Overview
Contract Research Organizations (CROs) in the USA are specialized entities that provide essential services to the pharmaceutical, biotechnology, and medical device industries, facilitating research studies through project management, regulatory compliance, and data management. The article illustrates their growing importance in drug development, particularly in the context of personalized medicine and the challenges faced by medical device startups, highlighting how CROs enhance efficiency and compliance in research processes.
Introduction
The landscape of clinical research is rapidly evolving, with Contract Research Organizations (CROs) at the forefront of this transformation. These specialized entities play a crucial role in supporting the pharmaceutical, biotechnology, and medical device industries, particularly in the management and execution of clinical trials. As the demand for innovative therapies and personalized medicine escalates, CROs are increasingly recognized as indispensable partners, enabling sponsors to focus on their core research activities while navigating complex regulatory environments.
This article delves into the multifaceted functions of CROs, the current market trends shaping the industry, and the collaborative partnerships that enhance the efficiency and effectiveness of clinical trials. As the sector embraces technological advancements and patient-centric approaches, understanding the pivotal role of CROs becomes essential for stakeholders aiming to drive successful research outcomes.
Defining Contract Research Organizations: An Overview
Contract Research Organizations in the USA are specialized entities that offer crucial assistance to the pharmaceutical, biotechnology, and medical device industries in the management and execution of research studies. Their comprehensive service offerings encompass:
- Project management
- Clinical trial monitoring
- Regulatory compliance
- Data management
- Statistical analysis
- Trial set-up
- Detailed reporting processes
As the demand for personalized medicine rises, contract research organizations in the USA are increasingly viewed as vital partners in drug development, allowing pharmaceutical companies to focus on core research activities.
Furthermore, the challenges faced by medical device startups, such as regulatory hurdles, competition, and recruitment issues, highlight the importance of CROs in streamlining these processes. Significantly, the partnership between bioaccess™ and Caribbean Health Group in Barranquilla, backed by Colombia's Minister of Health, aims to establish the region as a premier location for clinical research in Latin America. This initiative exemplifies how strategic partnerships can enhance trial capabilities and improve recruitment efficiency.
For instance, GlobalCare Clinical Trials' partnership with bioaccess™ has achieved over a 50% reduction in recruitment time and a remarkable 95% retention rate, illustrating the effectiveness of CRO services in addressing industry challenges. According to recent statistics, the Asia-Pacific region's CRO market is expected to grow at the fastest pace over the next few years, further underscoring the expanding role of these organizations. By delegating these essential functions to contract research organizations in the USA, sponsors not only improve the efficiency and effectiveness of their projects but also ensure strict compliance with regulatory requirements.
This trend is depicted by the increasing need for personalized medicine, driving market expansion and emphasizing the crucial role that contract research organizations in the USA fulfill in connecting the gap between innovation and market introduction. As the sector adopts novel methods like decentralized studies and digital twins, contract research organizations will progressively tackle recruitment obstacles and enhance study design, greatly influencing patient care and results in the changing environment of medical inquiry.
