Introduction
As the demand for innovative medical technologies surges, the role of Contract Research Organizations (CROs) in the Medtech sector becomes increasingly vital. These specialized entities provide essential support to medical technology companies throughout the intricate processes of product development and commercialization.
In Colombia, a burgeoning hub for clinical research, Medtech CRO services are not only transforming the landscape of healthcare but also presenting unique opportunities and challenges for stakeholders.
With a favorable regulatory environment and advancements in technology, companies like bioaccess® are at the forefront, offering comprehensive services that streamline clinical trials and ensure compliance with rigorous standards.
This article delves into the multifaceted world of Medtech CRO services in Colombia, exploring their significance, the evolving regulatory landscape, and the technological innovations that are shaping the future of clinical research.
Understanding Medtech CRO Services: Definition and Scope
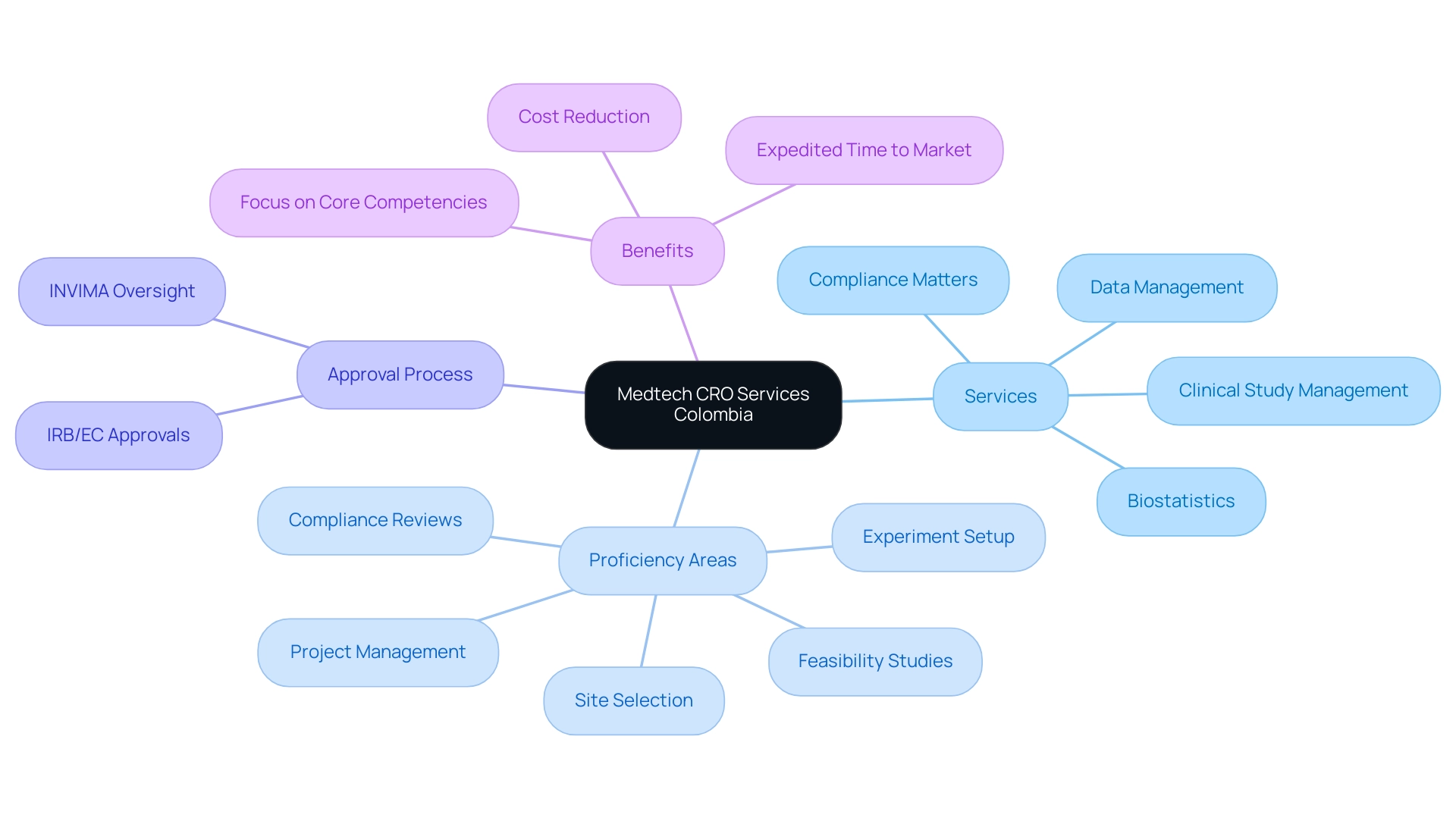
Medtech CRO Services Colombia provide specialized support to medical technology companies during the development and commercialization of their products. At bioaccess®, we provide a complete range of services that includes:
- Clinical study management
- Compliance matters
- Data management
- Biostatistics, customized specifically for medical device studies
Our proficiency encompasses:
- Feasibility studies
- Site selection
- Compliance reviews
- Experiment setup
- Project management
This ensures conformity to standards and speeds up the process to market.
