Introduction
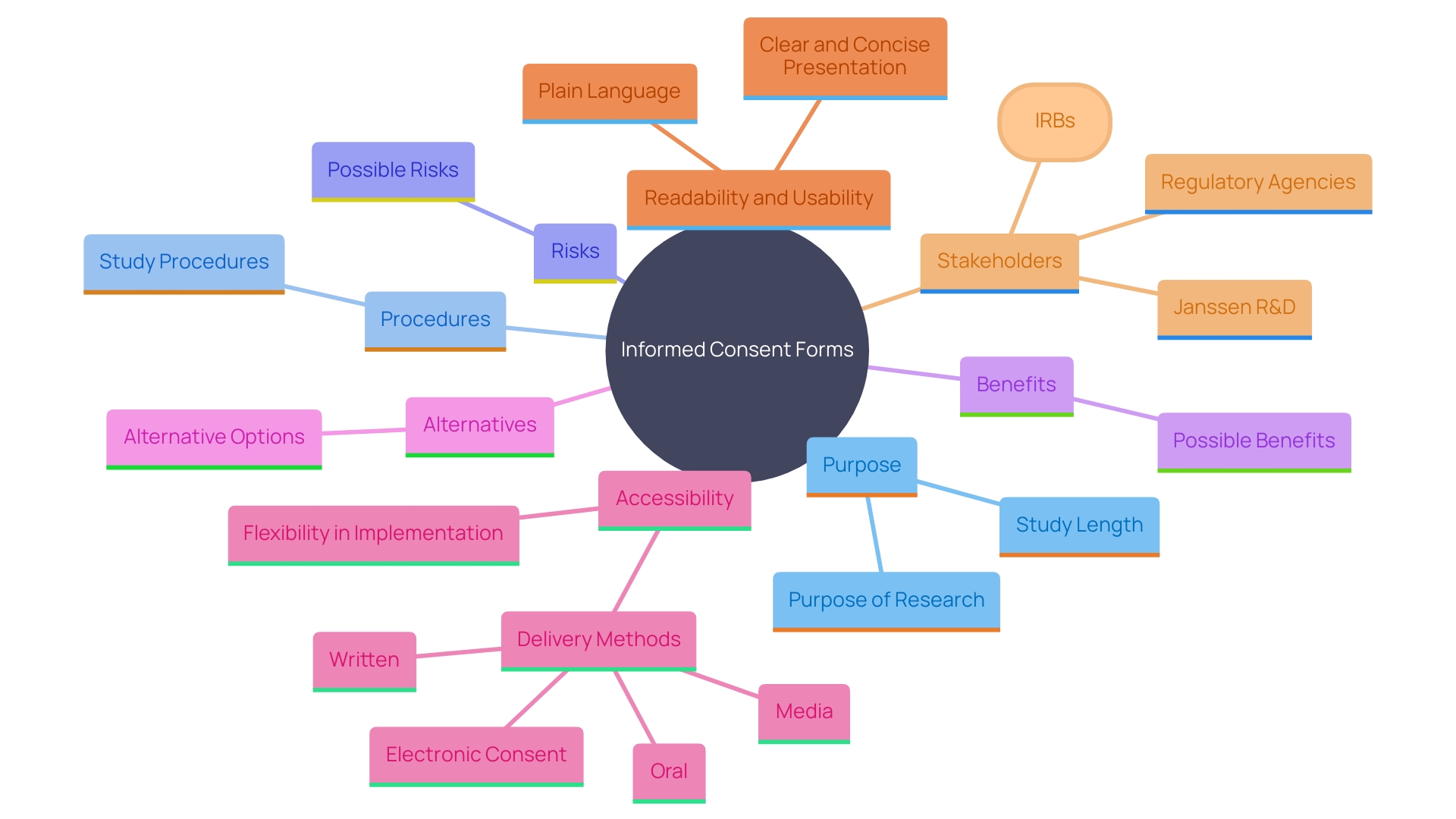
In clinical research, the Informed Consent Form (ICF) stands as a pivotal document designed to provide potential participants with essential information about a study in a clear and concise manner. This form encompasses key details such as the study's purpose, procedures, potential risks, benefits, and alternatives, enabling individuals to make well-informed decisions regarding their participation. Recent guidance underscores the importance of presenting this information upfront to enhance comprehension and ensure that participants can make choices aligned with their personal and health needs.
Efforts are underway to improve the accessibility and readability of ICFs, including the use of multimedia elements to aid those with language barriers or sensory impairments. By refining these documents, the aim is to foster greater understanding and willingness among participants, thereby supporting ethical research practices and maintaining the integrity of clinical trials.
