Introduction
The Role of Consultant Medical Devices in Innovation
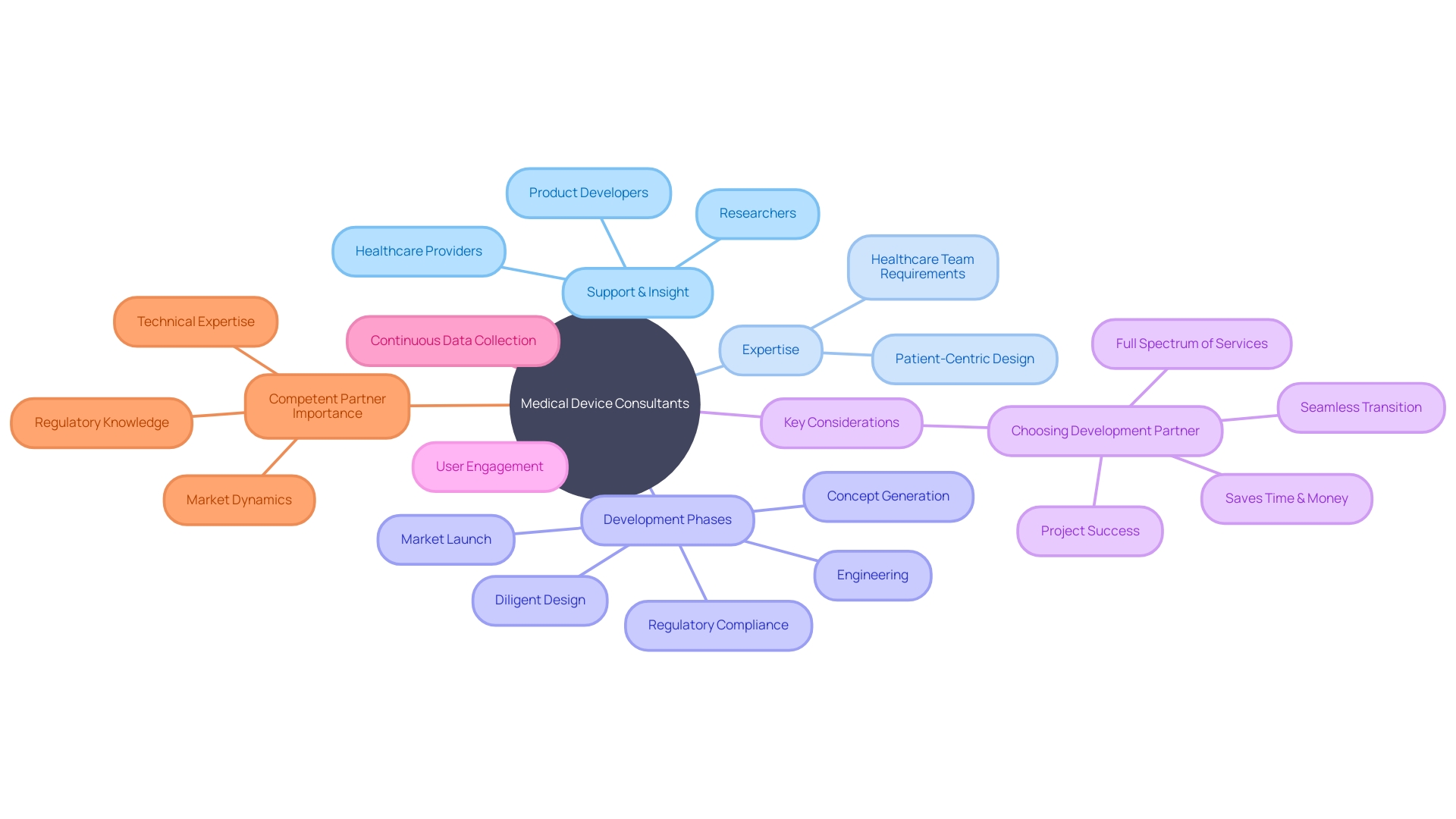
Medical device consultants play a crucial role in the ever-evolving world of medtech. They provide essential expertise and support to healthcare providers, researchers, and product developers, helping to harness the full potential of innovation within the medical technology field. From concept generation to navigating regulatory hurdles, these experts bring a deep understanding of the factors involved in developing and launching successful medical devices.