The approval process includes acquiring required approvals from the institutional review board (IRB)/ethics committee (EC) and Colombia's oversight agency (INVIMA). With Colombia rising as a leading location for first-in-human clinical trials thanks to its cost effectiveness, rapid oversight procedures, and high-quality healthcare, we offer Medtech CRO Services Colombia to assist medical technology companies in managing the intricacies of clinical research while taking advantage of these competitive benefits.
We also provide essential reporting processes, including:
- Study status updates
- Adverse event reporting
These are critical for compliance with regulations.
By outsourcing these critical functions to a CRO like bioaccess®, companies can focus on their core competencies, reduce operational costs, and expedite the time to market for their innovations. In essence, Medtech CRO Services Colombia are integral to the successful development of medical technologies, bridging the gap between research and practical application in healthcare settings.
BOOK A MEETING.
The Landscape of Medtech CRO Services in Colombia: Opportunities and Challenges
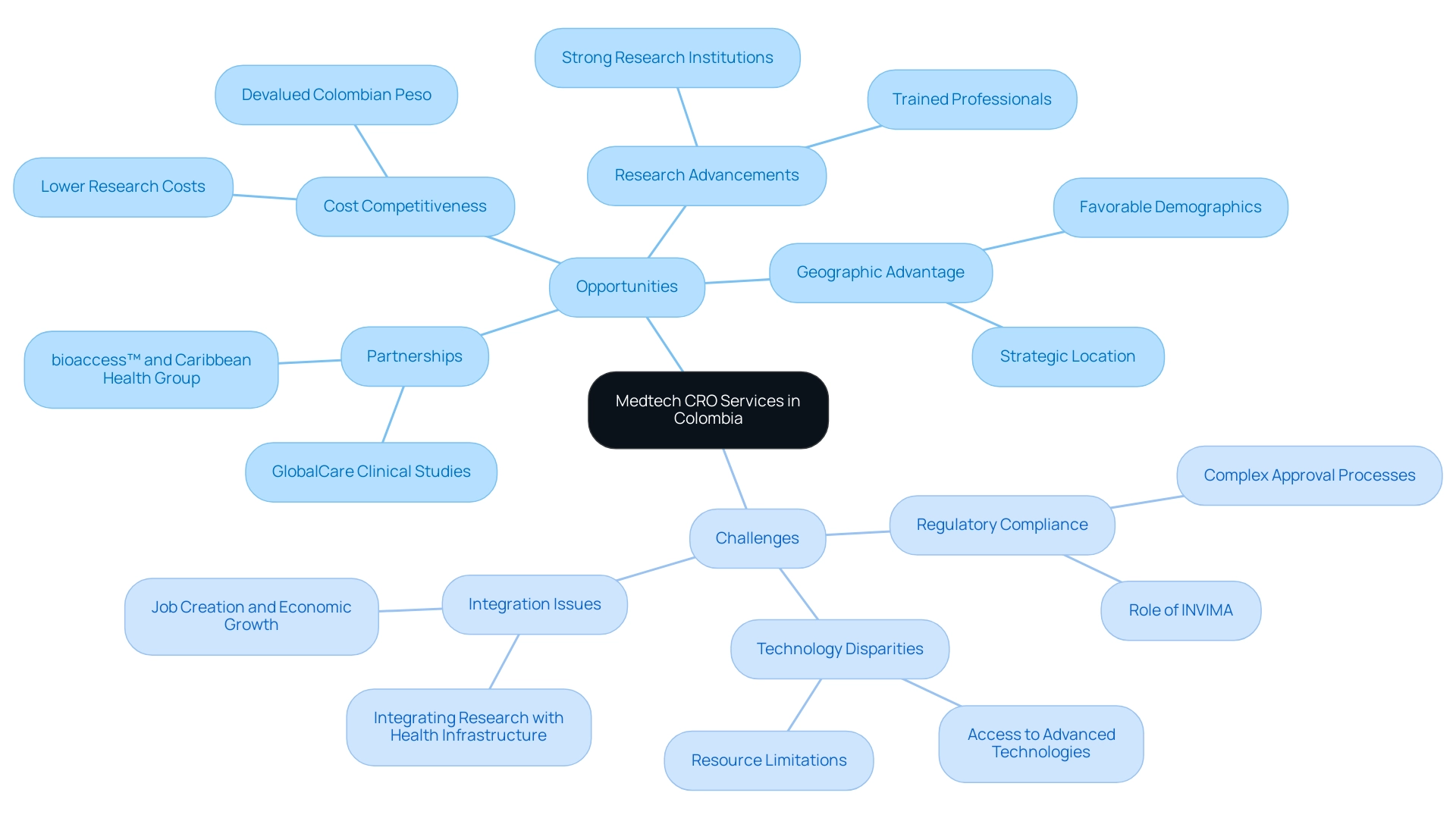
Colombia is rapidly emerging as a significant market for Medtech CRO Services Colombia, fueled by an increasing demand for innovative medical technologies and a regulatory environment that is becoming more accommodating. Significantly, bioaccess™ and Caribbean Health Group's partnership seeks to establish Barranquilla as a premier location for trials in Latin America, a vision endorsed by Colombia's Minister of Health. The Colombian peso, being the most devalued currency in Latin America, contributes to competitive costs for medical research, making it an appealing choice for Medtech companies.
