Introduction
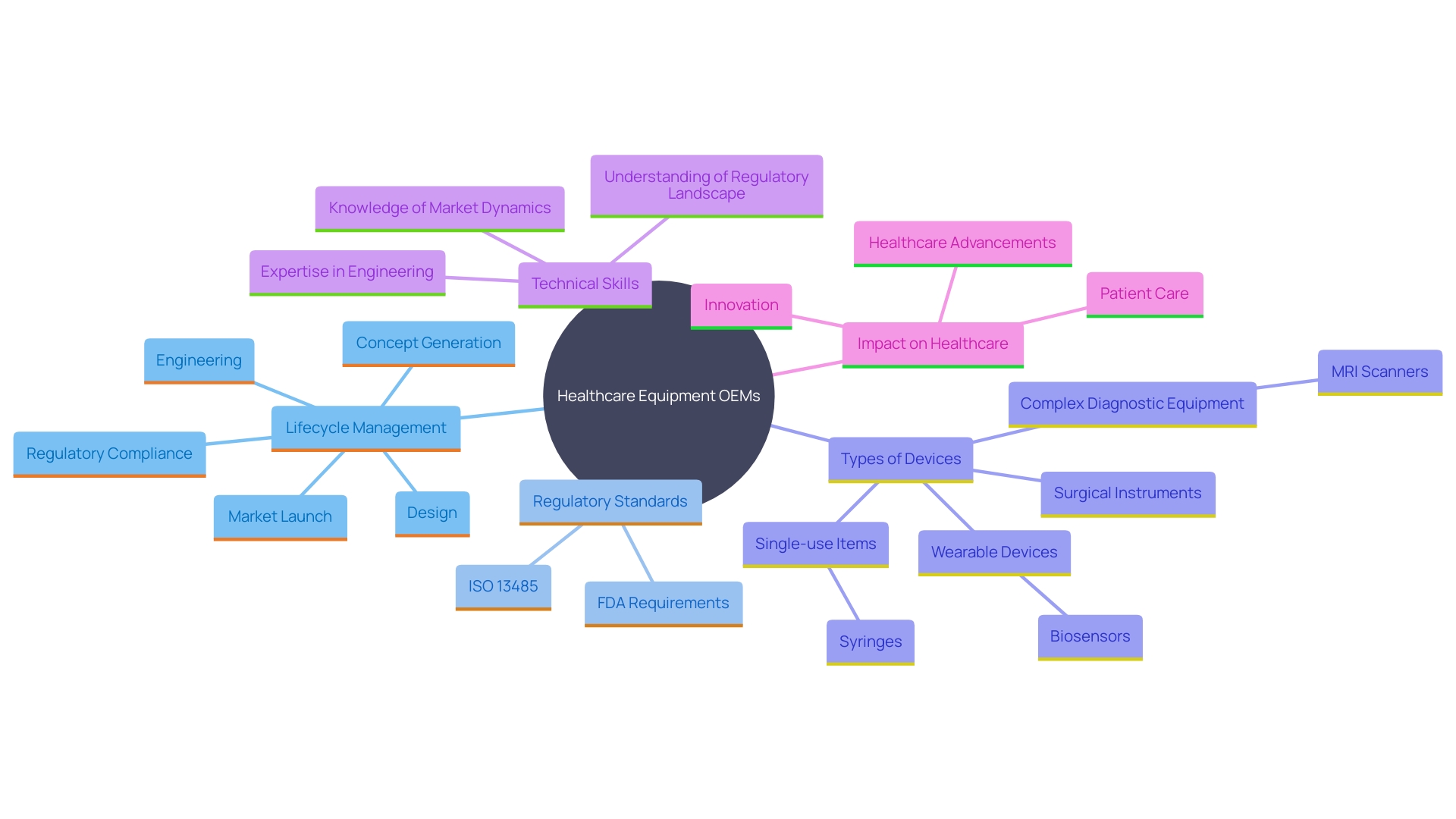
A Medical Device Original Equipment Manufacturer (OEM) is integral to the healthcare ecosystem, specializing in the design, development, and production of medical devices that another company markets under its brand. These OEMs manage the entire lifecycle of medical products, from initial concept generation through to regulatory compliance and market launch. Their role is pivotal in ensuring that devices meet stringent standards such as ISO 13485 and FDA requirements, which is crucial for safety, performance, and quality assurance.
