Overview
Clinical trial supply companies are essential for the logistics and distribution of materials necessary for medical research, ensuring that investigational drugs and devices are delivered efficiently to research sites. The article highlights their critical role in managing various services, such as regulatory compliance and temperature control, which are vital for maintaining the integrity of studies and advancing medical knowledge, particularly as the demand for research grows globally.
Introduction
In the realm of clinical research, the success of trials hinges significantly on the efficiency and reliability of clinical trial supply companies. These specialized organizations are not just logistical support; they are the backbone that ensures investigational drugs and medical devices reach their intended destinations on time and in optimal condition.
As the landscape of clinical trials continues to evolve, marked by increasing financial stakes and technological advancements, the role of these companies becomes even more critical. From managing complex supply chains to navigating regulatory challenges, clinical trial supply companies are at the forefront of driving innovation in healthcare.
This article delves into the multifaceted operations of these entities, exploring their essential services, the challenges they face, the impact of technology, and the future trends shaping the industry.
Defining Clinical Trial Supply Companies: An Overview
The successful implementation of medical studies relies on clinical trial supply companies, which are vital for the logistics and distribution of necessary materials, such as investigational drugs and medical devices. Organizations such as bioaccess®, Latin America’s top CRO, improve this process by partnering with Medtech startups to speed up study results. They guarantee that resources are sent to research locations swiftly and effectively, while also offering essential services such as regulatory authorization, research site initiation, and participant recruitment.
For instance, the cardiovascular diseases sector produced revenue of USD 982.2 million in 2020 and is anticipated to attain USD 1,198.7 million by 2023, emphasizing the substantial financial interests involved in medical studies. Furthermore, the MEA medical research logistics market, valued at USD 162.90 million in 2023, is projected to expand to around USD 302.14 million by 2033, at a CAGR of 6.37%. This growth highlights the rising need for efficient logistics oversight in research studies.
As noted by McKinsey & Company,
- 'Working with P&S Intelligence and their team was an absolute pleasure – their awareness of timelines and commitment to value greatly contributed to our project's success. Eagerly anticipating future collaborations.'
Furthermore, the biologics segment is projected to grow at a CAGR of 6.8%, driven by the rising need for safe and effective drugs.
This growth necessitates advancements in cold storage and distribution technologies, as illustrated by the case study titled 'Biologics Growth in Clinical Trials,' which emphasizes the effect of biologics on chain management. Recent advancements, like Bomi Group's purchase by UPS, further emphasize the changing environment of healthcare logistics and its effects on clinical trial supply companies that provide services for research projects. By overseeing the intricacies of the logistics chain and addressing the specific challenges faced by Medtech companies, organizations like bioaccess® not only help maintain the integrity of studies but also contribute significantly to the advancement of medical knowledge.
Key Services Offered by Clinical Trial Supply Companies
Clinical trial supply companies play a crucial part in the effective administration of research studies by providing an extensive range of services. These services include:
- Feasibility Studies and Site Selection: Identifying suitable sites and principal investigators is crucial for trial success, ensuring that research is conducted in compliant and capable environments.
- Logistics Management: This entails the careful supervision of the whole chain process, from sourcing materials to their distribution across medical sites.
- Inventory Management: Effective monitoring and management of inventory levels are crucial, ensuring that research sites are consistently equipped with the necessary supplies to facilitate uninterrupted research activities.
- Labeling and Packaging: Companies provide compliant labeling and packaging solutions tailored for investigational products, ensuring that all materials are clearly identified and meet regulatory standards.
- Temperature Control: For temperature-sensitive materials, maintaining appropriate storage and transportation conditions is essential for preserving efficacy, thus safeguarding the integrity of the study.
- Regulatory Compliance and Reviews: Clinical trial supply companies assist in navigating the complex landscape of regulatory requirements, providing compliance reviews and feedback on study documents to ensure adherence to country-specific regulations.
- Import Permits: They also facilitate the importation of investigational devices, ensuring that all necessary permits are obtained.
- Project Management and Reporting: Comprehensive project oversight and detailed reporting on study status, inventory, and adverse events help maintain transparency and accountability throughout the research process.
The importance of these services cannot be overstated, as they are instrumental in preserving the integrity of studies. The cardiovascular diseases therapeutic area alone generated revenue of USD 1,198.7 million in 2023, highlighting the substantial financial implications of effective logistics management. Significantly, North America is anticipated to dominate the global research and logistics market throughout the forecast phase, highlighting the area's importance in this sector.
As Aditi Shivarkar notes,
With over 14 years of experience, I ensure that every piece of data and content meets our rigorous standards.
Such commitment is essential as the industry evolves, particularly with recent advancements and partnerships. For example, Catalent's facility enlargement in Singapore demonstrates how companies are improving their capacities to fulfill the increasing need for clinical research resources.