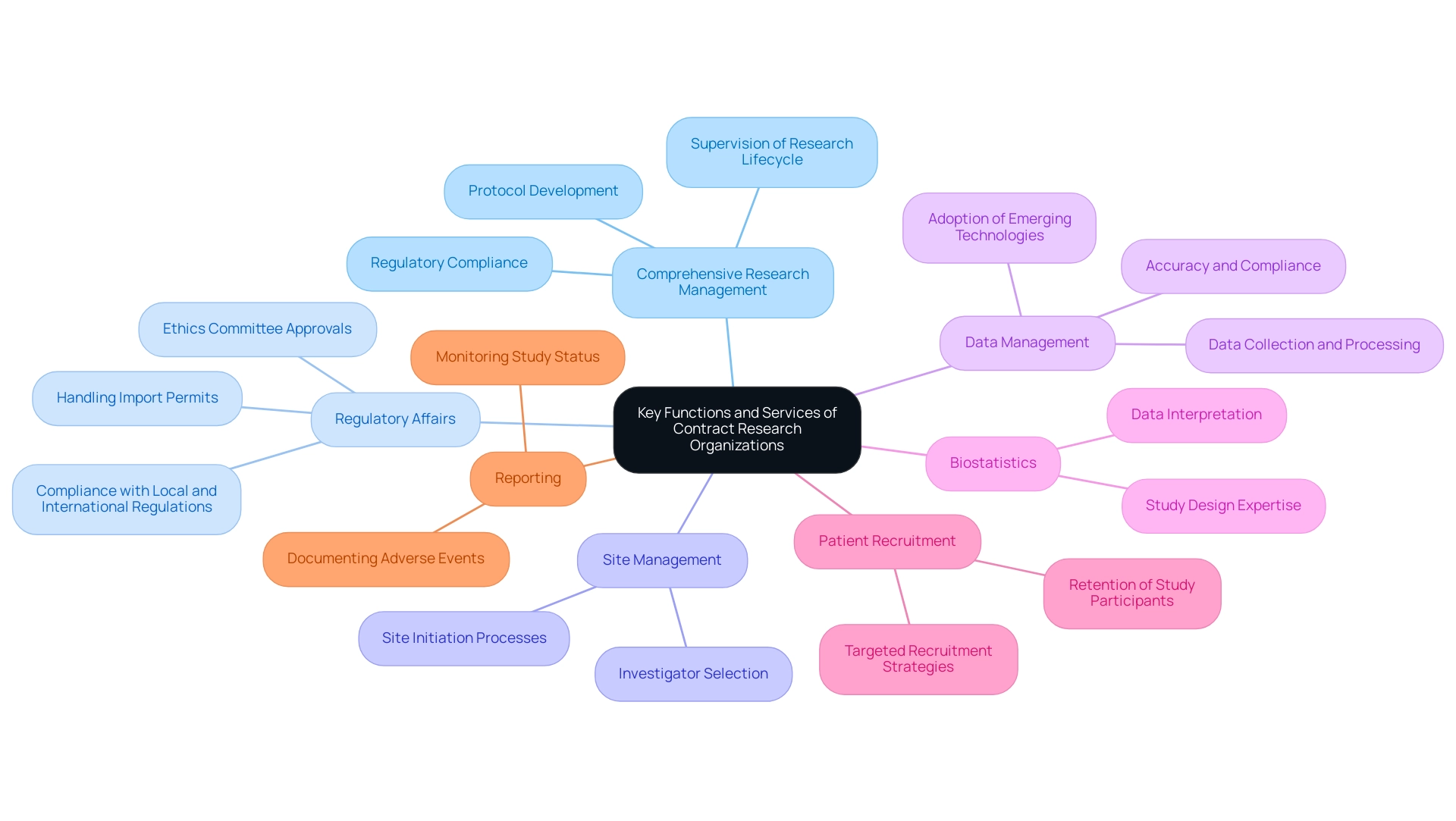
Key Functions and Services of Contract Research Organizations
Contract Research Organizations in USA offer an important array of services crucial to the study process, enabling effective and compliant investigations. The market size for contract research organizations in the USA was estimated at USD 13.95 billion in 2023 and is projected to hit around USD 27.44 billion by 2033, growing at a CAGR of 7.0% from 2024 to 2033. These services include:
- Comprehensive Research Management: CROs supervise the entire research lifecycle, from initial planning and protocol development through to execution and close-out, ensuring that studies adhere to regulatory standards and timelines. Their capabilities encompass the evaluation and choice of research locations and principal investigators (PIs), along with thorough assessment and feedback on study documents to guarantee adherence to country requirements.
