Introduction
The 510(k) clearance process provides a streamlined pathway for medical device manufacturers to bring new products to market by demonstrating substantial equivalence to a legally marketed device in the United States. This submission, required by the FDA before marketing, ensures that new devices meet safety and effectiveness standards. It's important to first determine the correct classification of the device, which falls into one of three FDA classifications based on patient risk.
Once classified, the appropriate registration pathway—such as the Premarket Notification (510(k)), Pre-Market Approval (PMA), or De Novo process—must be chosen.
Interestingly, the 510(k) process has been scrutinized for allowing some devices to bypass clinical trials, as highlighted in the 2018 documentary 'The Bleeding Edge.' This fast-tracking has led to concerns over patient safety, with some devices causing injuries such as bleeding and organ puncture. Despite these criticisms, the FDA continues to enhance its regulatory processes to balance safety with innovation.
For instance, a recent report shows that more than half of the 15 new decision summaries posted for De Novos in August 2023 were from this year, showcasing a commitment to timely updates and transparency in medical device regulation.
What is 510(k) Clearance?
The 510(k) clearance process offers an efficient route for medical product manufacturers to introduce new offerings to the market by showing substantial equivalence to a legally marketed item in the United States. 'This submission, required by the FDA before marketing, ensures that new products meet safety and effectiveness standards.'. It's important to first determine the correct classification of the apparatus, which falls into one of three FDA classifications based on patient risk. Once classified, the appropriate registration pathway—such as the Premarket Notification (510(k)), Premarket Approval (PMA), or De Novo process—must be chosen.
'Interestingly, the 510(k) process has been scrutinized for allowing some products to bypass clinical trials, as highlighted in the 2018 documentary 'The Bleeding Edge.' 'This fast-tracking has led to concerns over patient safety, with some equipment causing injuries such as bleeding and organ puncture.'. Despite these criticisms, the FDA continues to enhance its regulatory processes to balance safety with innovation. For instance, a recent report reveals that over fifty percent of the 15 new decision summaries posted for De Novo in August 2023 were from this year, highlighting a commitment to timely updates and transparency in medical equipment regulation.
Eligibility Criteria for 510(k) Clearance