In an industry where accuracy and efficacy are paramount, medtech developers must balance these priorities with a focus on enhancing the end-user experience. Choosing a competent medical device development partner with technical prowess and a profound grasp of the regulatory landscape and market demands is essential. As the medical devices industry continues to grow rapidly, it is clear that strategic collaborations and partnerships are key in overcoming the complexity of medtech advancements and regulatory landscapes.
By embracing collaboration and harnessing the power of innovative technologies, the future of healthcare holds great promise for transformative solutions that prioritize patient well-being.
The Role of Consultant Medical Devices in Innovation
Medical device consultants serve as critical conduits of expertise in the rapidly evolving medtech landscape, providing vital support, and insight to healthcare providers, researchers, and product developers. These experts are integral to harnessing the full potential for innovation within medical technology, aiding in ensuring devices are designed not only with patient needs in mind but also the requirements and workflow of an entire healthcare team, including clinicians, nurses, and support staff. Their in-depth understanding of the myriad factors from early concept generation and diligent design to engineering, navigating regulatory hurdles, and successful market launch makes them invaluable.
With over 710,000 patents filed and granted in the medical devices industry in the past three years, it's clear that the pace of innovation is intense. Nevertheless, not every innovation follows an identical trajectory of success; comprehension of their development stage is crucial for evaluating their adoption rates and future impact. In a landscape where accuracy and efficacy are paramount, medtech developers must balance these priorities with a focus on the end-user experience, whether it be a patient or a healthcare professional.
This balance is achieved through rigorous engagement with end users and continuous data collection even after regulatory clearance, ensuring the product not only meets its intended use but also enhances user interaction and overall healthcare delivery. The significance of choosing a competent medical device development partner cannot be overstated, particularly one with technical prowess and a profound grasp of both the regulatory landscape and market demands. As the US Food and Drug Administration (FDA) and comparable agencies in Europe enforce strict regulatory control over medical devices ranked by potential risk, it is evident that the journey from conception to market is a complex and highly regulated pathway.
The goal? To deliver medical innovations that are not only technologically advanced and in compliance with rigorous standards but are also poised to reshape patient care for a digital future.
Case Study: Successful Implementation of Consultant Medical Devices
The symbiosis of a US-based medical device company and a Latin American research institution exemplifies a powerful strategy in medtech innovation. This alliance capitalized on each entity's strengths: the company's resource abundance and technical prowess paired with the institution's research acumen created a formidable partnership. The culmination of this collaboration was the development and deployment of a state-of-the-art medical device that has made significant strides in enhancing patient care.
The partnership followed a thorough development process, which is critical for success in the medical device industry. This process includes essential stages like concept creation, intricate design, rigorous engineering, and navigating through dense regulatory compliance frameworks before a successful market introduction. The successful launch of this device underscores the importance of engaging a medtech development company that offers comprehensive services, facilitating a smooth transition between each phase, ultimately resulting in time and financial savings while improving the likelihood of project triumph.
One of the remarkable advancements by this joint venture is the accolade of operational efficacy. A recent survey highlighted that 73% of radiology department leaders anticipate operational efficiency to be their primary challenge in the near future. The newly developed medical device evidently addresses such concerns, aiming to reduce wait times and enhance the patient journey from diagnosis to treatment.
Moreover, this case study resonates with a broader trend in the medical devices field where innovation and successful new product launches are driven by companies adept at fusing software with hardware functionalities. Such an approach not only maximizes digital health potentials but also provides a competitive advantage. Tech-forward medical device development, including devices capable of remote management and data integration, are pioneering this wave, asserting the significance of selecting a medical device development partner with extensive technological expertise and a visionary grasp of market dynamics.
In an era where the medtech industry is burgeoning with over 710,000 patents filed in recent years, this alliance showcases the prowess of collaborative innovation, reinforcing the notion that strategic partnerships are pivotal in surmounting the complexity of medtech advancements and regulatory landscapes—factors that are inextricably linked to the successful delivery of medical breakthroughs to the market and, ultimately, to the patients in need.