Moreover, major market participants like Pfizer Inc., Novartis International AG, Roche Holding AG, and Johnson & Johnson are leading these advancements, shaping trends in inventory oversight and operational practices, which foster job creation, economic growth, and healthcare enhancement in local economies.
Navigating Challenges in Clinical Trial Supply Management
Clinical study resource coordination presents a myriad of challenges that can profoundly affect the success of research initiatives. Among the most pressing challenges are:
-
Logistics Interruptions: Unexpected occurrences, like natural catastrophes or geopolitical conflicts, can cause considerable interruptions in the chain of distribution, leading to delays that threaten testing timelines.
For example, in a recent study, it was observed that 4 out of 10 women reacted to a new therapy, highlighting the essential aspect of prompt resource management, with a p-value of 0.49 suggesting the necessity for thorough statistical analysis.
-
Regulatory Changes: The rapidly evolving landscape of regulations, including oversight by INVIMA, Colombia's National Food and Drug Surveillance Institute classified as a Level 4 health authority by PAHO/WHO, poses a daunting challenge for clinical trial supply companies, necessitating constant vigilance and adaptability to maintain compliance.
-
Demand Forecasting: Accurately predicting the needs of diverse research sites is critical; however, failures in forecasting can lead to either shortages or surplus inventory, both of which are detrimental to progress. Consulting services from Supply Chain Wizard can assist in demand forecasting, logistics coordination, and data oversight to mitigate these issues.
-
Temperature Sensitivity: Numerous research materials are temperature-sensitive, necessitating strict controls that complicate logistics and subsequently increase costs.
-
Cost Management: Striking a balance between cost-effectiveness and the maintenance of quality and compliance remains a perpetual challenge for stakeholders. Tackling these challenges requires strong management techniques and efficient communication among all stakeholders engaged in the research process. Additionally, our service capabilities encompass feasibility and selection of research locations and principal investigators (PIs), comprehensive study setup, start-up, and approval processes, along with reporting on study status and inventory, which are essential for navigating these challenges.
The impact of Medtech clinical studies extends beyond the immediate research outcomes, contributing to local economies through job creation, economic growth, and improved healthcare services. As highlighted in the case study titled "Conclusions on COVID-19 Impact on Clinical Trials," the ongoing pandemic has underscored the importance of documenting any changes in Statistical Analysis Plans while striving to uphold original study objectives. Recommendations emphasize conducting thorough risk assessments to safeguard integrity of the experiment.
Such insights are vital as we navigate this complex landscape, where different approaches to defining pandemic periods in global studies may be necessary, and the rationale for these definitions must be meticulously documented. In the words of Scott R. Evans,
The author would like to thank Dr. Justin McArthur and Dr. John Griffin for their invitation to participate as part of the ANAs Summer Course for Clinical and Translational Research in the Neurosciences,
highlighting the collaborative spirit essential in overcoming these resource chain disruptions.
The Role of Technology in Enhancing Clinical Trial Supply Chains
Technology is essential for improving research distribution networks, promoting efficiencies and enhancing oversight processes. Several key advancements have transformed this landscape:
-
Supply Chain Management Software: Modern software solutions provide streamlined logistics, inventory management, and tracking capabilities, delivering real-time visibility into supply movements.
The adoption of such tools is increasing, allowing study sponsors to manage resources more effectively.
-
Blockchain Technology: This innovative technology enhances security and transparency in tracking trial materials. By ensuring data integrity, blockchain addresses concerns about traceability and compliance, which are essential in the research environment.
-
Data Analytics: Leveraging data analytics empowers organizations to forecast demand with greater accuracy, optimize inventory levels, and improve decision-making processes. This proactive approach is essential for managing the complexities of research trials.
-
Temperature Monitoring Solutions: Advancements in monitoring systems ensure that temperature-sensitive materials stay within specified ranges throughout the distribution chain. This is particularly vital for maintaining the efficacy of biologics and other sensitive products.
-
Automated Systems: Automation in packaging and labeling minimizes human mistakes and improves operational productivity, resulting in faster turnaround times for research materials.
By leveraging these technological advancements, clinical trial supply companies can significantly enhance their operational effectiveness, ultimately aiding the successful implementation of studies. For example, PCI Pharma Services’ growth in Berlin in March 2021 to enhance cold chain storage capabilities demonstrates the industry's dedication to fulfilling the increasing need for dependable medical research materials, particularly for temperature-sensitive items. This expansion is essential considering the size of medical studies, such as the Million Veteran Program and the All of Us study, both involving 1 million participants each, highlighting the significance of effective logistics management in managing large observational groups.