To qualify for 510(k) clearance, an item must meet specific criteria set by the FDA. Firstly, it must be intended for human use and fall under Class I or II, as Class III items require Pre-Market Approval (PMA). 'Secondly, the apparatus must show significant similarity to a reference instrument, indicating it should possess the same intended application and technological features. This often involves rigorous comparison through research literature, clinical studies, and examining the Summaries of Safety and Effectiveness (SSEs) available on the FDA’s 510(k) database. Comprehending the competitive environment and recognizing possible reference items are essential phases in this process. Ensuring adherence to these standards is crucial for the prompt and effective approval of medical equipment.
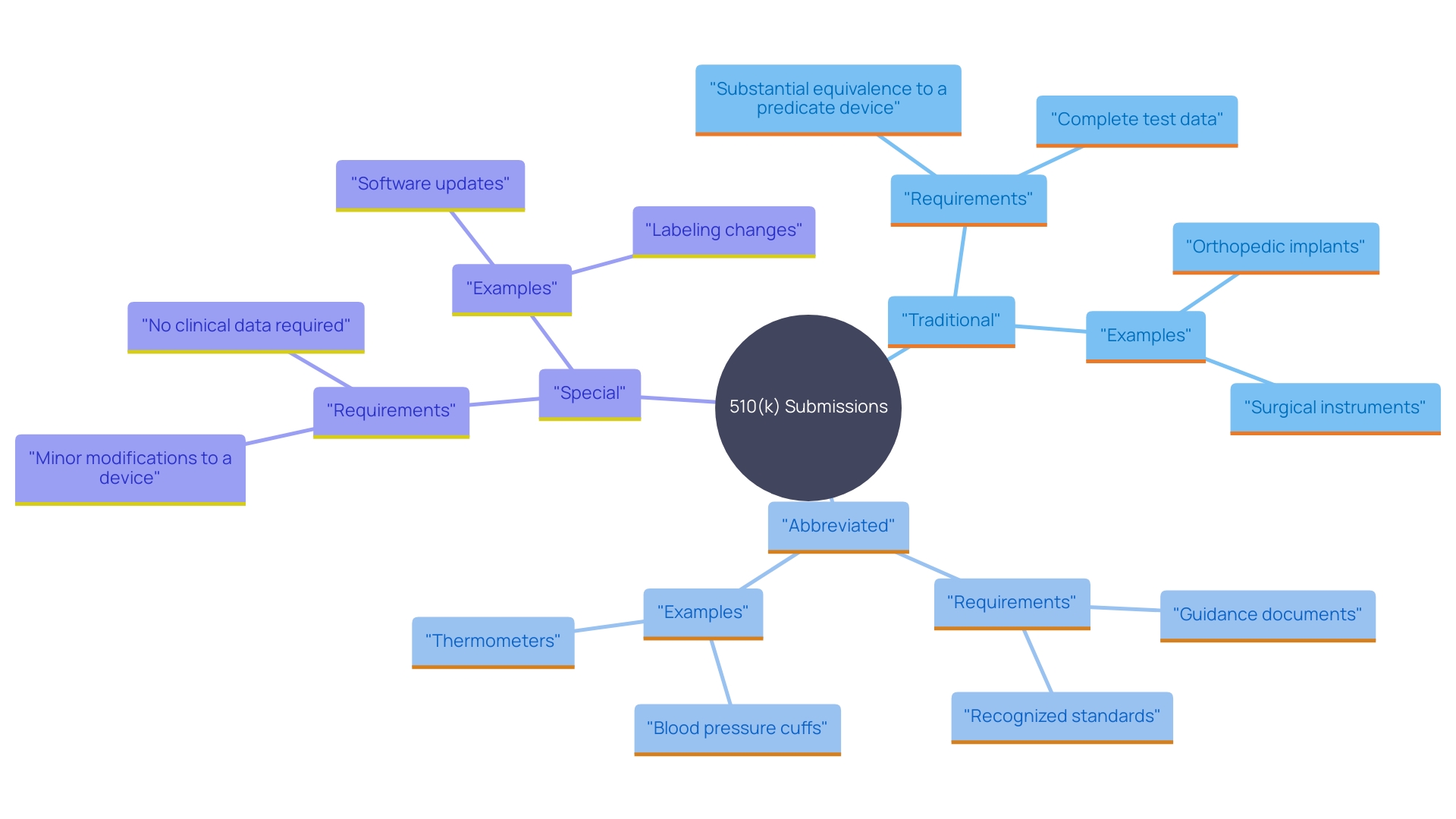
Types of 510(k) Submissions
The FDA recognizes three primary types of 510(k) submissions: Traditional, Abbreviated, and Special. Traditional 510(k) submissions are the most common and require comprehensive data to demonstrate that the new item is substantially equivalent to a legally marketed reference product. This includes detailed clinical and non-clinical data, such as bench performance testing and biocompatibility testing, to establish safety and effectiveness. For example, the FDA's recent draft guidance emphasizes the need for clinical data in certain scenarios to prove substantial equivalence.
Abbreviated 510(k) submissions are utilized when the item's substantial equivalence is supported by existing data, including conformity to recognized consensus standards. These standards, developed by Standards Development Organizations (SDOs), ensure regulatory quality by adhering to principles of transparency and stakeholder participation. The FDA's Federal Register Documents webpage provides a historical record of all recognition determinations, making it easier for manufacturers to identify applicable standards.
Special 510(k) submissions are intended for products that have undergone modifications but still maintain substantial equivalence to a reference product. This pathway is especially beneficial for minor modifications that do not influence the intended purpose or fundamental technology. The FDA's multipronged effort to modernize the 510(k) program includes scenarios where clinical data may be necessary to demonstrate equivalence, especially for products with technological differences from their predicates. By understanding the different submission types and their requirements, manufacturers can better navigate the 510(k) process and ensure compliance with FDA regulations.