- Regulatory Affairs: A vital function, this service ensures compliance with both local and international regulations overseeing research. As the U.S. pharmaceutical R&D expenditure is expected to hit USD 301 billion in 2023, regulatory expertise is increasingly essential to navigate the intricacies of drug development, including study setup, approval from ethics committees, health ministries, and the handling of import permits and nationalization of investigational devices.
- Site Management: This encompasses the identification and management of research sites, which involves meticulous investigator selection and site initiation processes. Effective site management is vital for study success and participant safety.
- Data Management: Contract Research Organizations excel in collecting, processing, and analyzing study data, ensuring its accuracy and compliance with regulatory standards. The necessity for contract research organizations in USA to adopt emerging technologies is emphasized by case studies showing that those who fail to leverage technological advancements risk falling behind in the competitive landscape of contract research.
- Biostatistics: Providing essential statistical expertise for study design and data interpretation, biostatistics ensures that the results are scientifically valid and can inform future research and clinical practices.
- Patient Recruitment: Implementing targeted strategies for recruiting and retaining study participants is essential for project viability. Given that the total cost of developing a drug in the U.S. can reach approximately USD 2.6 billion, effective patient recruitment can significantly impact study timelines and costs.
- Reporting: Comprehensive reporting is crucial for monitoring study status, inventory management, and documenting both serious and non-serious adverse events. This guarantees openness and adherence throughout the study process.
Through these comprehensive functions, contract research organizations in USA enable sponsors to focus on their main strengths while utilizing the specialized knowledge of these organizations, thereby improving the chances of successful study results. As Andrew MacGarvey, CEO of Phastar, states,
Increasingly, our customers and potential clients are approaching us with innovative demands, such as incorporating synthetic data into their upcoming studies.
This illustrates the changing environment of trial management and the essential function that contract research organizations in USA fulfill in addressing these new challenges.