Benefits of Consultant Medical Devices in Advancing Innovation
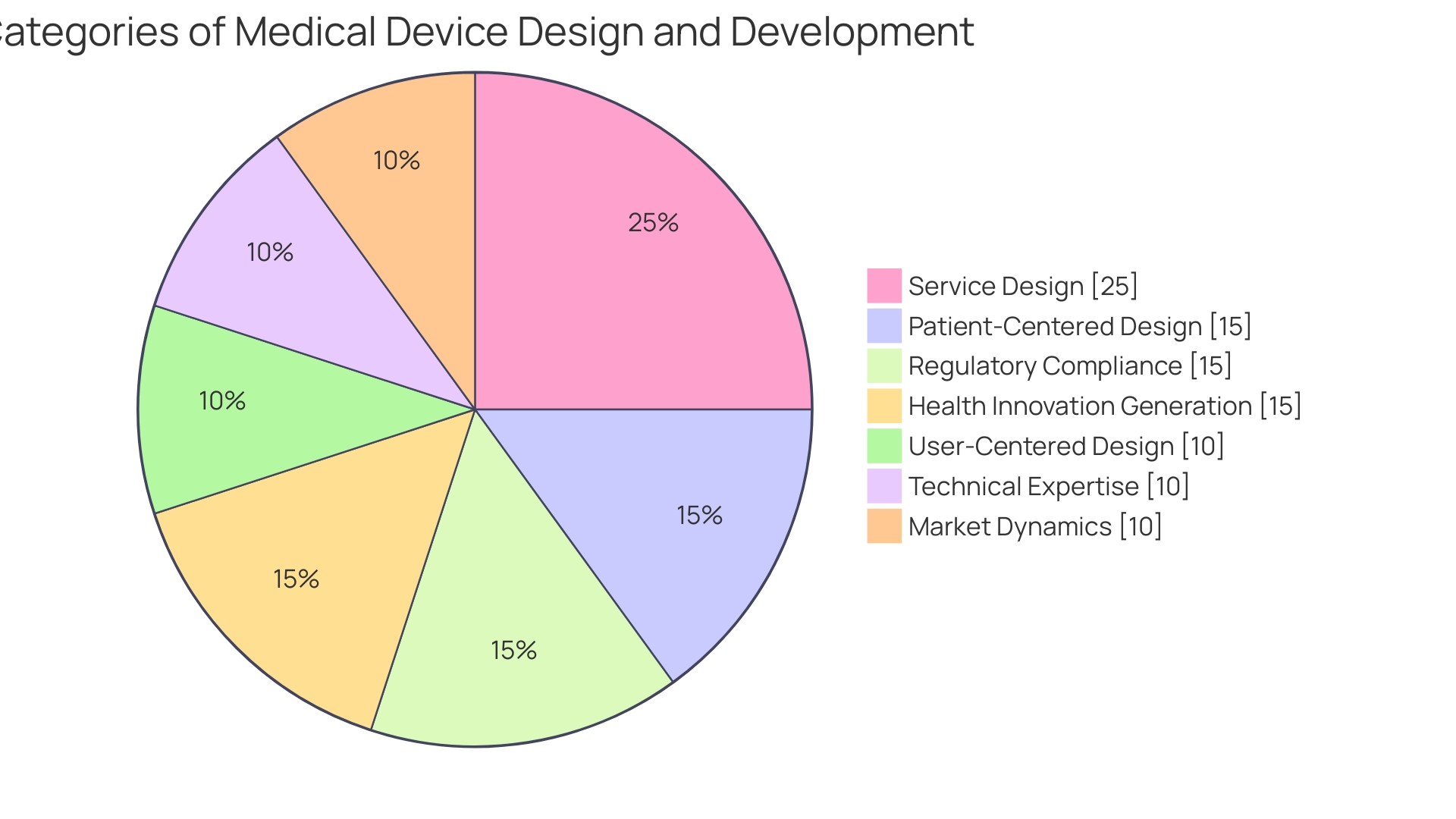
Harnessing the collective brainpower of a multidisciplinary team is pivotal to creating cutting-edge medical devices that truly meet patient needs. For example, Avation Medical put patient experience at the forefront during the development of the novel solution for overactive bladder. By investing months to understand how existing treatments impacted patients, the team ensured that their innovative device was not just medically sophisticated but also patient-centric.
Such collaborative efforts are supported further by medtech consultancies like Archetype. With statistics showing a high failure rate for medtech innovations, with around three quarters not reaching the market, Archetype's comprehensive approach to product development is invaluable. Their services span from concept to quality assurance to regulatory documentation, with a focus on market approval and patient delivery efficiency.
Principal Consultant Dr. Stuart Grant brings his 25-year experience and patent portfolio to provide a clear path through the regulatory labyrinth.
