Introduction
Endpoints in clinical trials are pivotal for assessing the efficacy and safety of treatments. Primary endpoints, such as hemostatic efficacy, are the primary outcomes that trials aim to evaluate. For instance, in a study involving 263 patients receiving andexanet for intracranial bleeding, the primary endpoints included hemostatic efficacy and the NIH Stroke Scale score.
These endpoints are crucial for determining whether a treatment meets its primary objectives.
Secondary endpoints provide broader insights into the treatment's effects. They allow researchers to explore additional benefits or adverse effects not captured by primary endpoints. For example, in the OCEANIC AF trial, while the primary focus was on stroke risk reduction in patients with atrial fibrillation, secondary endpoints would help understand other impacts of the treatment.
Understanding the distinction between primary and secondary endpoints is essential for interpreting study results accurately. It ensures that all relevant data are considered, providing a comprehensive evaluation of a treatment's effectiveness and safety.
Primary vs. Secondary Endpoints
Endpoints in clinical studies are pivotal for assessing the efficacy and safety of treatments. Main objectives, such as hemostatic efficacy, are the main results that trials aim to assess. 'For instance, in a study involving 263 patients receiving andexanet for intracranial bleeding, the primary goals included hemostatic efficacy and the NIH Stroke Scale score.'. These points are crucial for determining whether a treatment meets its primary objectives.
Additional measures offer wider perspectives on the treatment's effects. They permit researchers to investigate extra advantages or negative outcomes not recorded by main measures. For instance, in the OCEANIC AF research, while the main emphasis was on minimizing stroke risk in individuals with atrial fibrillation, additional measures would aid in comprehending other effects of the treatment.
Grasping the difference between primary and secondary objectives is crucial for interpreting study outcomes accurately. It ensures that all relevant data are considered, providing a comprehensive evaluation of a treatment's effectiveness and safety.
Role of Secondary Endpoints
'Secondary objectives are essential in clinical studies as they offer a complete insight into a treatment's effects beyond the primary measures.'. These endpoints help in identifying additional benefits and potential side effects, offering valuable insights into the overall quality of life of patients. For example, experimental emulation can be utilized to broaden the range of discoveries by using observational or simulated research to assess the effectiveness of treatments. This approach allows researchers to gather supplementary data that reinforces the results of traditional clinical trials.
Additionally, addressing alternative objectives contributes to a more comprehensive analysis of the disease or condition being examined. Researchers must ensure transparency and regulatory compliance when reporting these measures, as any methodological changes or outcome switching should be declared openly. By analyzing secondary measures, researchers can support primary findings, enhance the understanding of the treatment's effectiveness, and influence clinical decision-making processes.
Types of Secondary Endpoints
Secondary goals play a pivotal role in clinical trials, encompassing a broad spectrum of measures that extend beyond primary objectives. These can encompass safety measures, which meticulously monitor adverse effects of a treatment, and exploratory targets that delve into different mechanisms of action or specific patient subgroups. Moreover, important additional goals often include quality of life evaluations, biomarkers, and time-to-event results. The strategic choice of these targets is vital as they substantially enrich the dataset, offering a comprehensive understanding of the treatment's impact. For example, a recent study sponsored by Innovate UK emphasized the significance of alternative measures in assessing mental health changes in caregivers, illustrating their ability to uncover detailed insights that primary measures might miss. Moreover, openness in documenting these objectives is essential, following regulatory and ethical standards to uphold the integrity and reliability of the research results.
