Introduction
Navigating the regulatory landscape of medical device approval can be a daunting task, especially when it comes to understanding the intricacies of the 510(k) submission process. This critical pathway enables manufacturers to demonstrate that their medical devices are safe and effective by proving substantial equivalence to an existing, legally marketed device. The article delves into the fundamental aspects of 510(k) submissions, elucidating the different types tailored to specific scenarios and the key components essential for a comprehensive and successful submission.
By exploring detailed guidance on each submission type and providing practical tips for enhancing approval chances, the article serves as an indispensable resource for professionals seeking to ensure their devices meet U.S. Food and Drug Administration (FDA) expectations.
What is a 510(k) Submission?
A 510(k) submission is a premarket notification to the U.S. Food and Drug Administration (FDA) that ensures a medical product is safe and effective. Manufacturers must demonstrate that their product is substantially equivalent to an existing, legally marketed item, meaning it has the same intended use and similar technological characteristics. This involves a thorough understanding of the item's purpose, user instructions, and competitive landscape. Examining research literature, clinical studies, and competitor products assists in recognizing a predicate item for comparison. The FDA's definition of substantial equivalence requires that the new product either matches the technological characteristics of the predicate model or, if different, provides data proving it is as safe and effective without raising new safety or effectiveness concerns.
Types of 510(k) Submissions
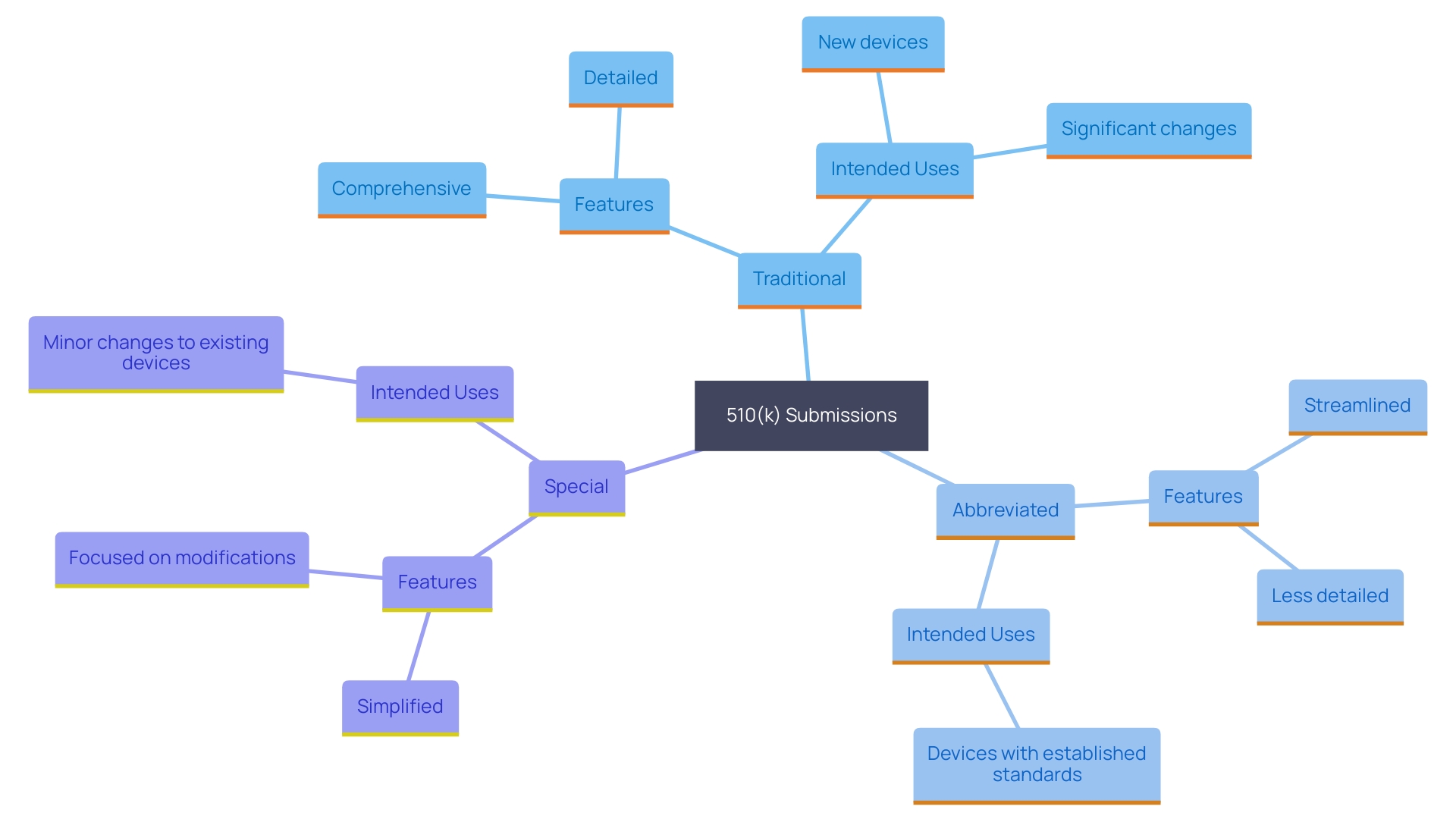
The FDA acknowledges three main categories of 510(k) submissions, each customized for particular situations depending on the item's features and the regulatory environment. The Traditional 510(k) is the most common pathway and is utilized when a product demonstrates substantial equivalence to a legally marketed predicate. This process involves a thorough comparison of the new item's intended use and technological characteristics with those of the predicate item. Industry experts highlight the significance of comprehending the subject apparatus, its users, and the competitive environment to identify appropriate predicate apparatuses.