Furthermore, leading CROs are concentrating on effective study techniques, talent acquisition, client engagement, and enhancing their domestic market presence, further highlighting their significance in the industry.
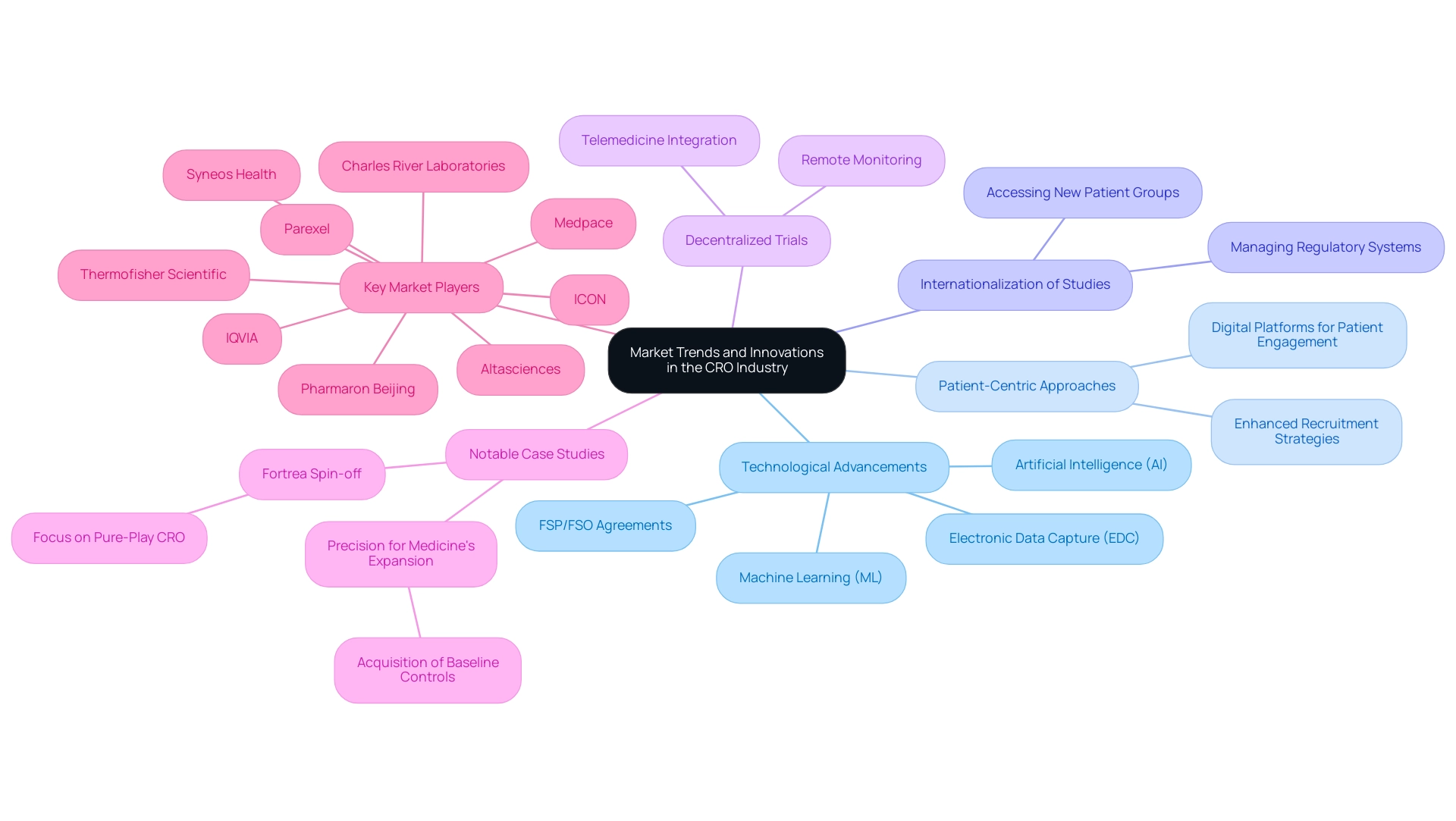
Market Trends and Innovations in the CRO Industry
The contract research organizations in USA sector are undergoing a transformative period, driven by significant market trends and innovations that are reshaping research studies. A key trend is the integration of technological advancements; tools like electronic data capture (EDC), artificial intelligence (AI), and machine learning (ML) are revolutionizing data collection and analysis, driving efficiencies in research studies. Significantly, 87% of drug creators are choosing Full-Service Provider (FSP) or hybrid FSP/FSO agreements, indicating the increasing dependence on external knowledge for research development outsourcing.
Extensive research management services are essential, including:
- Feasibility studies
- Site selection
- Compliance evaluations
- Setup
- Import permit processes
- Project oversight
- Detailed reporting on study status and adverse occurrences
The import permit process is critical, as it ensures that investigational devices are legally brought into the country, complying with local regulations. Additionally, effective reporting mechanisms are crucial for monitoring study progress, including the documentation of serious and non-serious adverse events, which is essential for regulatory compliance and patient safety.
In parallel, patient-centric approaches are gaining momentum as clinical research organizations prioritize strategies that enhance recruitment and retention. For example, numerous organizations are employing digital platforms to connect with patients more effectively, ensuring that the research process is more accessible and engaging. Industry leaders such as Peyton Howell, CEO of Parexel, stress that the contract research organizations in USA sector is poised for substantial growth, fueled by an increase in R&D funding throughout the pharmaceutical and biotech fields.
The internationalization of studies is another vital trend; as medical evaluations extend beyond borders, CROs skillfully manage various regulatory systems and cultural environments, allowing sponsors to access new patient groups. Additionally, the rise of decentralized trials—facilitated by advancements in remote monitoring and telemedicine—affords greater flexibility and accessibility for patients. This trend not only enhances participation rates but also reflects the industry’s commitment to innovation and efficiency.