OEMs offer a full spectrum of services, including engineering, prototyping, and final assembly, which ensures a seamless transition from one development phase to the next. This comprehensive approach saves time and money while enhancing the project's overall success. Their technical expertise and deep understanding of regulatory landscapes enable them to create a wide array of medical devices, ranging from simple daily-use items like syringes and bandages to highly complex equipment like MRI scanners and surgical robots.
The diversity of medical devices that OEMs produce reflects the broad spectrum of the healthcare field itself. Their products include diagnostic tools, therapeutic devices, surgical instruments, and patient monitoring systems. By adhering to rigorous regulatory standards, OEMs ensure that their devices are not only innovative but also reliable and safe for patient care.
In a rapidly evolving healthcare landscape, OEMs continue to play a critical role by leveraging advanced technologies and specialized manufacturing capabilities. This allows them to meet the growing demands for high-quality medical devices, ultimately contributing to improved patient outcomes and advancements in medical science.
What is a Medical Device OEM?
A Healthcare Equipment Original Equipment Manufacturer (OEM) is essential to the healthcare ecosystem, specializing in the design, development, and production of healthcare instruments that another company markets under its brand. These original equipment manufacturers oversee the complete lifecycle of medical products, from initial concept creation to regulatory adherence and market introduction. 'Their role is pivotal in ensuring that equipment meets stringent standards such as ISO 13485 and FDA requirements, which is crucial for safety, performance, and quality assurance.'.
Manufacturers provide a complete range of services, including engineering, prototyping, and final assembly, which ensures a seamless transition from one development phase to the next. This comprehensive approach saves time and money while enhancing the project's overall success. Their technical skills and profound knowledge of regulatory environments allow them to produce a diverse range of healthcare tools, from basic everyday items such as syringes and bandages to highly intricate machinery like MRI scanners and surgical robots.
The variety of medical instruments that manufacturers create mirrors the wide range of the healthcare sector itself. Their products encompass diagnostic tools, therapeutic equipment, surgical instruments, and patient monitoring systems. By following strict regulatory standards, manufacturers ensure that their products are not only innovative but also dependable and safe for patient care.
In a rapidly evolving healthcare landscape, original equipment manufacturers continue to play a critical role by leveraging advanced technologies and specialized manufacturing capabilities. This enables them to satisfy the increasing requirements for high-quality healthcare products, ultimately aiding in enhanced patient results and progress in healthcare science.