What is an Informed Consent Form (ICF)?
An Informed Consent Form (ICF) is a vital document in clinical research, intended to communicate important details to prospective individuals in a clear and concise manner. It includes key details about the study such as its purpose, procedures, risks, benefits, and alternatives, allowing individuals to make well-informed decisions about their involvement. The recent draft guidance highlights that the ICF should start with essential details to aid comprehension of why someone might or might not wish to take part. This approach ensures that potential individuals are better informed and can make decisions that align with their personal and health needs.
The guidance also highlights the importance of presenting information in accessible formats, which could include videos or illustrations, especially for those with language barriers, hearing or vision impairments, or other challenges. Industry-wide efforts are underway to improve the ICF's readability and usability, aiming to enhance the recruitment and retention of diverse populations in clinical trials. For instance, Janssen R&D is actively researching ways to optimize ICFs, focusing on study comprehension and the satisfaction of those involved. This empirical approach is crucial, as it moves beyond intuition and opinion, providing data-driven strategies to enhance informed agreement processes.
By simplifying and improving the communication of information in these documents, the goal is to enhance understanding and, consequently, the willingness to engage in clinical research. This initiative is supported by various stakeholders, including Institutional Review Boards (IRBs) and regulatory agencies, who are working together to ensure that informed consent processes are both effective and ethically sound.
The Importance of ICF in Clinical Trials
The Informed Consent Form (ICF) is crucial in protecting the rights and well-being of individuals involved in clinical trials. It guarantees clarity and promotes trust among researchers and subjects by offering clear and detailed insights about the study. This document enables individuals to comprehend the purpose, risks, benefits, and procedures involved, facilitating their informed decision-making. For example, the draft guidance suggests presenting key information in a clear and concise manner, making