Multiplicity and Statistical Considerations
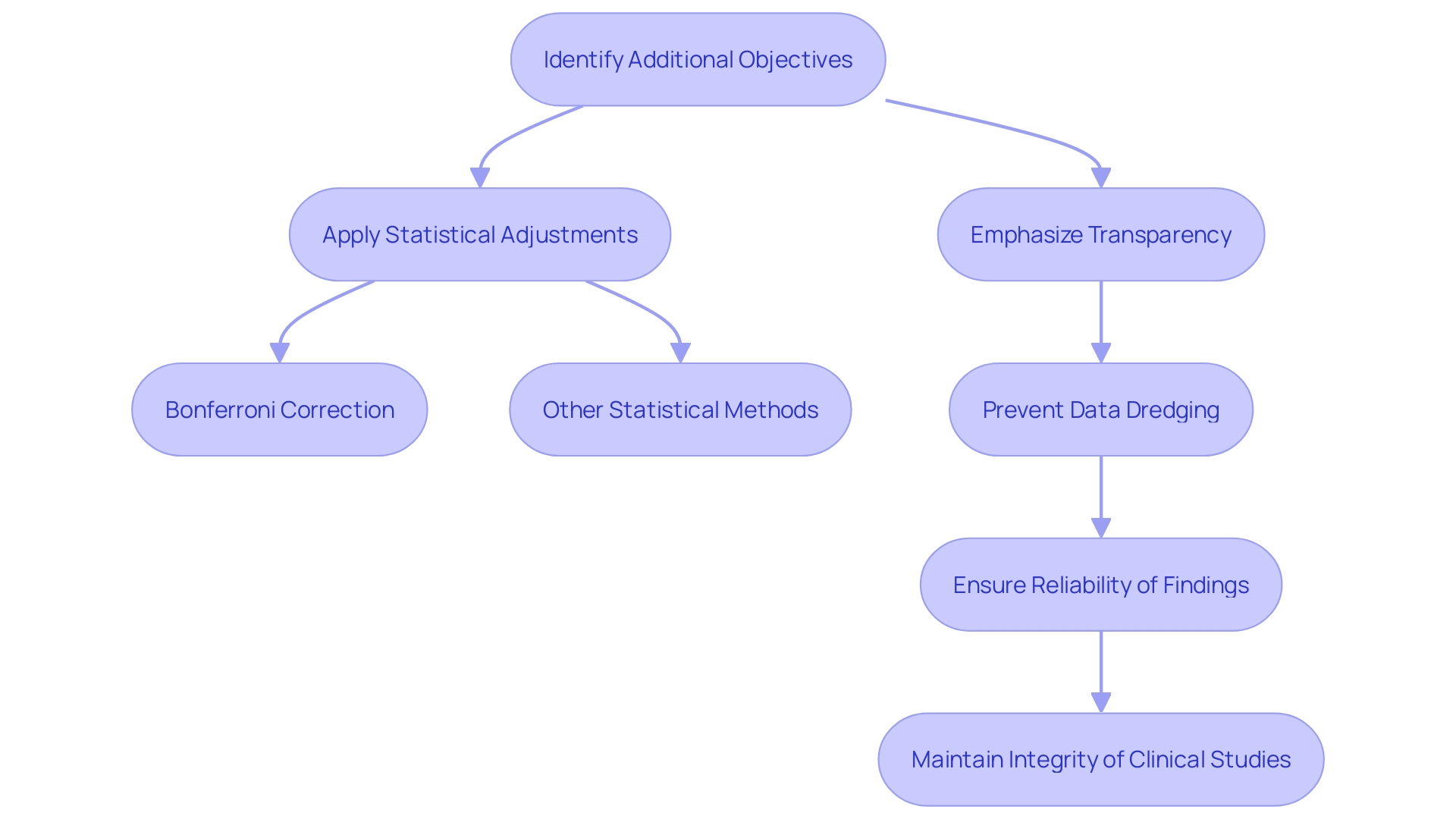
Including additional objectives in clinical trials requires careful consideration of multiplicity, which is the risk of heightened type I error rates when evaluating multiple hypotheses. This can compromise the reliability of research results if not properly managed. To mitigate this risk, statistical adjustments such as Bonferroni correction or false discovery rate procedures are essential. These adjustments help maintain the validity of the findings by controlling the overall type I error rate.