Role of OEMs in Medical Device Manufacturing
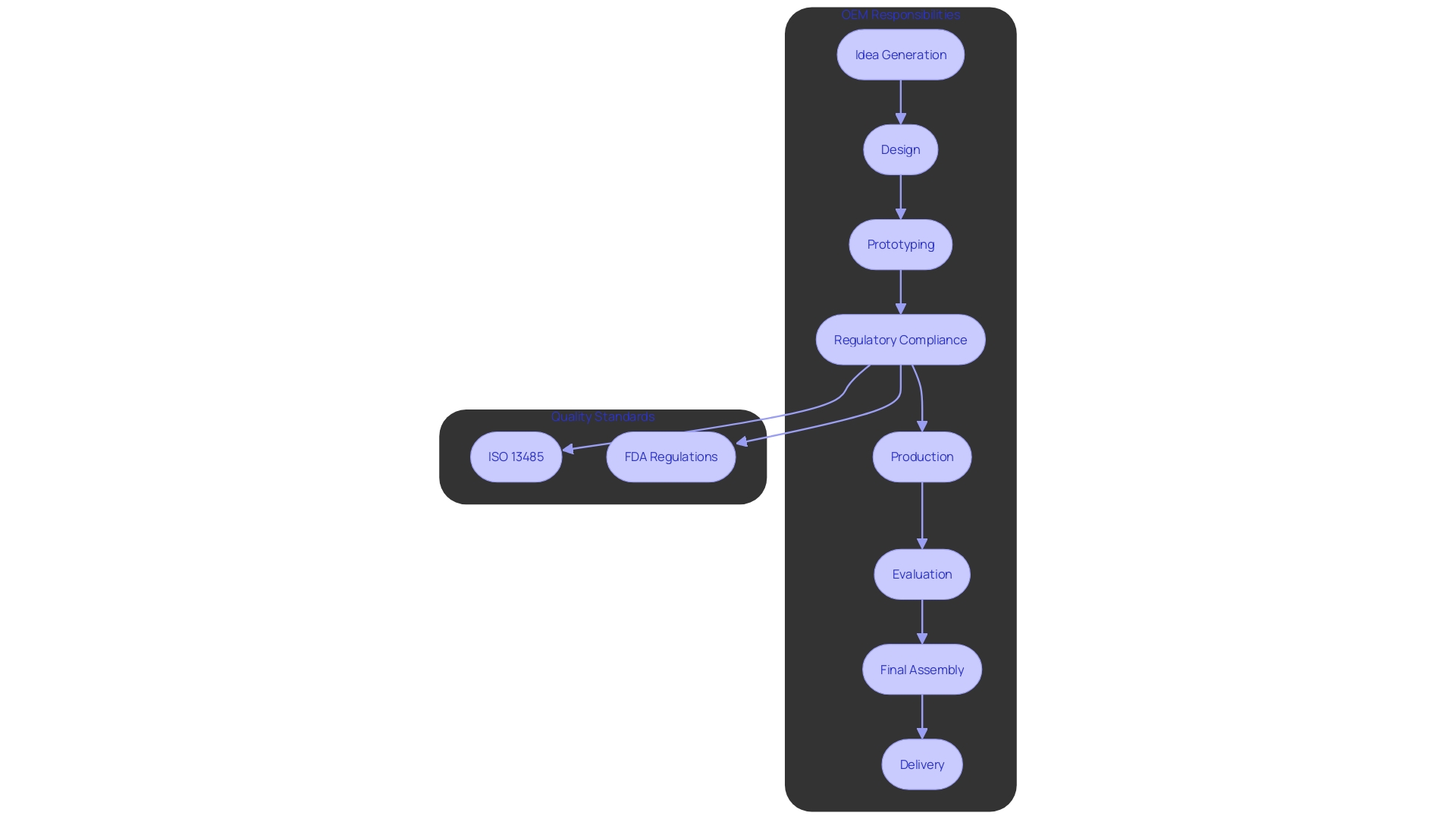
'Original Equipment Manufacturers play a crucial role in the medical apparatus manufacturing process, supplying the essential infrastructure, technical knowledge, and quality control to guarantee effective production and adherence to regulations.'. Manufacturers oversee the complete lifecycle of product creation, from idea and design to production and evaluation, ensuring items adhere to rigorous standards such as ISO 13485 and FDA regulations. This comprehensive approach guarantees high-quality, safe, and reliable products ready for market.
'Manufacturers create a wide variety of devices, including single-use items such as syringes, complex diagnostic equipment like MRI scanners, surgical instruments, and wearable devices like biosensors.'. Their role encompasses prototyping, regulatory compliance, final assembly, and delivery, addressing the diverse needs of the healthcare field.
This partnership enables healthcare firms to concentrate on marketing and distribution while depending on original equipment manufacturers for innovative production capabilities. As Medtronic exemplifies, with a global team of over 95,000 individuals across more than 150 nations, original equipment manufacturers are dedicated to providing technologies that change lives, addressing over 70 health conditions and ensuring improved results for patients around the globe.