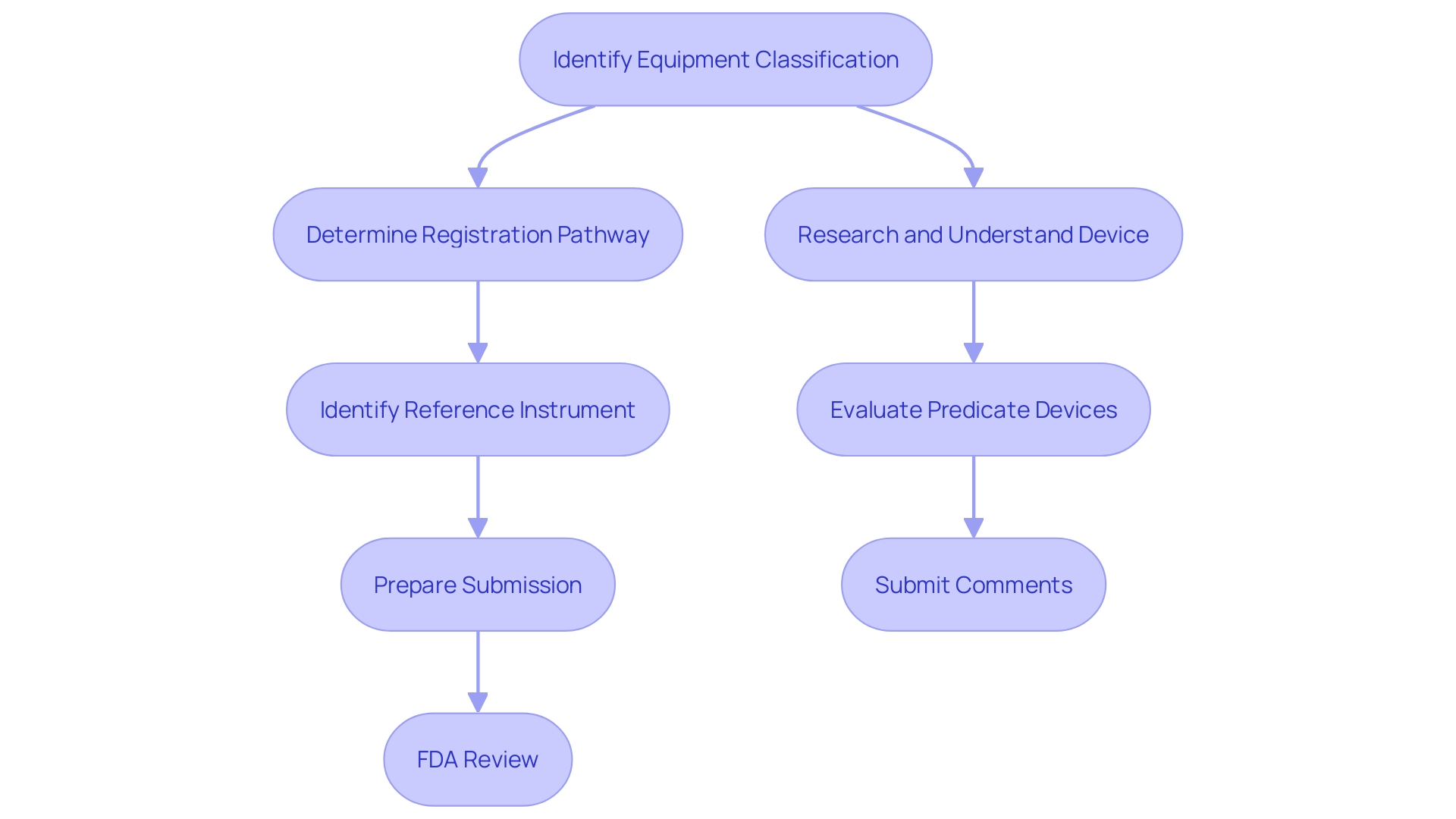
Step-by-Step Process for Obtaining 510(k) Clearance
The process for obtaining 510(k) clearance involves several critical steps. First, identify the appropriate classification of the equipment, as the FDA uses three levels of classifications for medical tools, each carrying a different patient risk value. Next, determine the proper registration pathway—Premarket Notification (510(k)), Premarket Approval (PMA), or De Novo process. This is crucial because before you can legally market your product in the US, it must be FDA Cleared, Approved, or Granted, depending on the pathway.
Once the classification and pathway are established, the next step is to identify a reference instrument. This involves understanding the intended use and technological characteristics of your equipment and comparing them to existing models. Employ resources including research literature, clinical studies, websites, brochures, and labeling to identify potential comparable products, and construct a comparative table to record similarities and differences.
After identifying the predicate product, you must prepare the 510(k) submission. This includes gathering detailed information about the gadget, its intended use, technological characteristics, and compliance with applicable standards. Pay the necessary FDA fees and submit the application.
Once submitted, the FDA reviews the application, ensuring that it meets all regulatory requirements. The FDA may request additional information or clarification to address any concerns. Understanding the evolving landscape of regulatory demands and practical approaches used by industry professionals can enhance the efficiency of this process.
