, designated as a Level 4 health authority by PAHO/WHO, demonstrates this dedication to guaranteeing safety, efficacy, and quality in clinical trials through its systematic methodologies for monitoring compliance and enforcing standards.
The partnership between ethics boards and regulatory bodies like INVIMA highlights The Role of Ethics Committees in Latin American Clinical Trials.
It reinforces the ethical framework within which clinical trials function, thereby improving the overall quality of medical studies.
Significantly, a recent assessment showed that only 2% of projects were either rejected or underwent substantial changes due to input from these groups.
This statistic emphasizes the significance of regulatory organizations in enhancing investigative practices, as it suggests that most projects can move forward with required modifications instead of complete dismissal, illustrating the positive role of ethical review boards.
As mentioned by a prominent scholar,
Ethical review boards operate from a standpoint of how can I assist in making this project function ethically?
This perspective emphasizes the proactive strategy that regulatory bodies promote in their collaboration with ethical boards.
Furthermore, the request for a thorough evaluation of Institutional Ethics Committees (IECs) indicates the persistent necessity for clear definitions of success and standards for assessment, intending to enhance understanding of their influence on clinical studies.
Significantly, studies involving children necessitate careful moral consideration, balancing the need for protection with children's rights to participate.
This aspect is vital as it highlights the dedication of advisory groups to uphold the rights and welfare of vulnerable populations.
In Latin America, although the methods of regulatory agencies may vary, their primary objective remains the same: to improve the moral standards of research for the advantage of participants and the progress of medical science, which underscores The Role of Ethics Committees in Latin American Clinical Trials.
Moreover, the case study titled 'Challenges in Ethics Consultation' demonstrates the difficulties encountered by ethical review groups, where differing views on who can request guidance and how transparent the process should be have resulted in continued discussions and the creation of parallel nursing ethical review groups.