Regulatory Compliance and Quality Assurance
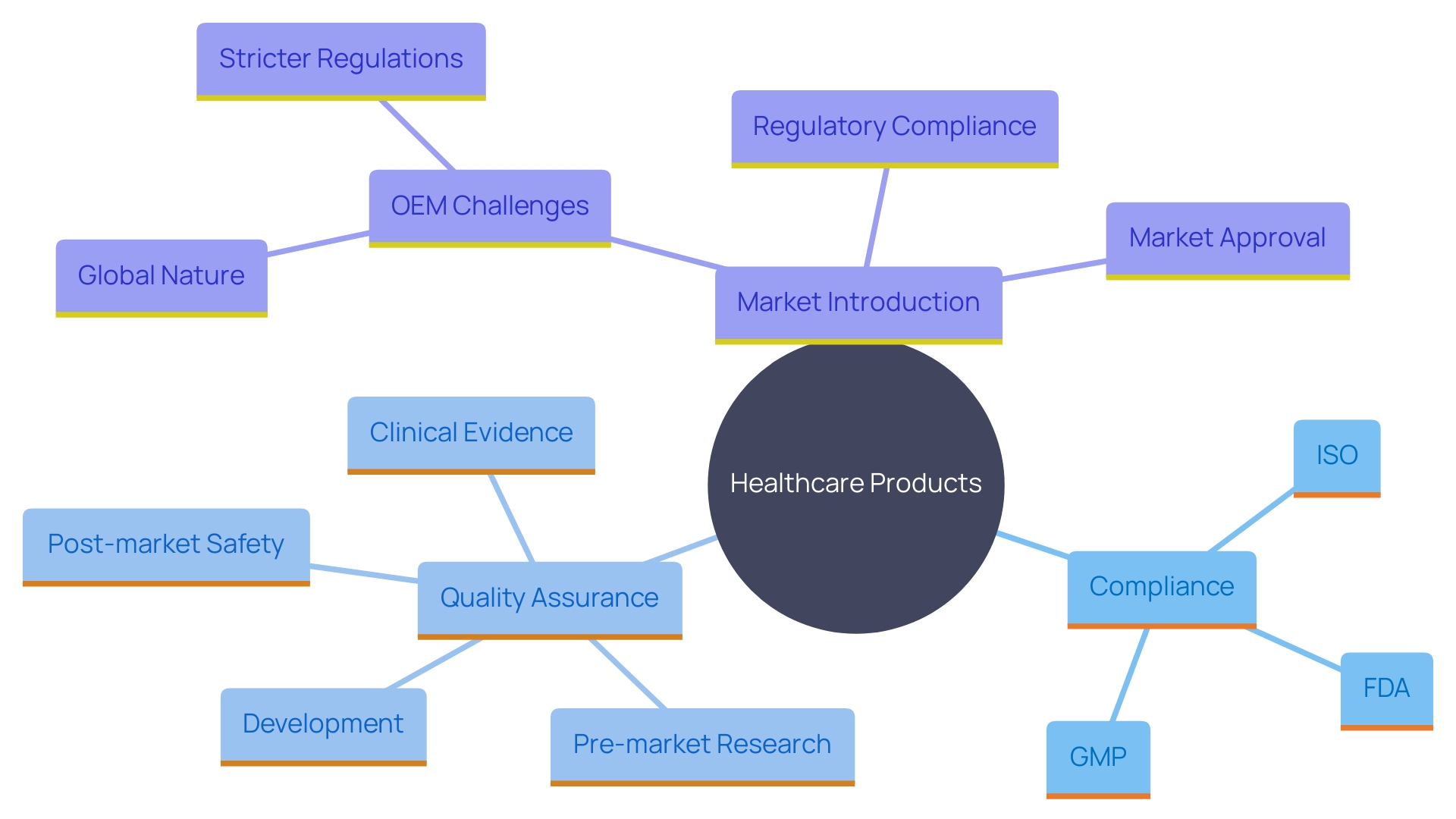
Adhering to guidelines is essential in the healthcare equipment sector, impacting every stage from creation to market launch. OEMs (Original Equipment Manufacturers) must follow strict standards established by governing organizations like the FDA and ISO, ensuring that all products meet the essential safety and effectiveness criteria. This includes following Good Manufacturing Practices (GMP), which provide a framework for consistent quality and compliance with international standards like ISO 13485. GMP is crucial in the qualification process of healthcare instruments, aiming to ensure that every item is validated and tested thoroughly.