A notable case is Precision for Medicine, which, in 2023, acquired Baseline Controls to bolster its biomanufacturing capabilities. This strategic move is anticipated to accelerate the drug development process, demonstrating how technological advancements can convert into tangible advantages for research. Furthermore, the recent spin-off of Fortrea from Labcorp in 2023 highlights a strategic focus on becoming a pure-play CRO, as it plans to divest its Endpoint Clinical and Fortrea Patient Access businesses for $345 million. This shift underscores the ongoing evolution within the industry.
Key market players such as Medpace, ICON, IQVIA, and Syneos Health, recognized as contract research organizations in USA, are also adapting to these trends, reinforcing the competitive landscape. As these trends develop, they highlight the CRO industry's adaptability to changing market needs and its commitment to improving the efficiency and effectiveness of research studies.
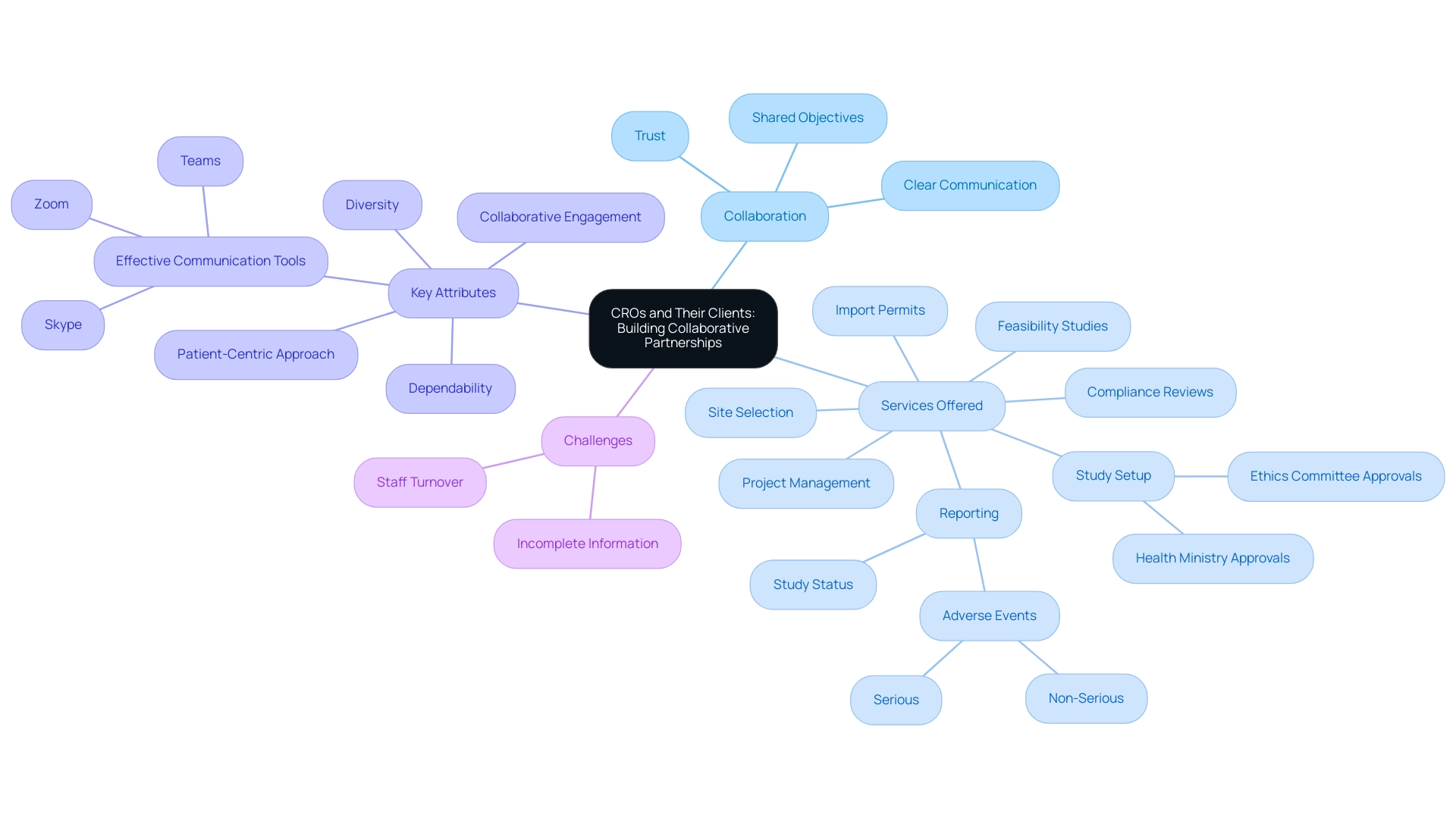
CROs and Their Clients: Building Collaborative Partnerships
The relationship between Contract Research Organizations and their clients—comprising pharmaceutical companies and academic institutions—is fundamentally rooted in collaboration and mutual benefit. Successful partnerships hinge upon clear communication, trust, and shared objectives. Contract research organizations offer an extensive array of research management services, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup (including ethics committee and health ministry approvals)
- Import permits and nationalization of investigational devices
- Project management
- Reporting (study status, inventory, serious and non-serious adverse events)
This specialized expertise allows clients to optimize resources while concentrating on their core business operations. Statistics reveal that 58% of respondents regularly utilize applications such as Zoom, Skype, or Teams, underscoring the importance of effective communication tools in fostering these relationships. By engaging a CRO to manage a Phase III clinical trial, a pharmaceutical company can capitalize on the CRO's proficiency in regulatory compliance and patient recruitment strategies.
Furthermore, contract research organizations often partner with academic institutions to improve study efforts, effectively merging scientific insight with practical application. This synergistic approach not only enhances the quality of studies but also significantly speeds up the timeline for introducing new therapies to the market. Dependability, diversity, a patient-centric approach, and collaborative engagement are essential non-negotiables in a CRO partner, reinforcing the critical attributes necessary for successful collaborations.