Additionally, a worldwide cooperative group from the University of Texas at Austin examined 1 billion potential molecules and chose the 30 most promising candidates for further drug development evaluation, highlighting the crucial role of advanced technology in medical testing. As the market keeps changing, the incorporation of these technologies will be essential in managing the intricacies of medical research logistics.
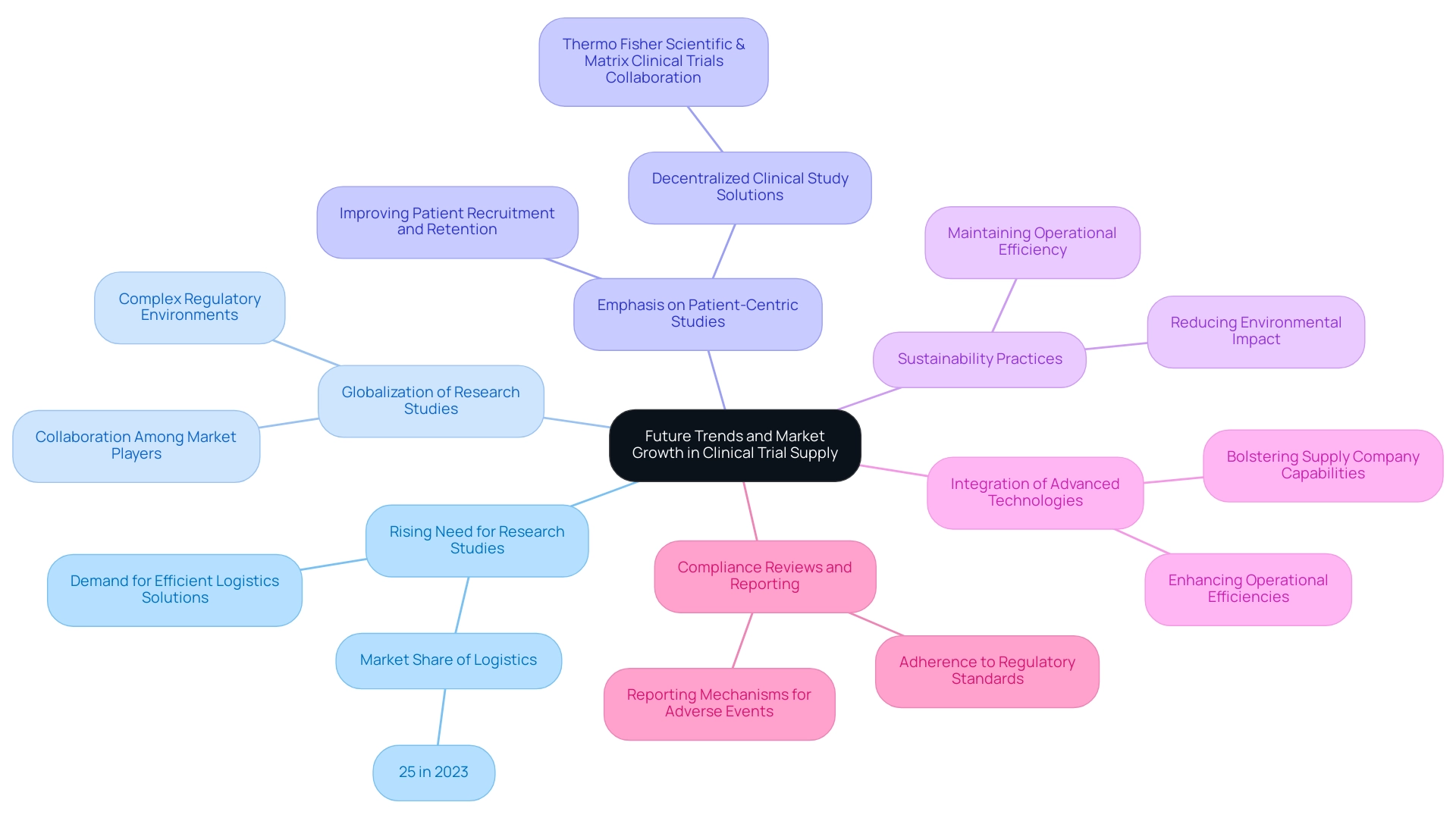
Future Trends and Market Growth in Clinical Trial Supply
The research study provision market is ready for significant expansion, driven by key trends that highlight the significance of thorough research study oversight services:
- Rising Need for Research Studies: The worldwide drive for healthcare advancement is heightening the demand for research studies, intensifying the necessity for efficient logistical solutions. This trend is evident as the logistics and services sector captured the largest market share of 25% in 2023, highlighting its critical role in meeting these demands.
- Globalization of Research Studies: As research studies expand into emerging markets, the complexity of varied regulatory environments requires strong logistics management. This globalization fosters collaboration among key market players, enhancing strategies to navigate these challenges effectively.