This highlights the necessity for clear guidelines and practices in the consultation process, further stressing the role of regulatory bodies, such as INVIMA, in supporting ethical review boards.
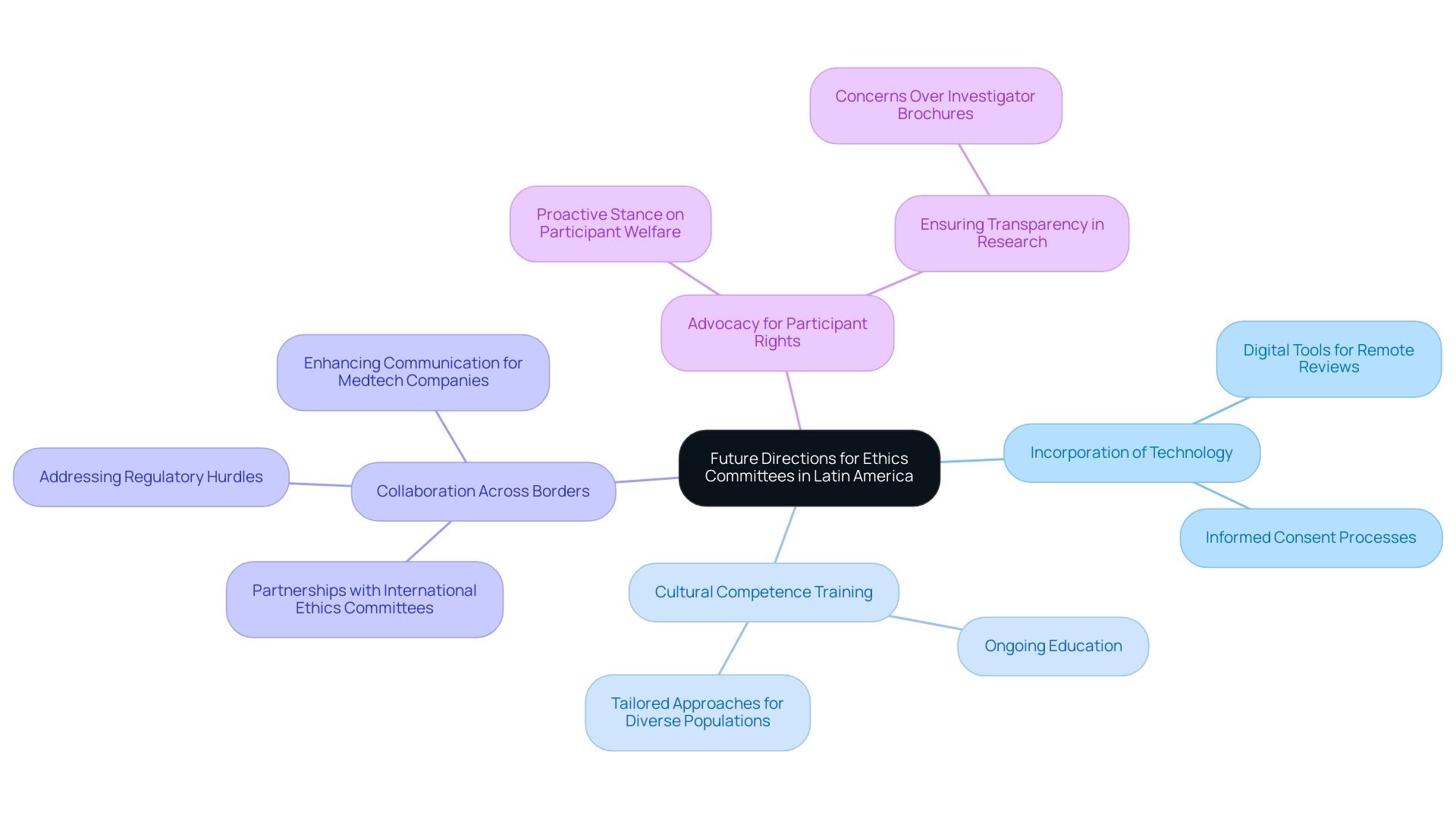
Future Directions for Ethics Committees in Latin America
As the landscape of clinical trials changes, review boards in Latin America face both challenges and opportunities that necessitate strategic foresight. Future directions for these groups may encompass the following key areas:
- Incorporation of Technology: The adoption of digital tools for remote reviews and informed consent processes is essential. Such innovations not only enhance accessibility but also increase the efficiency of the review process, aligning with the trend of innovative clinical trial designs that are increasingly popular for their adaptability.
- Cultural Competence Training: Ongoing education in cultural competence will enable advisory groups to better comprehend and respond to the varied needs of participants. This understanding is critical, particularly in a region where trials often engage patients from approximately 171 different geographical areas. This statistic highlights the necessity for tailored approaches in participant engagement to ensure proper practices are upheld across diverse populations.
- Collaboration Across Borders: Strengthening partnerships with international ethics committees and regulatory bodies, such as INVIMA—the Colombian National Food and Drug Surveillance Institute—can facilitate knowledge sharing and the adoption of best practices. INVIMA’s role as a Level 4 health authority by PAHO/WHO emphasizes the significance of strong regulatory frameworks that direct proper conduct in clinical trials. Improved cooperation can result in stronger moral structures that benefit all parties involved in clinical studies. Furthermore, addressing the regulatory hurdles and language barriers faced by Medtech companies can enhance communication and collaboration in clinical trials.
- Advocacy for Participant Rights: Ethics groups must take a proactive stance in advocating for the rights and welfare of participants. Ensuring that participant voices are heard throughout the research process is paramount to ethical integrity. The results from a case study on Investigator Brochures (IBs) show that fewer than 5% of preclinical efficacy studies contained crucial information such as randomization and sample size calculations, raising significant concerns about the transparency needed for informed decision-making by review boards.