it easier for participants to understand why they might want to take part in the study. The ICF also supports ethical investigation practices and enhances the credibility and validity of study results. In recent years, the incorporation of patient engagement throughout the study lifecycle has been increasingly recognized as vital, with many funding organizations now requiring it. This shift reflects the growing importance of making research more patient-centered and ensuring that findings are meaningful and relevant to those who participate. Additionally, the use of innovative methods such as electronic consent (eConsent) has simplified the process, making it more accessible and easier to comprehend. By utilizing digital formats like audio, video, and multimedia elements, eConsent engages different demographics and enhances understanding, ensuring individuals fully grasp the study's scope and expectations.
Key Elements of an ICF
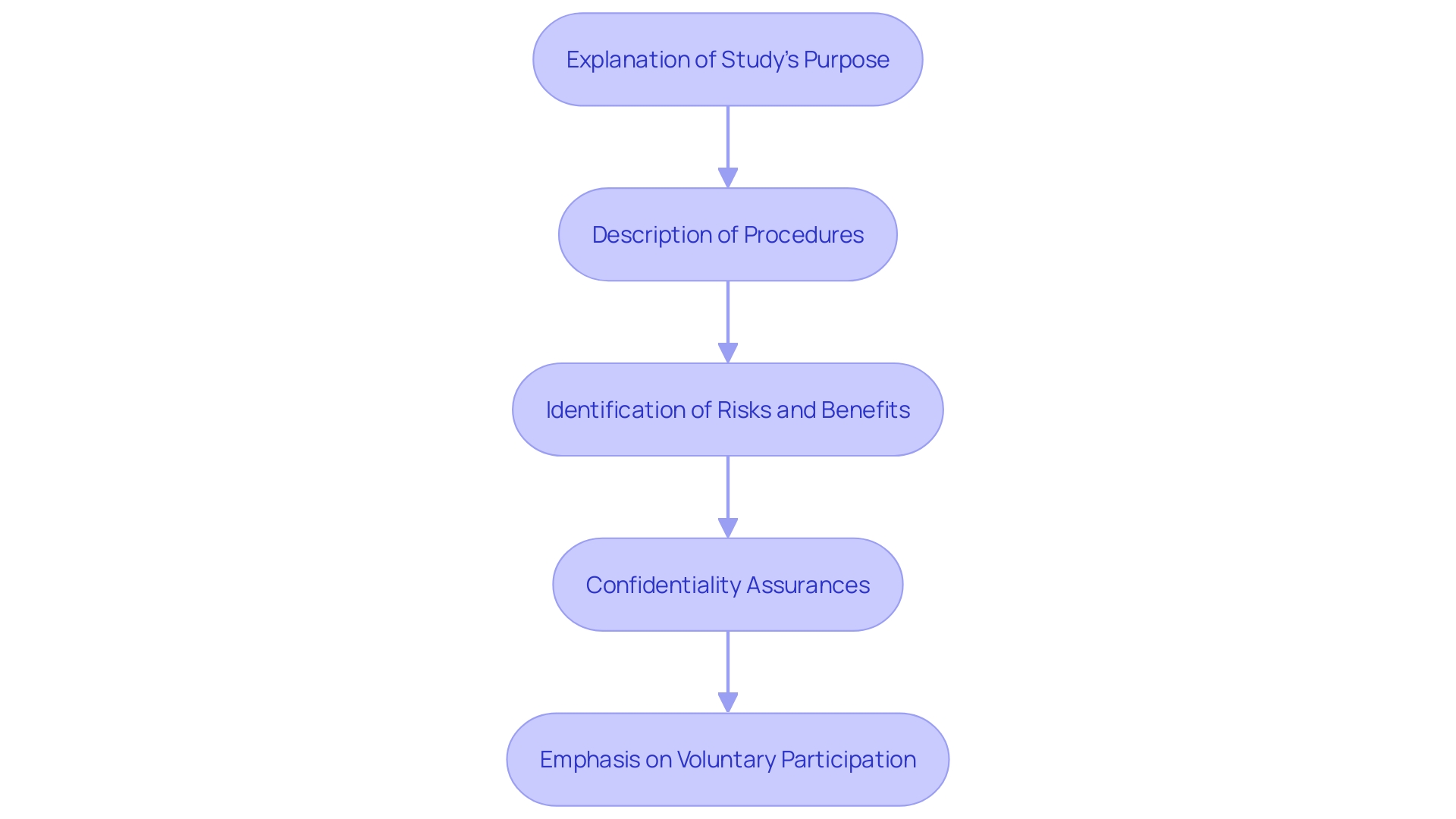
An effective Informed Consent Form (ICF) comprises several essential elements that ensure individuals are fully informed and can make a knowledgeable decision about their involvement in a study. The form begins with a clear explanation of the study's purpose, providing context and rationale for the research. Detailed descriptions of the procedures involved are included, outlining what individuals can expect throughout the study's duration.
Potential risks and benefits are clearly identified, allowing individuals to weigh the pros and cons of their involvement. Confidentiality assurances are provided to ensure that individuals understand how their personal information will be protected. The voluntary nature of involvement is emphasized, reiterating that individuals can withdraw from the study at any time without any repercussions.
These components collectively assist individuals in understanding their roles and the implications of their involvement in the research. The addition of essential details at the start of the informed agreement document is especially advantageous, as it aids the discussion between researchers and prospective individuals. This organized method improves the availability and clarity of the agreement process, simplifying it for individuals to make informed choices regarding their involvement.
Regulatory Requirements for ICF