- Emphasis on Patient-Centric Studies: There is a notable shift toward designing studies that prioritize patient needs, significantly influencing supply chain strategies. Collaborations, like the one between Thermo Fisher Scientific and Matrix Clinical Trials, seek to create decentralized clinical study solutions, improving patient recruitment and retention. This illustrates the sector's dedication to innovative solutions that enhance efficiency and patient experiences.
- Sustainability Practices: The integration of sustainable methods within logistics is gaining momentum, with companies striving to reduce their environmental impact while maintaining operational efficiency.
- Integration of Advanced Technologies: Continuous investment in advanced technologies is anticipated to enhance operational efficiencies and bolster the capabilities of supply companies involved in research, improving their contributions to the research landscape.
- Compliance Reviews and Reporting: A critical aspect of study management includes thorough compliance reviews to ensure adherence to regulatory standards, alongside robust reporting mechanisms for serious and non-serious adverse events. These elements are essential for preserving the integrity of research studies.
As these trends develop, the role of clinical trial supply companies in supporting successful clinical research and advancing medical science will become increasingly critical.
Insights from McKinsey & Company highlight the value of collaborations, as noted in a recent testimonial: "Working with P&S Intelligence and their team was an absolute pleasure – their awareness of timelines and commitment to value greatly contributed to our project's success. Eagerly anticipating future collaborations." This emphasizes the importance of partnerships in facilitating timely project execution and fostering future advancements.
Conclusion
The pivotal role of clinical trial supply companies in the success of clinical research cannot be overstated. These organizations ensure that investigational drugs and medical devices are delivered efficiently and reliably, directly impacting the integrity and outcomes of clinical trials. Through a comprehensive suite of services, including:
- Supply chain management
- Regulatory compliance
- Project oversight
they facilitate seamless operations that are critical to the advancement of medical science.
As the industry faces numerous challenges, such as:
- Supply chain disruptions
- Regulatory changes
the adaptability and strategic management of these companies become essential. The introduction of advanced technologies, including:
- Supply chain management software
- Blockchain
further enhances their ability to navigate these complexities, ensuring that trials proceed without unnecessary delays or complications.
Looking ahead, the clinical trial supply market is on the brink of significant growth, driven by the increasing demand for clinical trials, globalization, and a shift toward patient-centric approaches. The integration of sustainable practices and advanced technologies will not only improve operational efficiencies but also ensure that these companies continue to play a vital role in shaping the future of healthcare. As the landscape evolves, the contributions of clinical trial supply companies will remain crucial, underscoring their importance in fostering innovation and improving patient outcomes in the realm of clinical research.
Frequently Asked Questions
What is the role of clinical trial supply companies in medical studies?
Clinical trial supply companies are vital for the logistics and distribution of necessary materials, such as investigational drugs and medical devices, ensuring efficient administration of research studies.
How do organizations like bioaccess® contribute to clinical trials?
Organizations like bioaccess® partner with Medtech startups to improve the logistics of clinical trials, ensuring resources are sent to research locations swiftly and effectively while providing services like regulatory authorization, research site initiation, and participant recruitment.
What financial significance do medical studies hold?
The cardiovascular diseases sector generated revenue of USD 982.2 million in 2020 and is projected to reach USD 1,198.7 million by 2023, highlighting the substantial financial interests involved in medical studies.
What is the projected growth of the MEA medical research logistics market?
The MEA medical research logistics market, valued at USD 162.90 million in 2023, is expected to grow to around USD 302.14 million by 2033, with a compound annual growth rate (CAGR) of 6.37%.
What services do clinical trial supply companies provide?
They provide a range of services including feasibility studies and site selection, logistics management, inventory management, labeling and packaging, temperature control, regulatory compliance, import permits, and project management and reporting.
Why is inventory management important in clinical trials?
Effective inventory management ensures that research sites are consistently equipped with the necessary supplies, facilitating uninterrupted research activities.
How do clinical trial supply companies ensure regulatory compliance?
They assist in navigating regulatory requirements by providing compliance reviews and feedback on study documents, ensuring adherence to country-specific regulations.
What advancements are being made in cold storage and distribution technologies?
Recent advancements, such as Bomi Group's acquisition by UPS, highlight the evolving healthcare logistics environment and its impact on clinical trial supply companies.
Which regions are expected to dominate the global research and logistics market?
North America is anticipated to dominate the global research and logistics market throughout the forecast phase, emphasizing its importance in this sector.
How do major market players influence clinical trial logistics?
Major market participants like Pfizer, Novartis, Roche, and Johnson & Johnson are shaping trends in inventory oversight and operational practices, which contribute to job creation, economic growth, and healthcare enhancement in local economies.