Establishing additional objectives and the statistical techniques for their examination is essential for maintaining the research's integrity. This practice ensures transparency and helps prevent data dredging or selective reporting, which can skew results. The FDA emphasizes the importance of well-designed studies and reliable data, as these are critical for informed decision-making about the benefits and risks of medical products. By adhering to these principles, researchers can contribute to the generation of high-quality evidence that informs clinical practice and improves patient outcomes.
Interpreting Secondary Endpoint Results
Analyzing outcomes from secondary measures requires a nuanced approach. While these endpoints can yield valuable insights, they are inherently exploratory unless pre-specified and sufficiently powered. Researchers must exercise caution in drawing conclusions from these results, particularly when they were not the main focus.
To ensure scientific rigor, it is essential to contextualize additional findings within the broader scope of the clinical study. Real-world evidence studies, for instance, often rely on secondary data generated by the routine operations of healthcare systems. These data sources can introduce unavoidable idiosyncrasies, such as algorithm underperformance, which can lead to misinterpretations if not properly explored and adapted. For example, an initial algorithm might underestimate the prevalence of a condition, alerting researchers to the need for modifications to ensure accuracy.
Furthermore, the new European Good Clinical Practice (GCP) guidance emphasizes the importance of data governance, including processes to protect participant data, manage computer systems, and support key decision-making activities. This updated guidance aims to enhance the reliability of clinical study results and underscores the need for careful evaluation of scientific objectives and associated risks.