Another example of the medtech field benefiting from collaboration is the use of Computational Modelling & Simulation (CMS). Companies leverage Ansys CMS software for a variety of multiphysics problems, streamlining device development and ensuring submissions to regulatory bodies are robust. CMS can conduct Computational Fluid Dynamics (CFD) to anticipate problems, such as high fluid shear areas potentially damaging to DNA or proteins, improving design before physical prototypes are even made.
Furthermore, data play an essential role in medtech innovation. Medical device regulations by the FDA and EMA are intricate, especially for Class III high-risk devices. Medical device consulting firms maneuver these challenges by advising on research, development, compliance, and marketing communication initiatives—helpful services considering the burgeoning volume of patents and the digital health trend on the rise.
As medical device consultants often handle the complex journey from ideation to market, their expertise is fundamental for any organization aiming to navigate the regulatory landscape and optimize product success.
Overall, the journey from innovative idea to market entry for medical devices is marked by in-depth collaboration, data insight, and expert consultancy, driving the medtech industry towards transformative healthcare solutions.
Challenges and Solutions in Implementing Consultant Medical Devices
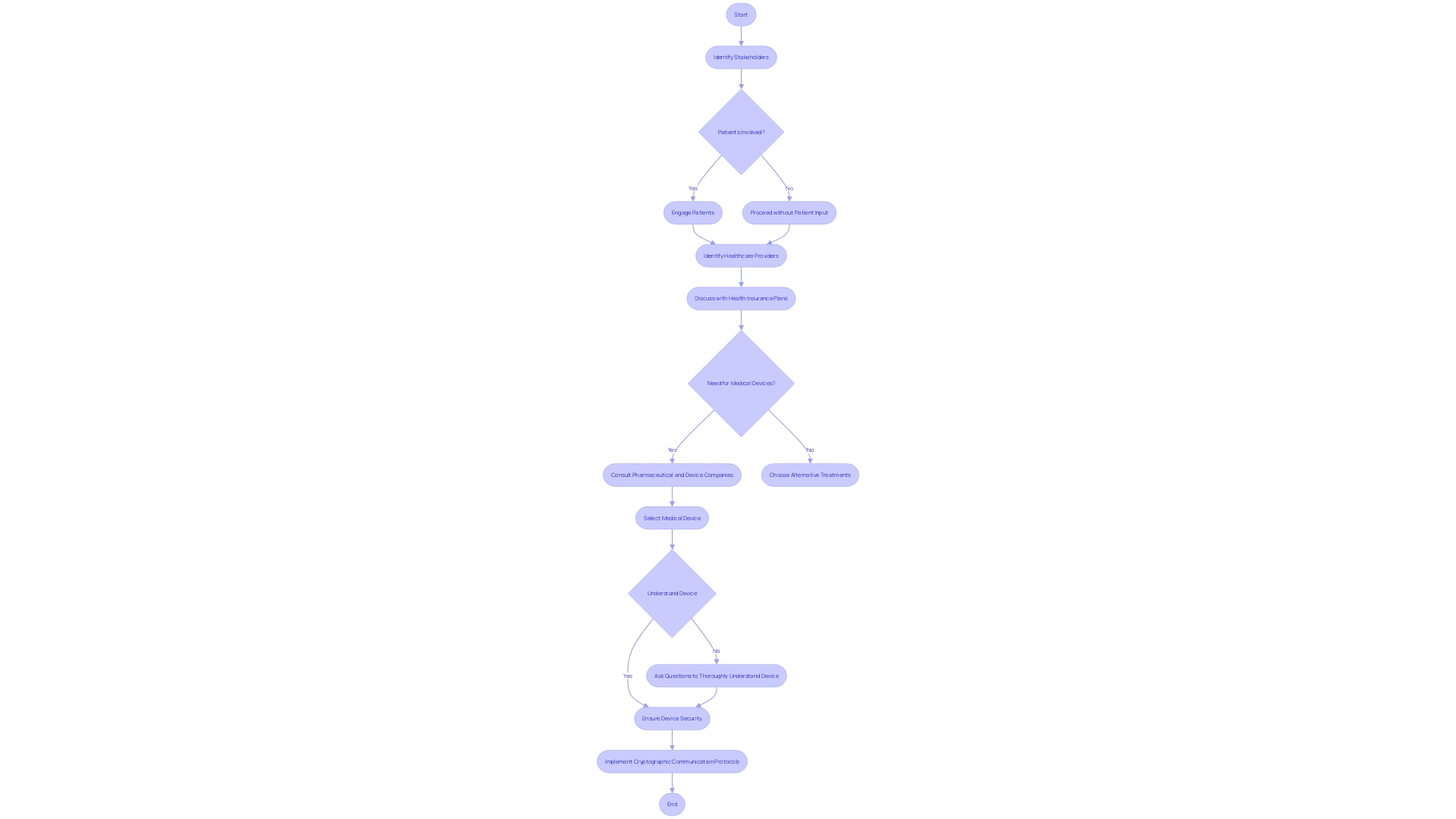
The integration of medical devices within healthcare systems presents its unique set of challenges, most notably the necessity of seamless communication and collaboration among various stakeholders. The healthcare delivery model, akin to a four-legged chair, encompasses patients, healthcare providers, insurance plans, and medical device companies. Each group has its interests, often leading to shifting alliances and opposing forces.