The changing environment of compliance requirements greatly influences the creation and market introduction of healthcare products. Industry experts have observed that obtaining market approval for new products and ensuring adherence to governing bodies are becoming more difficult due to stricter legislation and the increasing amount of literature to evaluate. For instance, a recent poll emphasized that 47% of healthcare equipment leaders recognized adherence to governing organizations as a top priority.
Adapting to these changes requires practical approaches to streamline processes and ensure efficiency. Many companies are moving towards more automated validation processes, encouraged by bodies such as the FDA, to reduce manual efforts and improve accuracy. This shift not only satisfies compliance expectations but also boosts productivity and reduces errors.
Quality assurance goes beyond adherence to rules; it is essential for guaranteeing that healthcare tools are safe and effective for patient use. This commitment to quality maintains the integrity of the healthcare system and fosters trust among stakeholders. As James Pink, Senior Director of Medical Technologies at Element Materials Technology, emphasizes, focusing on the next generation of software to support the safety and performance of medical devices is a key priority. His extensive experience underscores the importance of adapting to and thriving amidst changing regulatory landscapes, ensuring both compliance and efficiency.
Benefits of Partnering with OEM Manufacturers
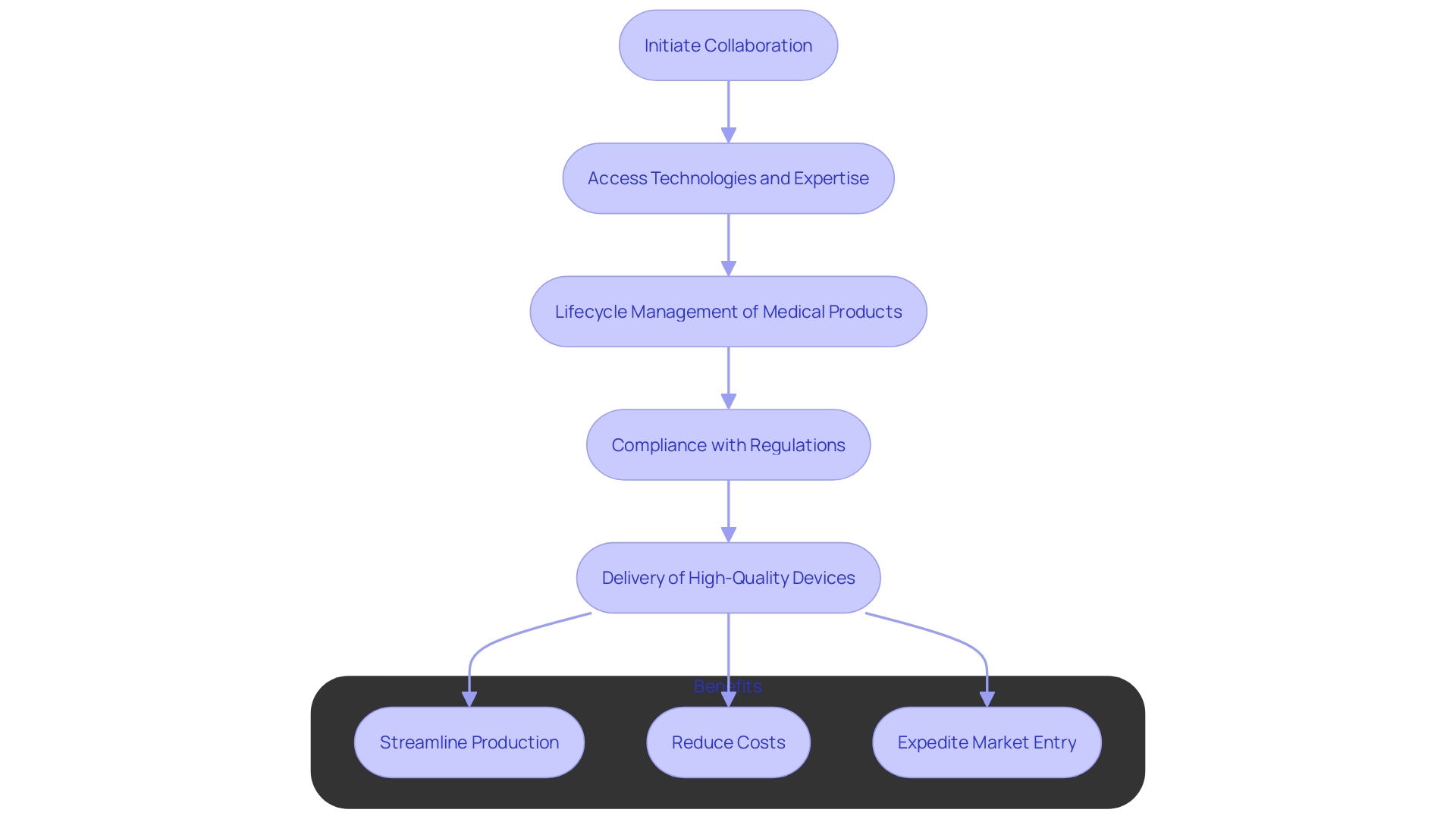
Collaborating with Original Equipment Manufacturers offers significant advantages for healthcare firms. These partnerships provide access to advanced technologies, specialized expertise, and established manufacturing processes, effectively reducing time to market for new healthcare devices. Manufacturers are skilled in overseeing the complete lifecycle of medical products, from prototyping and compliance to final assembly and delivery, which guarantees high-quality, safe, and dependable products.