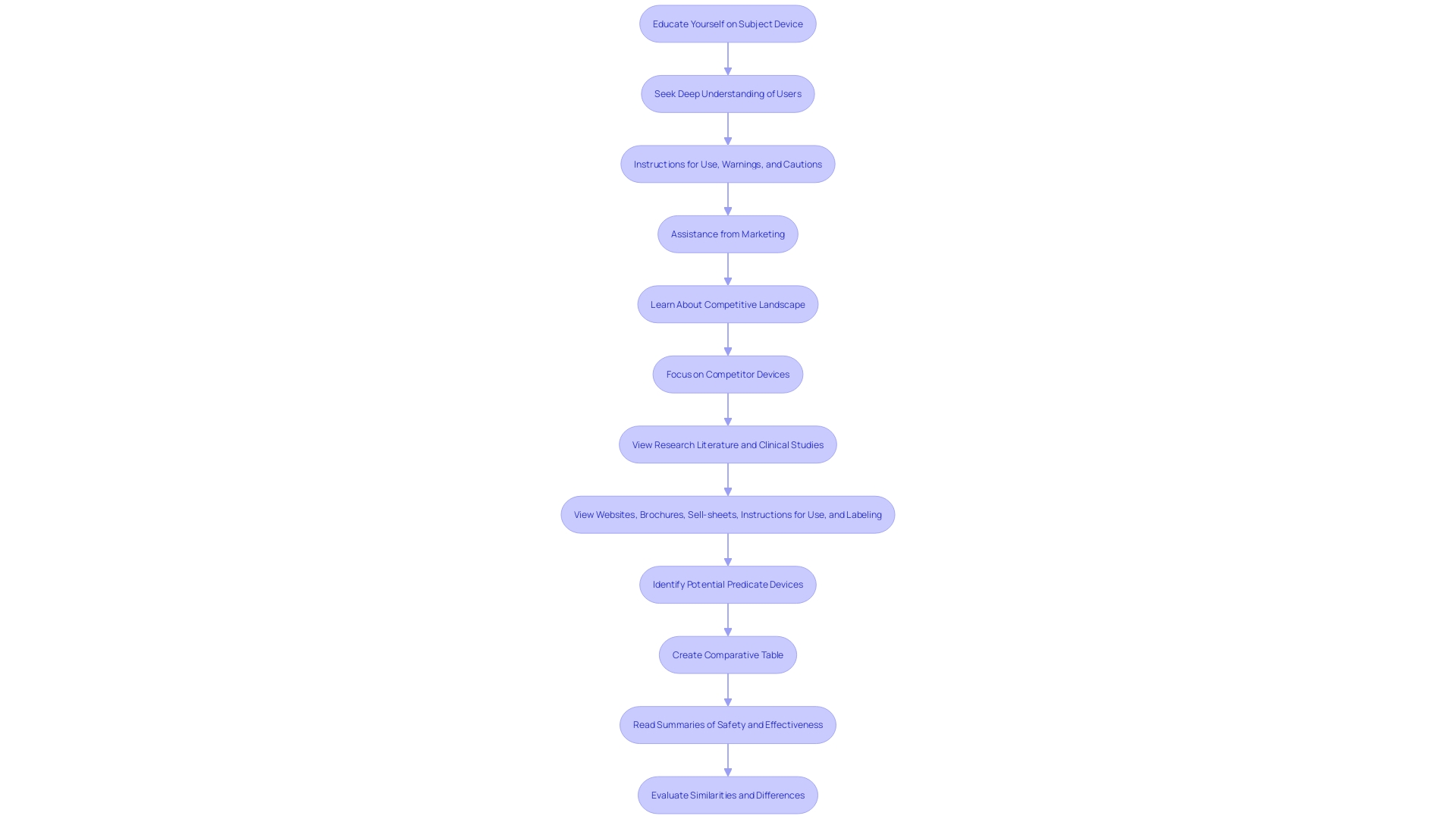
Learning about the topic tool and its purpose is essential. Aim to seek a deep understanding of users—clinicians, physicians, dentists, patients, etc.—and the instructions for use, including warnings and cautions. Work together with Marketing to understand the competitive environment and concentrate on rival products. 'Examining materials like Summaries of Safety and Effectiveness (SSEs) accessible on FDA’s 510(k) database can also offer valuable insights into the similarities and differences between your product and the predicate products.'.