Regulatory frameworks require that informed agreement forms (ICFs) adhere to specific guidelines to ensure participant protection. Authorities such as the FDA and EMA outline stringent requirements for content, readability, and the process of obtaining consent. Adhering to these regulations is vital for the ethical execution of clinical trials and for maintaining the integrity of the investigative process.
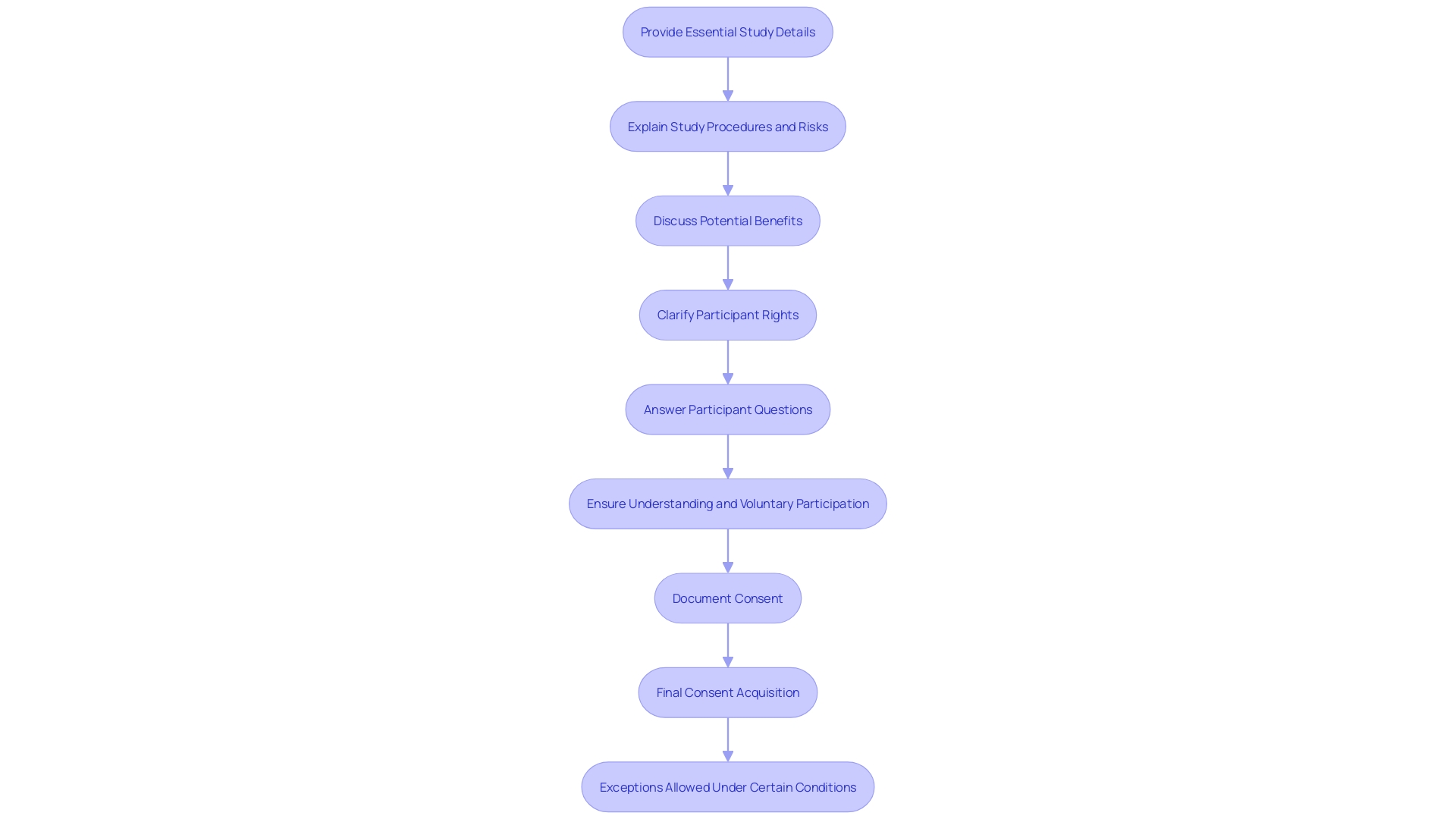
The FDA, specifically, has made important strides to align clinical study regulations, collaborating closely with the U.S. Department of Health and Human Services (HHS) Common Rule. This harmonization aims to make clinical studies more efficient while protecting the rights of those involved. A final rule issued by the FDA permits an exception from acquiring informed agreement when a clinical investigation poses no more than minimal risk, provided there are appropriate safeguards in place to protect participants.
Knowledgeable agreement starts with providing essential details about the study in a straightforward and succinct way. This includes the purpose of the research, potential risks and benefits, and the study's procedures and duration. The FDA and OHRP suggest utilizing straightforward phrases, clear language, and structured formatting to enhance the clarity of consent details. For example, presenting information in a bubble format has been found to facilitate consumer understanding.
These regulatory efforts highlight the importance of transparency and protection for those involved in clinical trials. By ensuring that data is rigorous and reliable, and promoting open access to clinical trial data, the FDA aims to advance medical product development without compromising the safety of those involved.
Ethical Considerations in ICF
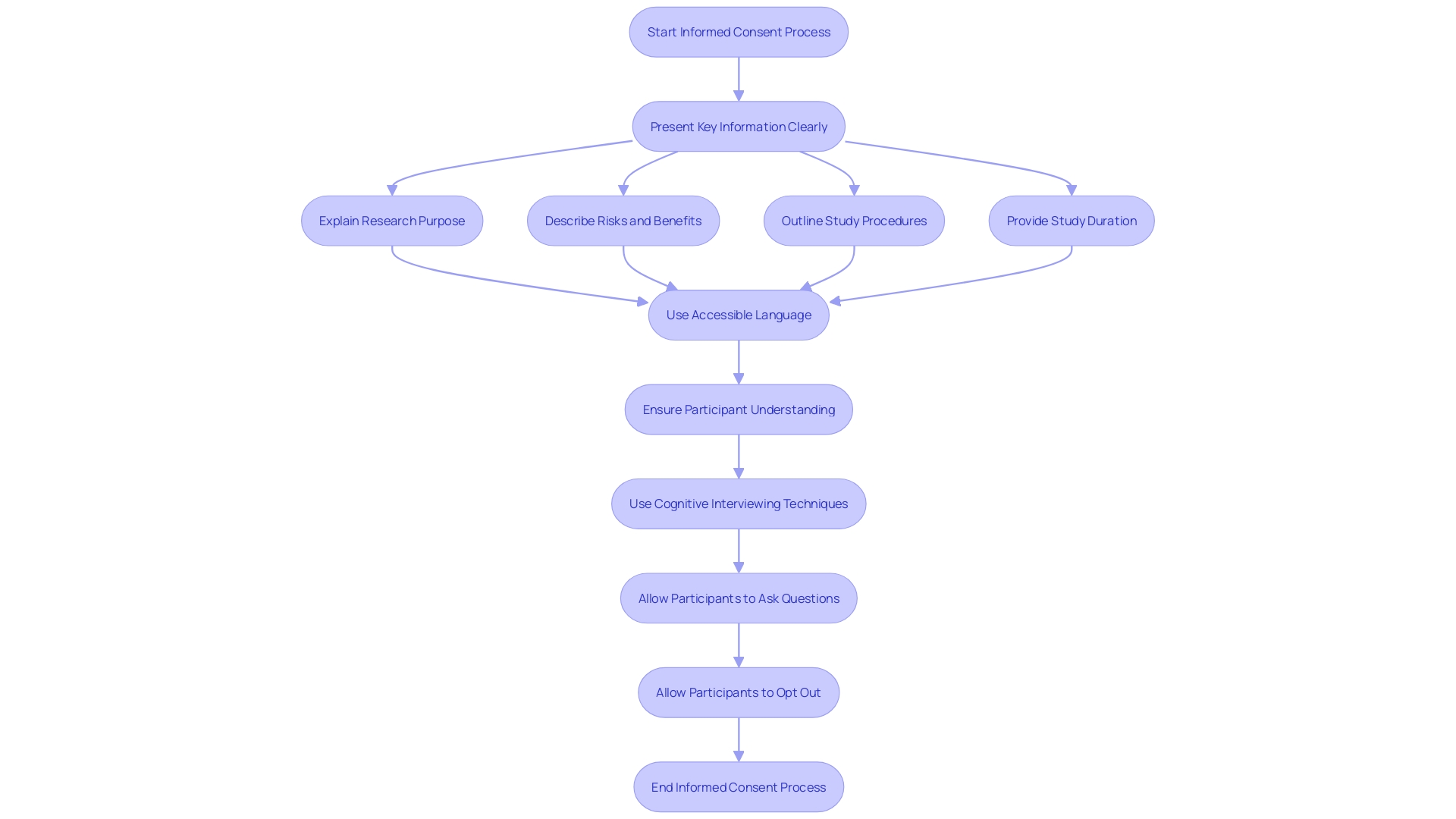
Ethical factors regarding informed agreement highlight the importance of transparency, understanding, and regard for individuals' independence. Researchers must ensure that the language used is accessible, avoiding technical jargon that may obscure understanding. The draft guidance outlines the importance of presenting key information at the beginning of the document, including the purpose of the research, possible risks and benefits, and the study’s length and procedures. This method enhances comprehension and assists individuals