The Abbreviated 510(k) leverages guidance documents, special controls, and recognized standards to streamline the review process. This type is particularly useful when there is existing guidance that can be directly applied to the item in question, thereby reducing the amount of data and documentation required. The aim here is to improve efficiency in regulatory and safety document preparation, a priority highlighted by surveyed professionals.
Lastly, the Special 510(k) is intended for modifications to a product that has already been cleared through the 510(k) process. This submission category emphasizes modifications that do not change the intended application or essential scientific technology. It enables producers to refresh their products while ensuring ongoing adherence to regulatory standards. By understanding these pathways and their specific requirements, professionals can better navigate the evolving regulatory landscape and ensure their products meet FDA expectations.
Key Components of a 510(k) Submission
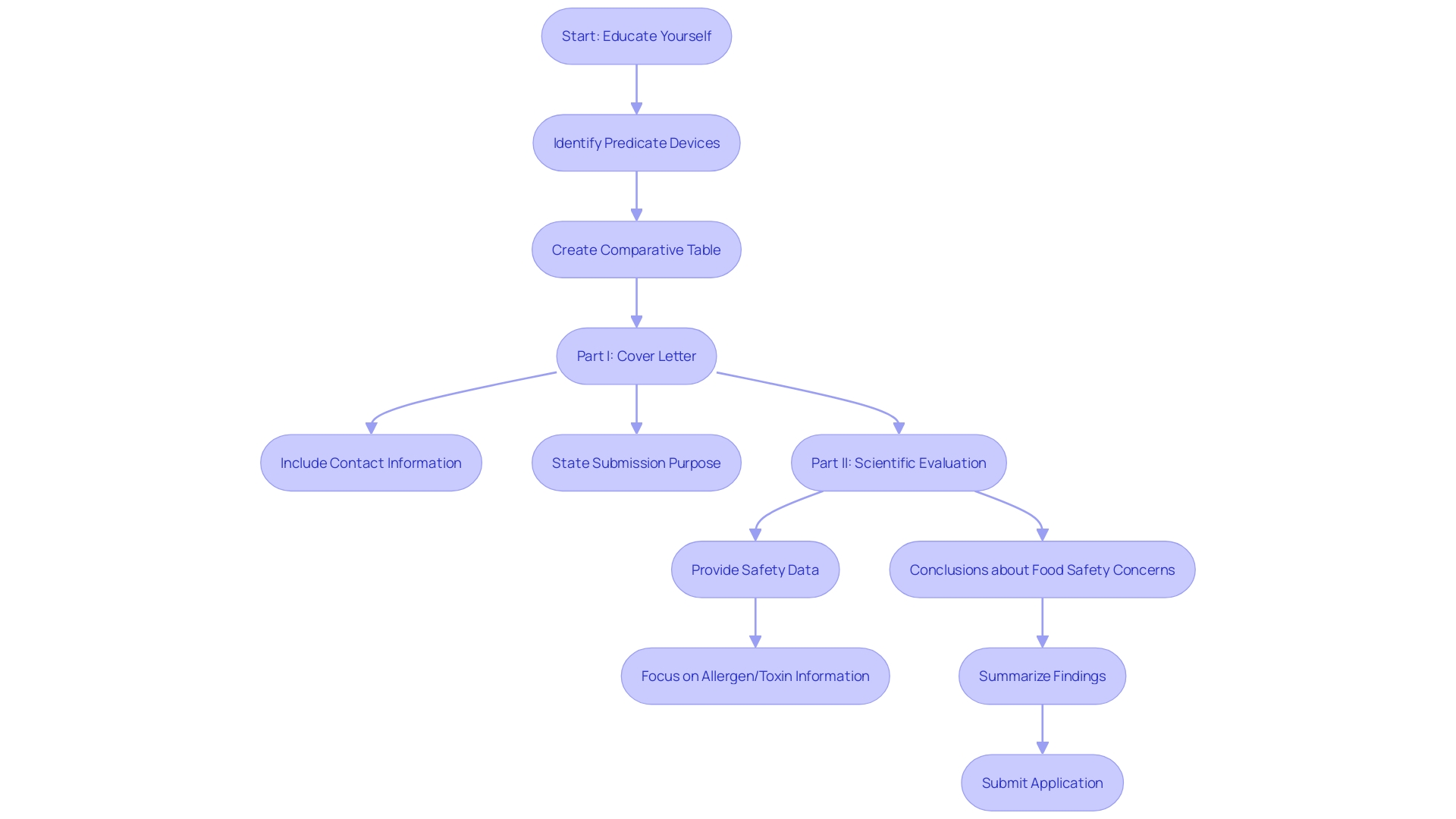
A comprehensive 510(k) application must encompass several critical components. Central to this are the cover letter, which acts as an introduction and summary of the proposal, and a statement indicating intended use that clearly outlines the product's purpose and target audience, including clinicians, physicians, and patients. Furthermore, a comprehensive description of the apparatus is essential to provide a thorough understanding of the product's design and functionality.
Furthermore, the submission must include a substantial equivalence discussion. This section is pivotal as it involves a comparative analysis between the new product and a predicate item already on the market. This comparison is not merely a cursory overview but a detailed examination that highlights similarities in intended use and technological characteristics. It