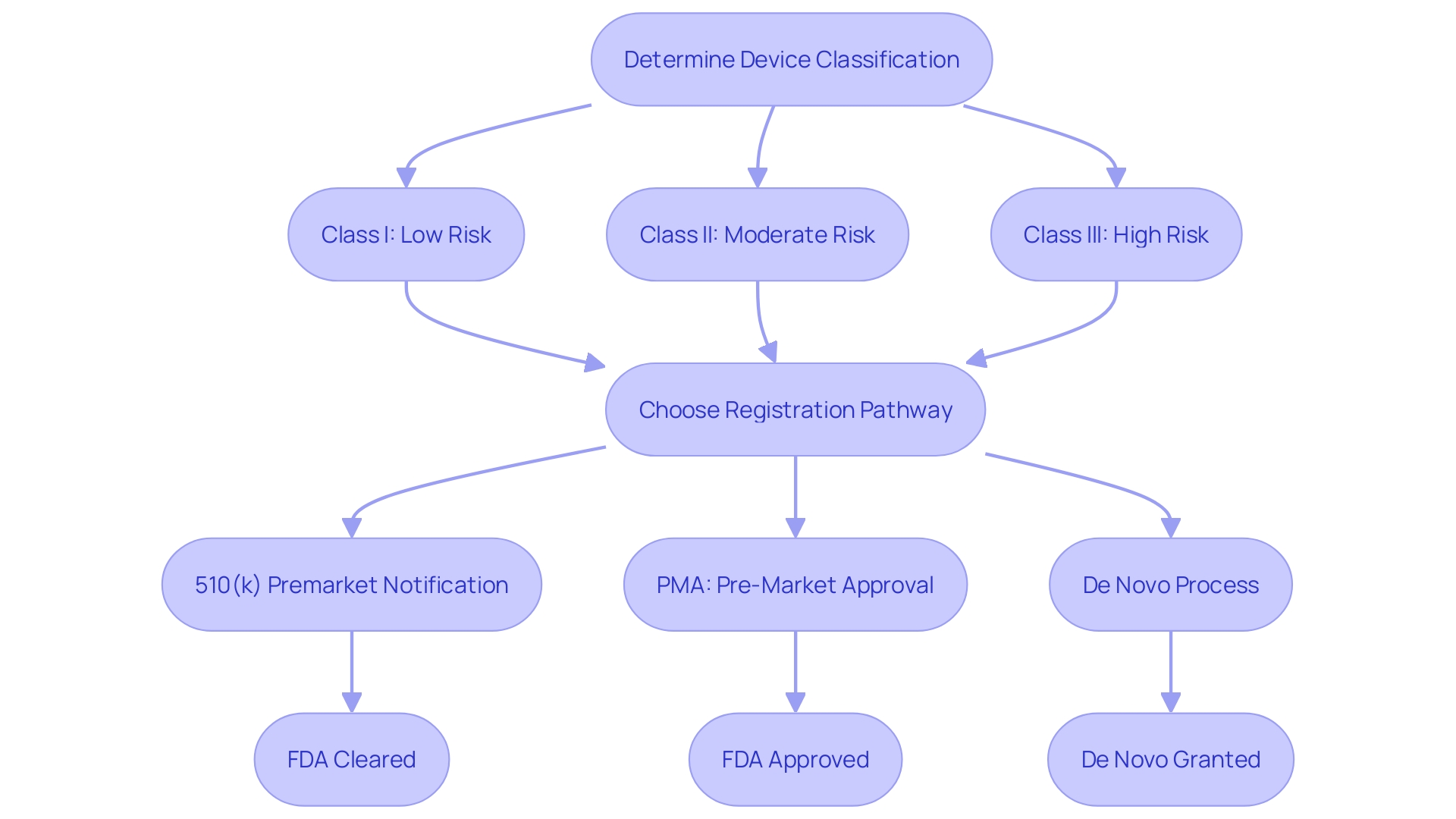
Determining Device Class and Submission Type
Comprehending the categorization of a medical instrument is a fundamental step in determining the appropriate regulatory pathway for FDA approval. Medical instruments are classified into three categories according to their risk level and the extent of regulatory oversight required to guarantee safety and efficacy.
-
Class I products are regarded as low risk and are typically exempt from premarket notification requirements. These tools consist of items such as bandages and handheld surgical instruments. 'The regulatory oversight for Class I items is minimal, concentrating on general controls to ensure safety.'.
-
Class II items present a moderate risk to patients and typically require a 510(k) submission. The 510(k) process involves demonstrating that the new product is substantially equivalent to a legally marketed item. 'Examples of Class II items include powered wheelchairs and infusion pumps.'. The FDA's acknowledgment of voluntary consensus standards, as mentioned in the Federal Register, plays a crucial role in the conformity evaluation of these items.
-
Class III items are high-risk products that support or sustain human life or present a potential unreasonable risk of illness or injury. These instruments require a more rigorous Premarket Approval (PMA) process, which includes providing valid scientific evidence of their safety and effectiveness. Examples include implantable pacemakers and brain-computer interface systems. According to the FDA's final guidance issued on May 20, 2021, for implanted brain-computer interface systems, non-clinical testing and clinical considerations are essential for PMA submissions.
To ascertain the categorization of a specific instrument, one can consult the FDA's classification database or 21 CFR Parts 862-892, which organizes over 1,700 distinct types of equipment by medical specialty. Once the correct classification is identified, choosing the proper registration pathway—510(k), PMA, or De Novo process—becomes clear. Ensuring adherence to the appropriate standards and regulatory requirements is critical for bringing a medical product to market successfully.
Identifying Predicate Devices
To establish substantial equivalence, manufacturers must identify a reference apparatus that has already received FDA approval. This classification tool should have comparable intended use and technological features to the new item being submitted. The process starts by confirming that the potential item is a legally marketed product, currently registered with the FDA.