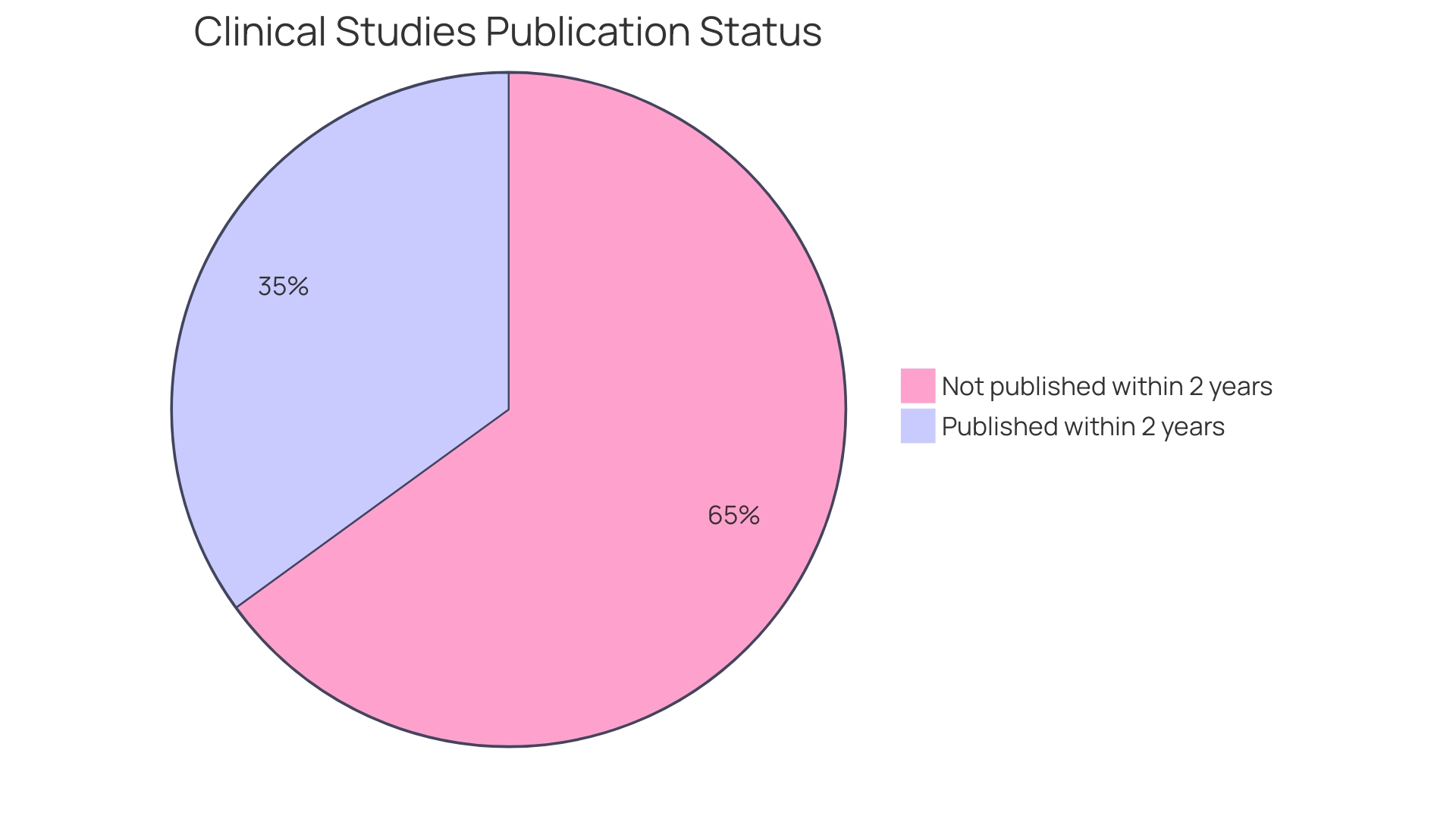
The 2008 Declaration of Helsinki and the World Health Organization's 2015 Statement on Public Disclosure of Clinical Trial Results also highlight the importance of transparency and publication, regardless of the statistical significance of outcomes. Despite these guidelines, publication bias continues to be a significant issue, with only 39% of clinical studies in German university medical centers being published within two years of completion between 2009 and 2013. This highlights the importance for researchers to not only perform thorough analyses but also guarantee that all results, including those from additional measures, are accurately reported and publicly available.
Challenges and Best Practices for Secondary Endpoints
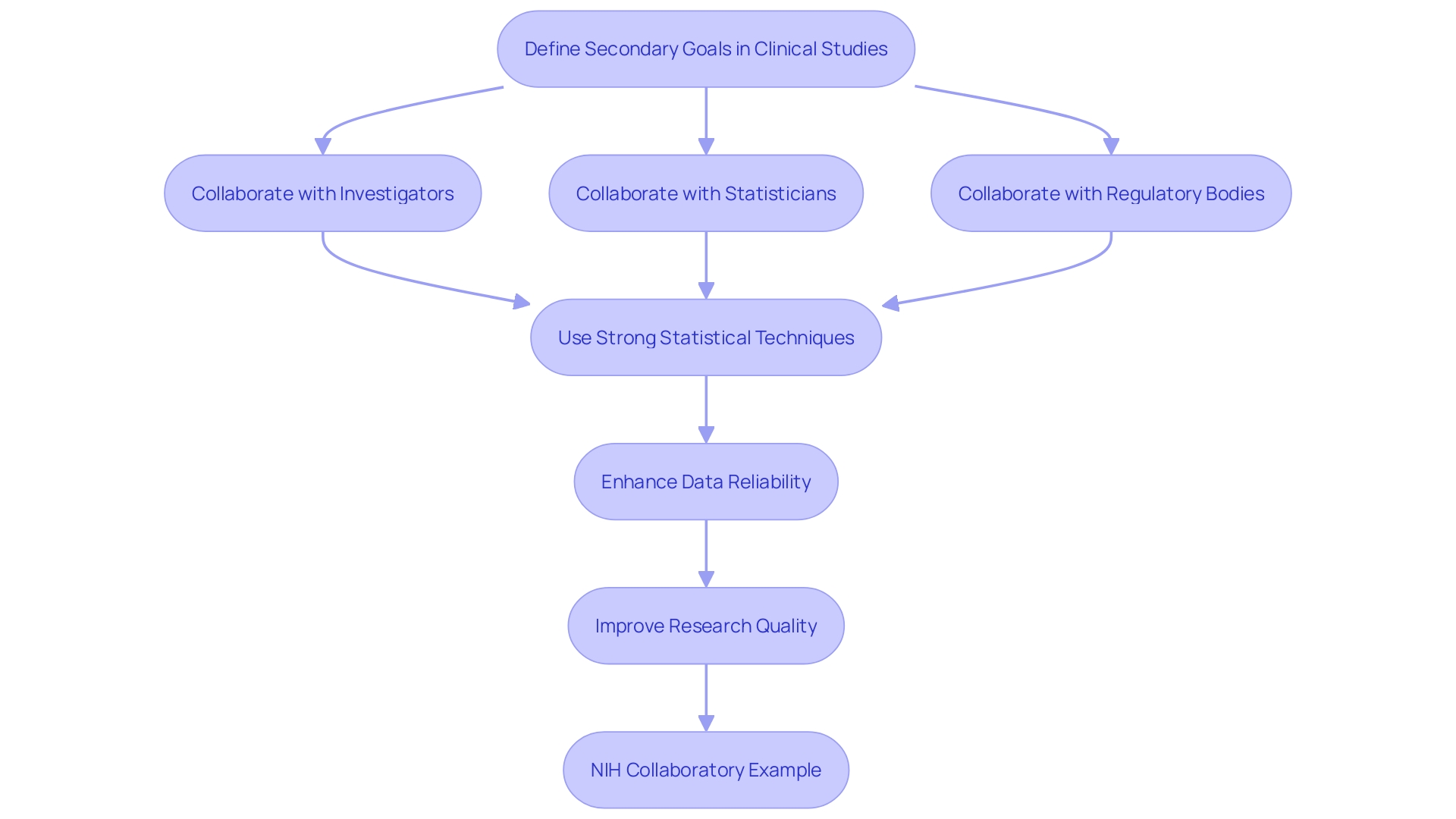
The application of alternative measures in clinical studies introduces multiple difficulties, such as the danger of overinterpretation and the intricacies of data analysis. To reduce these risks, it is essential to clearly define secondary goals at the outset. 'Utilizing strong statistical techniques, like those described in the PRINCIPLED process for inferential research, can enhance the reliability of data analysis.'. Making certain that all outcomes are understood in relation to the main objective is essential for preserving the validity of the research. Collaboration among investigators, statisticians, and regulatory bodies can enhance the integration of secondary endpoints into the trial design and analysis, ultimately improving the quality of the research. This collaborative approach is exemplified by the NIH Collaboratory, which has contributed significantly to the peer-reviewed literature with insights from various core working groups and study designs, highlighting the importance of effective teamwork in clinical research.