The nation has made significant advancements in improving its research capabilities, indicated by an increasing number of trained professionals and strong research institutions. 'GlobalCare Clinical Studies' collaboration with bioaccess™ further improves ambulatory services in Colombia, leading to more than a 50% decrease in recruitment duration and an impressive retention rate exceeding 95%. Experts, including Julio G. Martinez-Clark, CEO of bioaccess™, emphasize that the favorable conditions in Colombia contribute to lower dropout rates, which are one-third of those observed in the U.S. and the EU.
However, challenges persist, particularly in navigating a compliance landscape that can often be intricate and time-intensive, impacting the efficiency of Medtech CRO Services Colombia. INVIMA, Colombia's National Food and Drug Surveillance Institute, plays a critical role in overseeing medical device regulation and ensuring compliance as a Level 4 health authority recognized by PAHO/WHO. This regulatory environment can pose hurdles for companies, as they must navigate complex approval processes, which can delay the start of research trials.
Additionally, disparities in access to advanced technologies and resources when compared to more developed markets may further complicate efforts. A case study titled 'Integrating Research with Existing Health Infrastructure' illustrates how efforts to integrate new studies into local health systems can not only strengthen care delivery but also enhance the overall healthcare landscape while conducting research. The integration of clinical studies can lead to job creation and economic growth, ultimately benefiting the local economy.
Nevertheless, Colombia's strategic geographic location, favorable demographic trends, and ongoing commitment to improving healthcare infrastructure present a compelling case for medical technology companies seeking Medtech CRO Services Colombia. This interplay of opportunities and challenges creates a vibrant environment for local and international stakeholders eager to leverage the potential within Colombia's medical technology sector.
Key Regulatory Considerations for Medtech CRO Services in Colombia
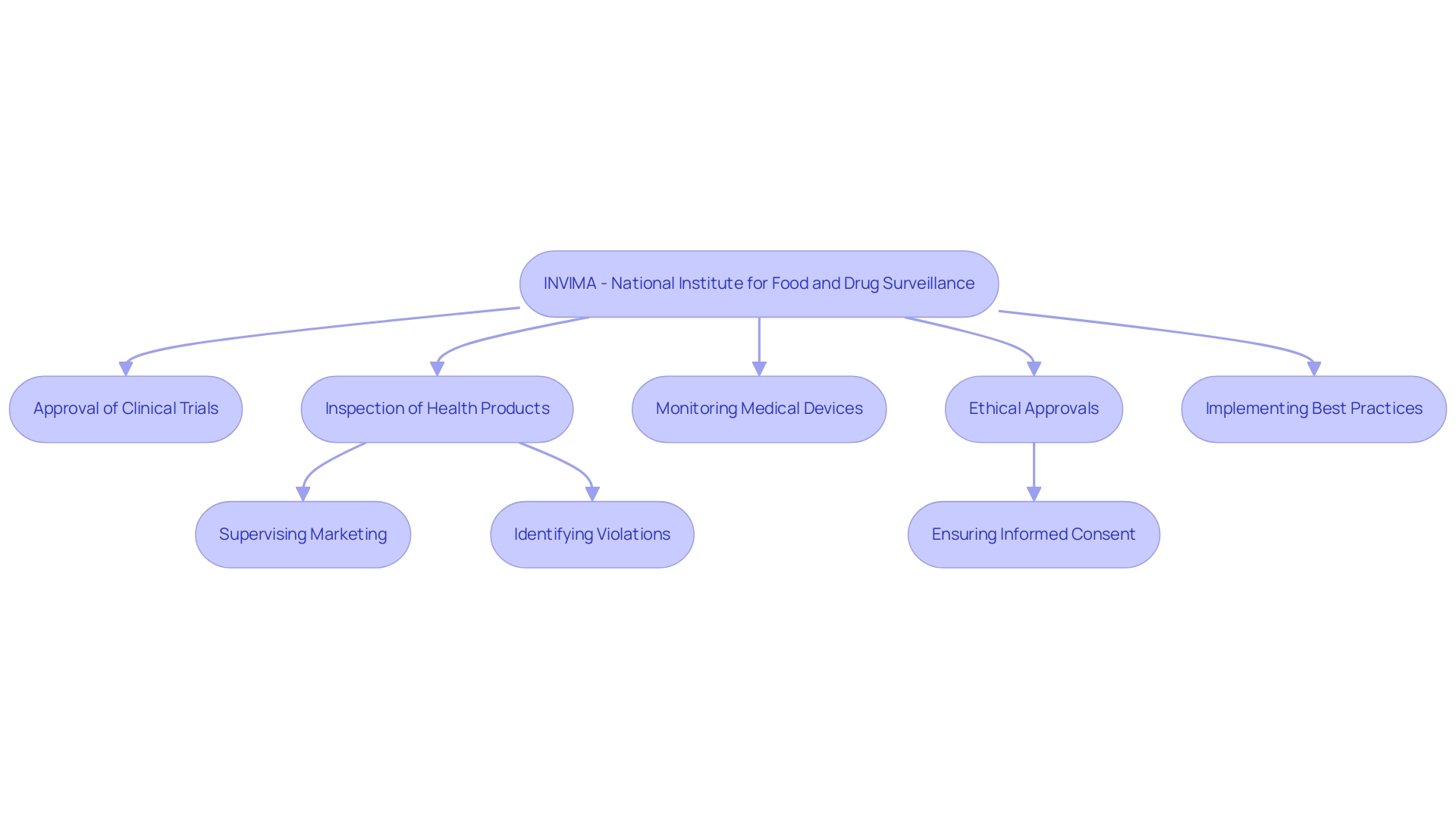