It is crucial to conduct a thorough investigation into the competitive landscape. This includes reviewing research literature, clinical studies, websites, brochures, and other relevant materials. Working together with marketing teams can offer insights into rival products. Constructing a comparative table can assist in recognizing potential terms with the same intended use and similar technological characteristics.
The FDA defines substantial equivalence as a product having the same intended use as the reference product and either the same technological characteristics or, if different, sufficient data to demonstrate that the product is as safe and effective as the reference. This ensures that any differing technological characteristics do not pose new questions of safety and effectiveness.
The FDA has also established optimal methods for choosing a reference product, highlighting the use of FDA-recognized consensus standards, guidance documents, and qualified medical product development tools. For instance, the agency’s draft guidance document released on September 7, 2023, highlights the importance of using well-established methods and ensuring that the chosen criteria have a robust history of safety data.
By following these guidelines and thoroughly researching potential predicate products, manufacturers can enhance their 510(k) submissions and boost the chances of FDA approval.
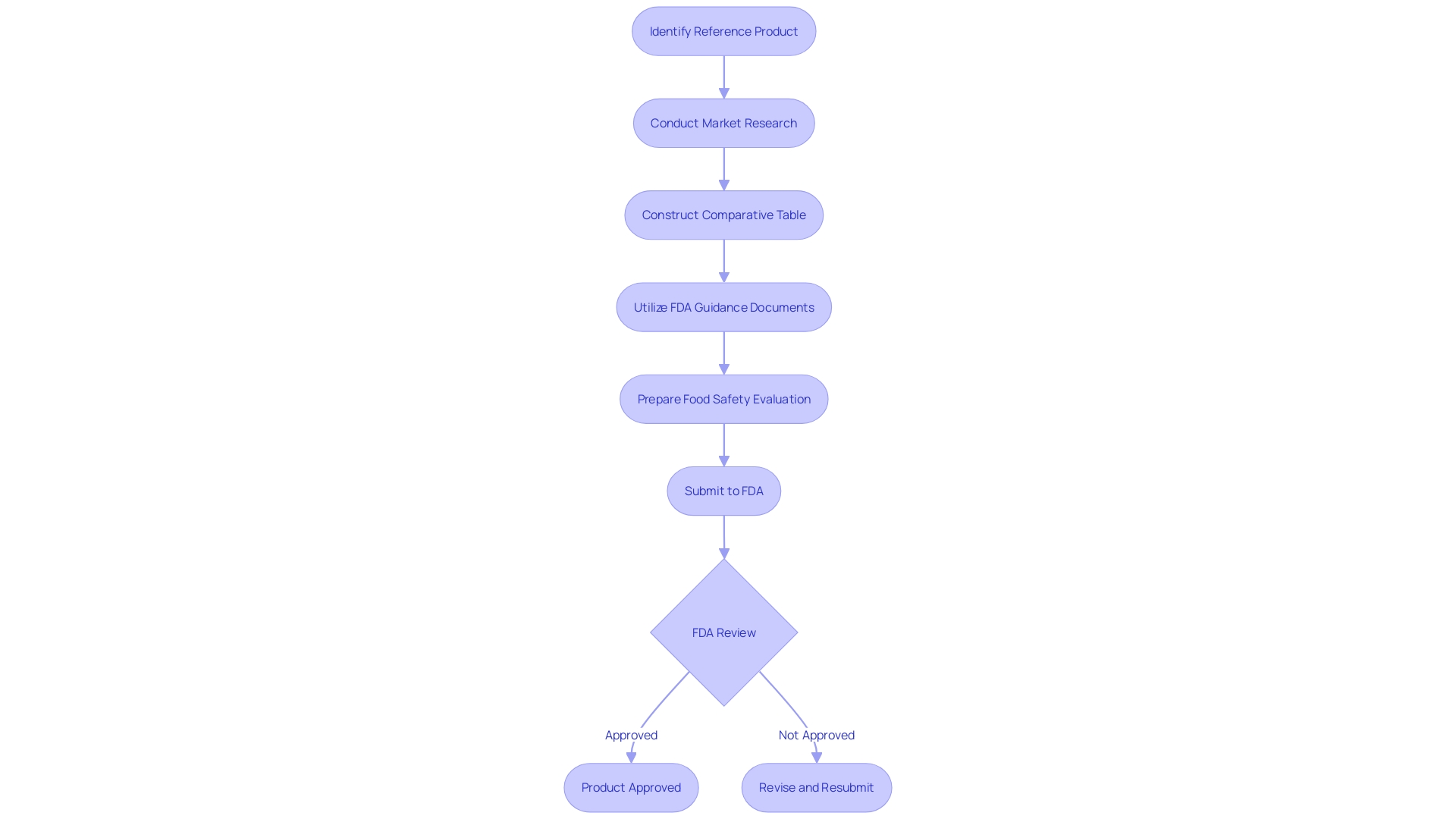
Preparing the 510(k) File
Preparing the 510(k) file involves compiling comprehensive documentation to support the substantial equivalence claim. This process begins with a thorough comprehension of the subject tool and its intended users, which may include clinicians, physicians, dentists, and patients. Thorough explanations of the apparatus, including guidelines for operation, alerts, and precautions, are vital. Working together with the marketing team can offer insights into the competitive environment, assisting in concentrating on rival products. Examining research literature, clinical studies, websites, brochures, sell-sheets, and labeling aids in recognizing potential predicate items with comparable intended uses and technological features. Creating a comparative table to evaluate these similarities and differences is a crucial step. Furthermore, reading the Summaries of Safety and Effectiveness (SSEs) available on the FDA's 510(k) database can provide additional insights. Ensuring that all performance testing results and other relevant data are accurately documented is vital for a successful submission.