Conclusion
The examination of endpoints in clinical trials reveals their critical role in evaluating treatment efficacy and safety. Primary endpoints, such as hemostatic efficacy, serve as the main focus of these studies, providing essential outcomes that determine whether a treatment meets its primary objectives. In contrast, secondary endpoints offer a broader perspective, capturing additional benefits and potential side effects that may not be evident through primary endpoints alone.
This distinction is vital for a comprehensive understanding of a treatment's overall impact.
Secondary endpoints significantly enrich clinical trial data by revealing insights into various aspects of patient care, including quality of life and safety. However, their interpretation requires caution, especially when results are exploratory in nature. The importance of pre-specifying secondary endpoints and employing appropriate statistical adjustments cannot be overstated, as these practices help maintain the integrity of the study and mitigate risks associated with multiplicity.
Transparency in reporting and adherence to regulatory standards are essential for ensuring that all findings contribute meaningfully to clinical decision-making.
The challenges associated with secondary endpoints, such as the potential for overinterpretation and complexities in data analysis, necessitate clear definitions and robust methodologies. Collaborative efforts among researchers, statisticians, and regulatory bodies can enhance the integration of secondary endpoints into trial design, ultimately leading to higher quality research outcomes. By embracing these best practices, researchers can better inform clinical practice and improve patient outcomes, ensuring that both primary and secondary endpoints are effectively utilized in the pursuit of medical advancements.