However, leveraging technological advances like digital health strategies, such as those seen in successful collaborations like Netsmart with Northstar Care Community and Hospice of Michigan, can enhance care quality and patient communication, setting a high standard for care delivery.
Additionally, the coordination of medical device implementation involves a myriad of technical considerations, necessitating analysis with first principles or optical design software to fully comprehend and optimize the relevant variables for success. With a multidisciplinary approach to complex problems—where optics intertwine with electrical, mechanical, and software development considerations—the focus shifts from an individual domain challenge to a comprehensive systems problem.
Finally, security remains paramount in medical device software development, where any communication must be secure and encrypted to adhere to regulations. Employing such rigor in planning and maintaining open channels for patient feedback paves the way for successful medical device utilization. This methodology is not only vital for enhancing healthcare outcomes but also emphasizes the importance of adopting digital technologies to keep pace with evolving healthcare models.
Future Trends and Opportunities in Consultant Medical Devices
The convergence of emerging technologies such as artificial intelligence and big data analytics with medical device consulting presents a vibrant panorama of progress and innovation. Artificial intelligence, in particular, is revolutionizing the field with its ability to interpret vast amounts of data, leading to improved diagnosis and disease detection. Medical imaging, enhanced by Machine Learning algorithms, is yielding early detection of diseases like cancer with remarkable accuracy.
The expansion of network connectivity in healthcare organizations supports such advancements, with a notable 74% already integrating over half of their medical devices to the network.
At the same time, the intertwining of US and Latin American efforts in medtech is nurturing a fertile ground for ingenuity. Collaborative efforts are pushing the boundaries of what's possible in cross-border partnerships, fostering shared knowledge and propelling the global innovation landscape.
The significance of data cannot be overstated in the quest for superior patient outcomes. Integration of data from Electronic Health Records (EHRs) and a variety of medical devices into unified dashboards enables comprehensive monitoring. The result is a paradigm shift towards early intervention, reducing diagnostic errors that account for up to 17% of adverse inpatient events.
Leveraging data is key to equipping clinical teams with actionable insights, which translates directly into improved patient care.
Consulting services are indispensable to navigating through the medtech ecosystem, which includes the rigorous process of bringing medical devices to market. Consultants ally with healthcare manufacturers offering an array of expertise from product conceptualization, design, legal counsel, and compliance, thus raising the bar for quality and value in healthcare solutions.
To encapsulate, the future of healthcare lies in embracing artificial intelligence and nurturing international cooperation, as these endeavors are pivotal in crafting a healthcare environment that is both innovative and secure, where patient well-being remains the central focus.
Conclusion
In conclusion, consultant medical devices are crucial for driving innovation in the medtech industry. These experts provide support and insight, helping to harness the full potential of medical technology. Choosing a competent development partner with technical prowess and regulatory knowledge is key.
Successful implementation of consultant medical devices is exemplified by powerful alliances and partnerships. Collaborations that fuse software with hardware functionalities maximize digital health potentials. Comprehensive services offered by medtech development companies facilitate a smooth transition to market launch.
Collaboration, computational modeling, and data insight are vital for advancing innovation. Medtech consultancies offer guidance through the complex journey from ideation to market success. Seamless communication and technological advancements enhance care quality.
The future of consultant medical devices lies in the convergence of emerging technologies such as artificial intelligence and big data analytics. These technologies revolutionize the field, improving diagnosis and disease detection. Integration of data enables comprehensive monitoring, leading to improved patient care.
In summary, consultant medical devices play a critical role in driving medtech innovation. By embracing collaboration, harnessing innovative technologies, and prioritizing patient well-being, the future holds great promise for transformative healthcare solutions.