Paying FDA Review Fees
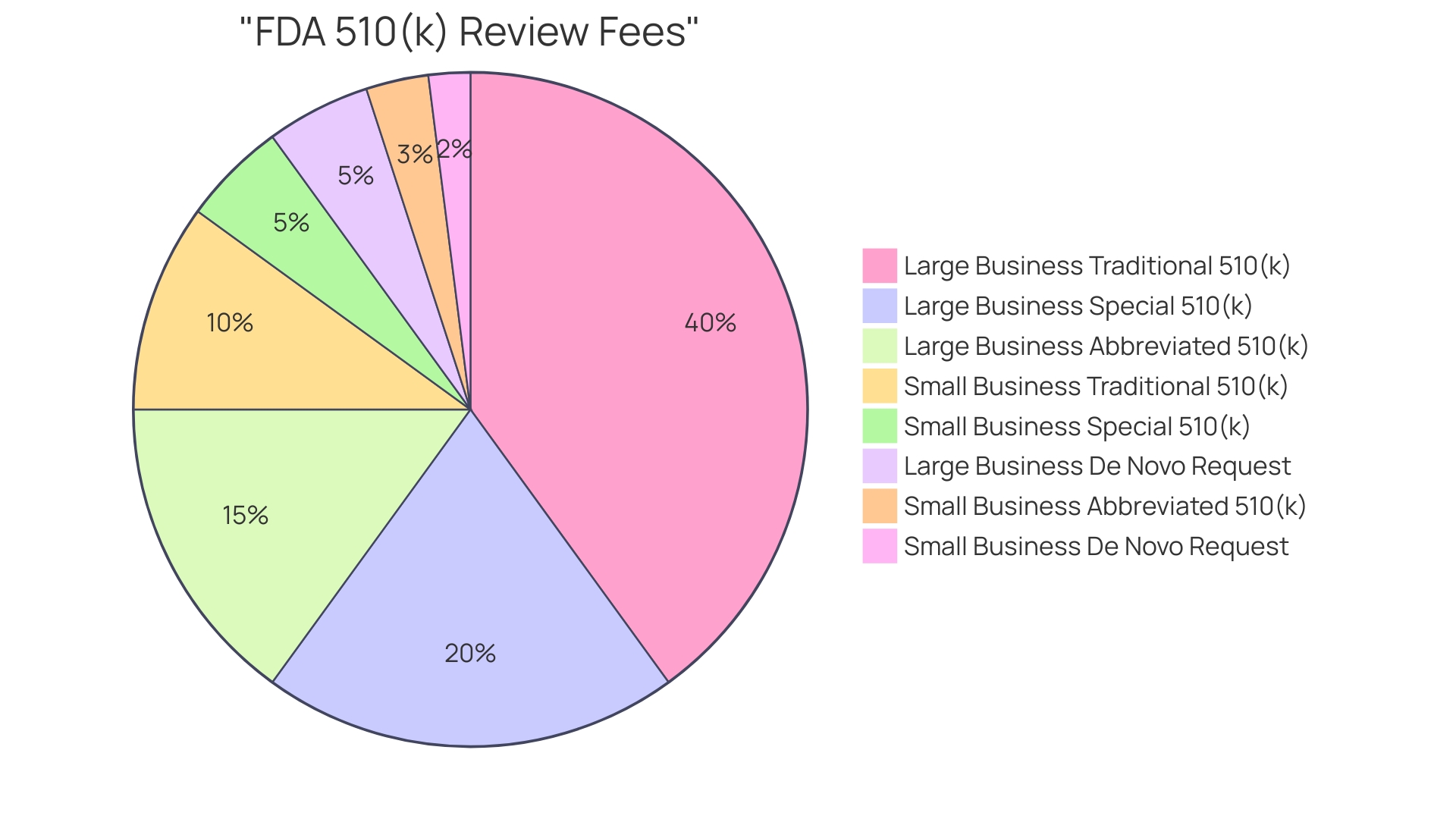
The FDA imposes fees for reviewing 510(k) submissions, which are essential for the evaluation and approval of medical products. These fees differ based on the manufacturer's status as a small business and the type of submission made. 'For small enterprises, the charges are significantly lowered, offering financial support and fostering innovation in the medical equipment sector.'. It is crucial to consult the current fee schedule on the FDA's official website to ensure accuracy and compliance. Submitting the correct fee along with the application is mandatory to avoid delays in the review process. The FDA continuously updates these fees, reflecting the most recent reauthorization of the Generic Drug User Fee Amendments (GDUFA), making it imperative for manufacturers to stay informed.