Furthermore, working together with original equipment manufacturers can result in substantial cost reductions by removing the necessity for large investments required in establishing and upkeeping production sites. OEMs comply with strict guidelines, such as ISO 13485 and FDA requirements, throughout the design and manufacturing process, ensuring that products meet all safety, performance, and quality standards before entering the market. This extensive experience in navigating regulatory landscapes accelerates the approval process for new medical devices, making it easier for healthcare companies to bring their innovations to market quickly and efficiently.
Conclusion
The role of Medical Device Original Equipment Manufacturers (OEMs) is crucial in the healthcare ecosystem, encompassing the design, development, and production of a diverse array of medical devices. By managing the entire lifecycle of these products, OEMs ensure compliance with stringent regulatory standards, such as ISO 13485 and FDA requirements, which are essential for guaranteeing safety, performance, and quality. Their comprehensive services, from engineering to final assembly, streamline the development process and enhance project success.
Partnering with OEMs offers significant advantages for healthcare companies, including access to advanced technologies and specialized expertise. This collaboration not only reduces time to market for new devices but also leads to considerable cost savings by alleviating the need for substantial capital investment in manufacturing facilities. As OEMs are well-versed in navigating complex regulatory landscapes, they facilitate a smoother approval process, enabling healthcare innovators to efficiently introduce their products to the market.
In an industry characterized by rapid advancements and evolving regulatory demands, the importance of OEMs cannot be overstated. Their commitment to quality assurance and adherence to regulatory compliance fosters trust within the healthcare system and ultimately contributes to improved patient outcomes. By leveraging the capabilities of OEMs, healthcare companies can focus on their core competencies while ensuring that they deliver safe, effective, and innovative medical devices to the market.