To further contextualize these discussions, media coverage of clinical trials in Latin America, particularly by sources like Clinical Leader, highlights ongoing developments and challenges in the field, providing valuable insights for stakeholders. By addressing these future directions, the role of ethics committees in Latin American clinical trials can be enhanced, ultimately ensuring that clinical trials are conducted ethically and responsibly across the region. This commitment reflects the sentiment that, 'the community in which research is conducted should collaborate in the research endeavor', reinforcing the need for stakeholder engagement to sustain the integrity of clinical trials.
Conclusion
Ethics committees, or Institutional Review Boards (IRBs), are crucial in ensuring the integrity and ethical standards of clinical trials. Their role encompasses a thorough review of research proposals, focusing on:
- Informed consent processes
- Risk-benefit assessments
- Adherence to regulatory frameworks
In regions like Latin America, where diverse cultural perceptions and regulatory challenges exist, these committees face unique hurdles that necessitate ongoing education and collaboration with local stakeholders. The complexity of these ethical landscapes underscores the importance of adapting practices to uphold participant rights and foster public trust in the research process.
Looking ahead, the future of ethics committees in Latin America hinges on:
- Embracing technological advancements
- Enhancing cultural competence
By incorporating digital tools for remote reviews and informed consent, committees can improve accessibility and efficiency. Furthermore, fostering international collaborations and advocacy for participant rights will be pivotal in addressing the evolving challenges of clinical research. Continuous adaptation to these dynamics will ensure that ethics committees not only protect participants but also contribute to the advancement of medical science.
Ultimately, the commitment of ethics committees to uphold ethical standards is paramount in reinforcing public confidence in clinical trials. As they navigate the complexities of regulatory environments and cultural contexts, their proactive approach will be essential in shaping a responsible research landscape that prioritizes the welfare of participants. The collective efforts of ethics committees, regulatory bodies, and community stakeholders will be instrumental in establishing a robust ethical framework that enhances the quality and integrity of clinical research across Latin America.
Frequently Asked Questions
What are Institutional Review Boards (IRBs) and their role in clinical trials?
Institutional Review Boards (IRBs), also known as ethics groups, are responsible for overseeing clinical trials to ensure the rights, safety, and well-being of participants are prioritized. They conduct comprehensive reviews of research proposals, focusing on scientific validity, informed consent processes, and the risk-benefit ratio.
How does INVIMA contribute to clinical trials in Colombia?
INVIMA, classified as a Level 4 health authority by PAHO/WHO, oversees medical devices and ensures compliance with health standards in Colombia. It also suggests technical standards for the manufacturing and marketing of these devices, which is crucial for maintaining quality in clinical trials.
What challenges do ethics committees face in Latin America?
Ethics committees in Latin America face challenges such as diverse regulatory frameworks across nations, cultural attitudes toward clinical research, and a general lack of public understanding of informed consent. These factors can lead to inconsistencies in oversight and complicate the review process.
Why is independent examination important for ethics committees?
Independent examination is vital for maintaining public trust in clinical research. It ensures compliance with international moral standards, such as the Declaration of Helsinki and Good Clinical Practice (GCP), and helps prevent unethical research conduct.
What gaps exist in the application of moral guidelines in clinical trials?
A study titled 'Call to Action for Standards in Inquiry' identified significant gaps in the application of moral guidelines across 22 nations, indicating a need for collaboration among health authorities, scientific agencies, and academic institutions to improve ethical standards in clinical trials.
How can ethics committees improve their effectiveness?
Ethics committees can enhance their effectiveness by prioritizing ongoing education, establishing collaborative relationships with researchers, and strengthening ties with community stakeholders. This approach helps ensure that local contexts are respected, standards of conduct are upheld, and integrity in inquiry is maintained.
What impact do cultural factors have on clinical trials in Latin America?
Cultural factors significantly influence public awareness and understanding of participant rights in Latin America. Many communities may not fully grasp informed consent, leading to moral dilemmas that ethics committees must navigate carefully.