in making informed choices regarding their involvement. Furthermore, the procedure of gaining approval should be handled with care, enabling individuals to inquire and opt out if they wish. For instance, studies have shown that many approval forms are written using complex language that might be hard for some patients to understand, highlighting the need for more accessible communication. Additionally, the use of cognitive interviewing techniques has proven effective in improving consent scripts, ensuring that participants fully comprehend what they are consenting to.
Conclusion
The Informed Consent Form (ICF) plays a critical role in clinical research by ensuring that potential participants are equipped with the necessary information to make informed decisions regarding their involvement. It encompasses key aspects such as the study's purpose, procedures, potential risks, benefits, and alternatives, thereby fostering transparency and trust between researchers and participants. Recent guidance emphasizes the importance of presenting this information clearly and upfront, which is essential for enhancing participant understanding and promoting ethical research practices.
The ongoing efforts to improve the accessibility and readability of ICFs, including the use of multimedia elements and electronic consent methods, demonstrate a commitment to inclusivity. These innovations aim to engage diverse populations, ensuring that individuals with language barriers or sensory impairments can comprehend the information provided. By refining the communication of essential details, researchers can enhance participant willingness and retention, ultimately supporting the integrity and credibility of clinical trials.
Furthermore, adherence to regulatory requirements is paramount in maintaining participant protection and upholding ethical standards. The emphasis on using plain language and organized formats aligns with the goal of making informed consent processes more effective and understandable. Ethical considerations also highlight the need for clarity and respect for participants' autonomy, ensuring they can engage meaningfully in the consent process.
Collectively, these elements underscore the importance of the ICF in clinical trials, reinforcing its role as a foundational document that supports ethical research and participant rights.