Submitting the 510(k) Application
Submitting a 510(k) application requires utilizing the FDA's electronic submission gateway. Ensuring adherence to the FDA’s guidelines for format and content is crucial to prevent delays in the review process. 'Become acquainted with the subject equipment, its intended users, and thorough guidelines for use, including warnings and cautions.'. It's also crucial to comprehend the competitive environment by reviewing research literature, clinical studies, and marketing materials to identify possible reference instruments that share the same intended use and similar technological features. This thorough preparation can aid in demonstrating substantial equivalence, a key component that often leads to 75% of initial 510(k) submissions being rejected, with 85% of those rejections due to issues related to substantial equivalence during the scientific review.
FDA Review Process
Upon submission of a 510(k) application, the FDA initiates a review process to evaluate whether the item is substantially equivalent to an existing, legally marketed product, known as a predicate. This review period can extend up to 90 days, during which the FDA may request further information from the applicant to ensure compliance with regulatory standards. 'The significance of this evaluation procedure is highlighted by the FDA's wider goal to safeguard public health by guaranteeing the safety and effectiveness of medical products.'. Successful clearance through the 510(k) pathway permits the product to be legally marketed in the United States, offering a streamlined route to market compared to the more rigorous Pre-Market Approval (PMA) or De Novo classification processes.
Common Challenges and Considerations
Producers frequently face considerable obstacles during the 510(k) submission procedure, mainly concerning the identification of a suitable comparison item, gathering extensive information, and complying with strict regulatory standards. Understanding the concept of substantial equivalence is critical, as 75% of 510(k) submissions are initially rejected, with 85% of those rejections due to issues related to substantial equivalence. The FDA defines substantial equivalence as having the same intended use and technological characteristics as a reference product, or differing characteristics that do not raise new safety or effectiveness concerns.
To navigate these challenges effectively, manufacturers should deeply educate themselves about their product, including its users and usage instructions. Collaboration with marketing departments to assess the competitive landscape and identify potential predicate devices is essential. This process involves reviewing research literature, clinical studies, and competitor materials to create a comparative table that highlights similarities and differences.
Improving efficiency in regulatory and safety document preparation is another priority. Streamlining these processes can minimize delays and errors, ensuring compliance with regulatory standards. This approach is vital in a landscape where regulatory demands are continuously evolving, and staying compliant is crucial for successful market entry.
Conclusion
The 510(k) clearance process serves as a vital mechanism for medical device manufacturers aiming to introduce new products to the market while ensuring safety and effectiveness. This pathway allows for expedited approval by demonstrating substantial equivalence to existing devices, yet it also raises concerns regarding potential risks associated with bypassing clinical trials. Despite ongoing scrutiny, the FDA remains committed to refining its regulatory processes to strike a balance between innovation and patient safety.
Eligibility for 510(k) clearance hinges on specific criteria, including intended use and classification of the device. Understanding the types of submissions—Traditional, Abbreviated, and Special—enables manufacturers to navigate the requirements effectively. Each type serves distinct purposes, and careful selection is crucial for successful outcomes.
The step-by-step process for obtaining 510(k) clearance emphasizes the importance of identifying the correct device classification, selecting appropriate predicate devices, and preparing comprehensive documentation. A thorough understanding of the competitive landscape and adherence to FDA guidelines is essential for minimizing common challenges faced during submission.
Ultimately, the 510(k) clearance process is a critical component of medical device regulation, facilitating timely access to innovative technologies while safeguarding public health. Awareness of the regulatory landscape, combined with diligent preparation and collaboration, can significantly enhance the likelihood of successful device approval and market entry.