is crucial to ensure that any differing technological features do not introduce new questions regarding safety and effectiveness. To substantiate this, reviewing the Summaries of Safety and Effectiveness (SSEs) available on the FDA’s 510(k) database can provide valuable insights into the comparative aspects of the products.
As James Pink, Senior Director of Medical Technologies at Element Materials Technology, emphasizes, understanding the intended use and comparative landscape of the apparatus is vital. This entails exploring research literature, clinical studies, competitor products, and creating a comparative table to identify potential predicate items. This process aids in creating a strong basis for the substantial equivalence discussion, ensuring that the new product meets all regulatory requirements for market approval.
Tips for a Successful 510(k) Submission
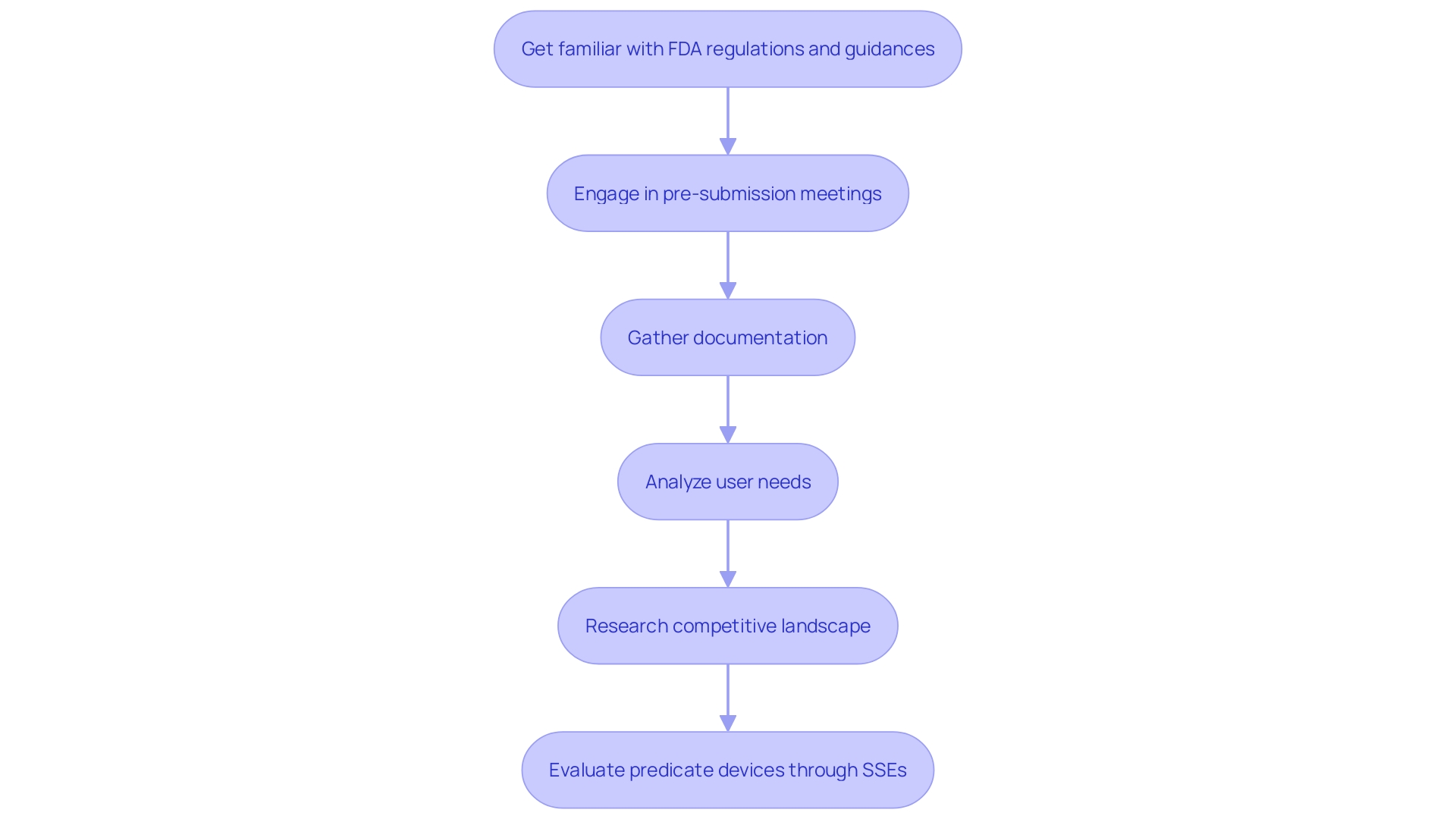
To enhance the likelihood of a successful 510(k) submission, it is crucial to thoroughly understand the requirements and guidelines set by the FDA. Engaging in pre-submission meetings with the FDA can provide valuable insights, and ensuring that all documentation is complete and accurate will facilitate a smoother review process. 'Learning about the topic and its purpose is extremely important.'. Aim to seek a deep understanding of users, instructions for use, including warnings and cautions. Next, with help from Marketing, learn about the competitive landscape and focus on rival products. View content such as research literature, clinical studies, and labeling to identify potential predicate devices with the same intended use and similar technological characteristics; and begin to create a comparative table. Once identified, read the Summaries of Safety and Effectiveness (SSEs) available on FDA’s 510(k) database to evaluate the similarities and differences. This thorough approach will significantly increase your chances of a successful submission.