However, challenges such as staff turnover and incomplete information can hinder these partnerships, emphasizing the need for robust communication and trust. Statistics suggest that successful partnerships between contract research organizations and pharmaceutical firms can result in enhanced success rates in medical studies, emphasizing the essential role of these collaborations in the evolving field of medical investigations.
The Future of Contract Research Organizations: Challenges and Opportunities
The medical research environment is experiencing considerable change, offering both challenges and opportunities for Contract Research Entities. As the industry navigates increasing complexity in testing and heightened regulatory scrutiny, the demand for continuous innovation has never been greater. With extensive research study management services, encompassing:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Study setup (including ethics committee approvals)
- Import permits
- Project oversight
- Thorough reporting on severe and non-severe adverse events
contract research organizations are positioned to advance global health enhancement.
The CRO industry has grown at a CAGR of 3.0% between 2019 and 2024, underscoring its expansion. Peyton Howell, CEO of Parexel, aptly notes,
The CRO industry is on the cusp of significant expansion driven by a surge in R&D investment across the pharma and biotech landscapes.
This expansion creates opportunities for contract research organizations to stand out by offering specialized services that cater to evolving client needs, particularly in the context of international collaboration and innovation in Medtech.
Leading companies in the market, such as Iqvia Holdings Inc., Charles River Laboratories International, Inc., and Pra Health Sciences, Inc., exemplify how organizations can thrive by adapting to these changes. For instance, with the rise of decentralized medical trials, organizations that successfully implement remote monitoring and data collection solutions can gain a substantial competitive advantage. Furthermore, the burgeoning emphasis on personalized medicine allows clinical research organizations to tailor their approaches to meet the unique requirements of specific patient populations.
Case studies showcasing the influence of Medtech research on local economies, including job creation and healthcare enhancement, illustrate the essential role that contract research organizations play in improving patient outcomes. By embracing AI and enhancing data quality, organizations can thrive by providing quicker results in the drug development process. By effectively addressing these challenges and harnessing emerging opportunities, CROs can solidify their roles as vital partners in advancing clinical research and improving healthcare globally.
Conclusion
The role of Contract Research Organizations (CROs) is increasingly vital in the evolving landscape of clinical research. By providing essential services such as:
- Project management
- Regulatory compliance
- Data management
- Patient recruitment
CROs allow sponsors to concentrate on their core research activities while ensuring efficient trial execution.
As the demand for personalized medicine rises, CROs navigate complex regulatory environments and enhance trial capabilities through strategic partnerships and technological advancements. The adoption of tools like artificial intelligence and electronic data capture is revolutionizing the clinical trial process, driving efficiencies and improving patient outcomes. Additionally, the rise of decentralized trials reflects the industry's commitment to innovation and accessibility for participants.
Successful collaborations between CROs and their clients are crucial, relying on trust and effective communication. By leveraging CRO expertise, sponsors can overcome challenges and accelerate the market introduction of new therapies.
In conclusion, as clinical research continues to evolve, the significance of CROs will only increase. Their ability to adapt to market trends and technological changes positions them as essential partners in advancing healthcare and improving patient care. Embracing these developments will enhance clinical trial efficiency and contribute to the success of the pharmaceutical and biotechnology industries.
Frequently Asked Questions
What are Contract Research Organizations (CROs) in the USA?
Contract Research Organizations in the USA are specialized entities that assist the pharmaceutical, biotechnology, and medical device industries in managing and executing research studies.
What services do CROs in the USA provide?
CROs offer a range of services including project management, clinical trial monitoring, regulatory compliance, data management, statistical analysis, trial set-up, and detailed reporting processes.
Why are CROs increasingly important in drug development?
As the demand for personalized medicine rises, CROs are viewed as vital partners that allow pharmaceutical companies to focus on core research activities while ensuring efficient and compliant study processes.
What challenges do medical device startups face that CROs help to address?
Medical device startups face challenges such as regulatory hurdles, competition, and recruitment issues, which CROs help to streamline.
Can you provide an example of a successful partnership involving CROs?
The partnership between bioaccess™ and Caribbean Health Group aims to establish Barranquilla as a premier location for clinical research in Latin America, demonstrating how strategic partnerships can enhance trial capabilities and improve recruitment efficiency.
What impact have CROs had on recruitment times in clinical trials?
For instance, GlobalCare Clinical Trials’ partnership with bioaccess™ has achieved over a 50% reduction in recruitment time and a 95% retention rate, showcasing the effectiveness of CRO services.
What is the projected market size for CROs in the USA by 2033?
The market size for contract research organizations in the USA was estimated at USD 13.95 billion in 2023 and is projected to reach around USD 27.44 billion by 2033, growing at a CAGR of 7.0% from 2024 to 2033.
What are some key functions of CROs regarding research management?
CROs supervise the entire research lifecycle, ensuring studies adhere to regulatory standards and timelines, including site selection and protocol development.
How do CROs ensure regulatory compliance?
CROs provide regulatory affairs services that ensure compliance with local and international regulations, which is crucial for navigating drug development complexities.
What role do CROs play in data management?
CROs excel in collecting, processing, and analyzing study data, ensuring its accuracy and compliance with regulatory standards.
How do CROs assist with patient recruitment?
CROs implement targeted strategies for recruiting and retaining study participants, which is essential for project viability and can significantly impact study timelines and costs.
What is the significance of reporting in CRO services?
Comprehensive reporting is crucial for monitoring study status, inventory management, and documenting adverse events, ensuring transparency and adherence throughout the study process.
How are CROs adapting to new demands in clinical trials?
Leading CROs are focusing on innovative demands such as incorporating synthetic data into studies, reflecting the changing environment of trial management.