Conclusion
Navigating the 510(k) submission process is critical for medical device manufacturers aiming to ensure their products meet FDA standards. Understanding the definition and purpose of a 510(k) submission is foundational, as it requires demonstrating substantial equivalence to an existing device. This involves a comprehensive analysis of the device's intended use, technological characteristics, and competitive landscape, ensuring compliance with regulatory expectations.
The article highlights the three primary types of 510(k) submissions: Traditional, Abbreviated, and Special. Each type serves distinct scenarios, allowing manufacturers to choose the most appropriate pathway based on their device's characteristics and any modifications made. By recognizing these pathways, professionals can streamline their submission process, enhancing efficiency and regulatory adherence.
Key components of a successful 510(k) submission include a clear cover letter, an indication for use statement, and a detailed device description. A thorough substantial equivalence discussion is essential, requiring in-depth comparisons with predicate devices. The importance of understanding the device's intended use and the competitive landscape cannot be overstated, as it forms the backbone of the substantial equivalence argument.
To increase the likelihood of approval, engaging in pre-submission meetings with the FDA and meticulously preparing documentation are vital steps. A thorough understanding of the device, its users, and the competitive environment will aid in identifying suitable predicate devices and crafting a robust submission. By following these guidelines, manufacturers can effectively navigate the complexities of the 510(k) submission process, ensuring their devices are positioned for success in the market.