Navigating the compliance landscape is paramount for Medtech CRO Services Colombia that are operating in the region. This landscape is governed primarily by the National Institute for Food and Drug Surveillance (INVIMA), recognized as a Level 4 health authority by the Pan American Health Organization/World Health Organization (PAHO/WHO). INVIMA oversees the approval of clinical trials and medical devices, playing a crucial role in ensuring compliance with safety, efficacy, and quality standards.
Its specific functions include:
- Inspecting and supervising the marketing and manufacturing of health products
- Identifying and evaluating violations of health standards
- Implementing best practices
The Directorate for Medical Devices and other Technologies within INVIMA is responsible for monitoring and controlling medical devices, tracking pre- and post-market programs, and suggesting technical standards for quality assurance. Essential legal considerations include obtaining necessary ethical approvals, ensuring informed consent from participants, and adhering to Good Clinical Practice (GCP) guidelines.
Between March 1, 2013, and December 31, 2016, a total of 356 research protocols were submitted to the Institutional Committee on Bioethics for Health (CIBS FM & MCH), resulting in 309 protocols receiving approval. This statistic not only emphasizes the significance of adherence to ethical standards but also mirrors the expanding network of trained professionals contributing to the framework. Katherine Ruiz, a compliance affairs expert in medical devices and in vitro diagnostics, has advised many foreign manufacturers on obtaining market clearance in Colombia, highlighting the value of expertise in navigating these complex regulations.
The training program at the University of Chile, which successfully trained 50 professionals over ten years, has played a significant role in promoting bioethical discourse and enhancing ethical standards in research. As Liza Dawson points out,
Transitioning from research ethics review to research ethics systems in low-income and middle-income countries
highlights the necessity for strong oversight frameworks in such environments. CROss must continuously monitor compliance updates to mitigate risks and ensure that studies conducted under Medtech CRO Services Colombia are carried out ethically while meeting both national and international standards.
The evolving regulations, including the latest updates from INVIMA regarding research studies in 2024, further emphasize the necessity for CROs to adapt their operational strategies to align with current ethical and regulatory expectations.
The Role of Technology in Enhancing Medtech CRO Services
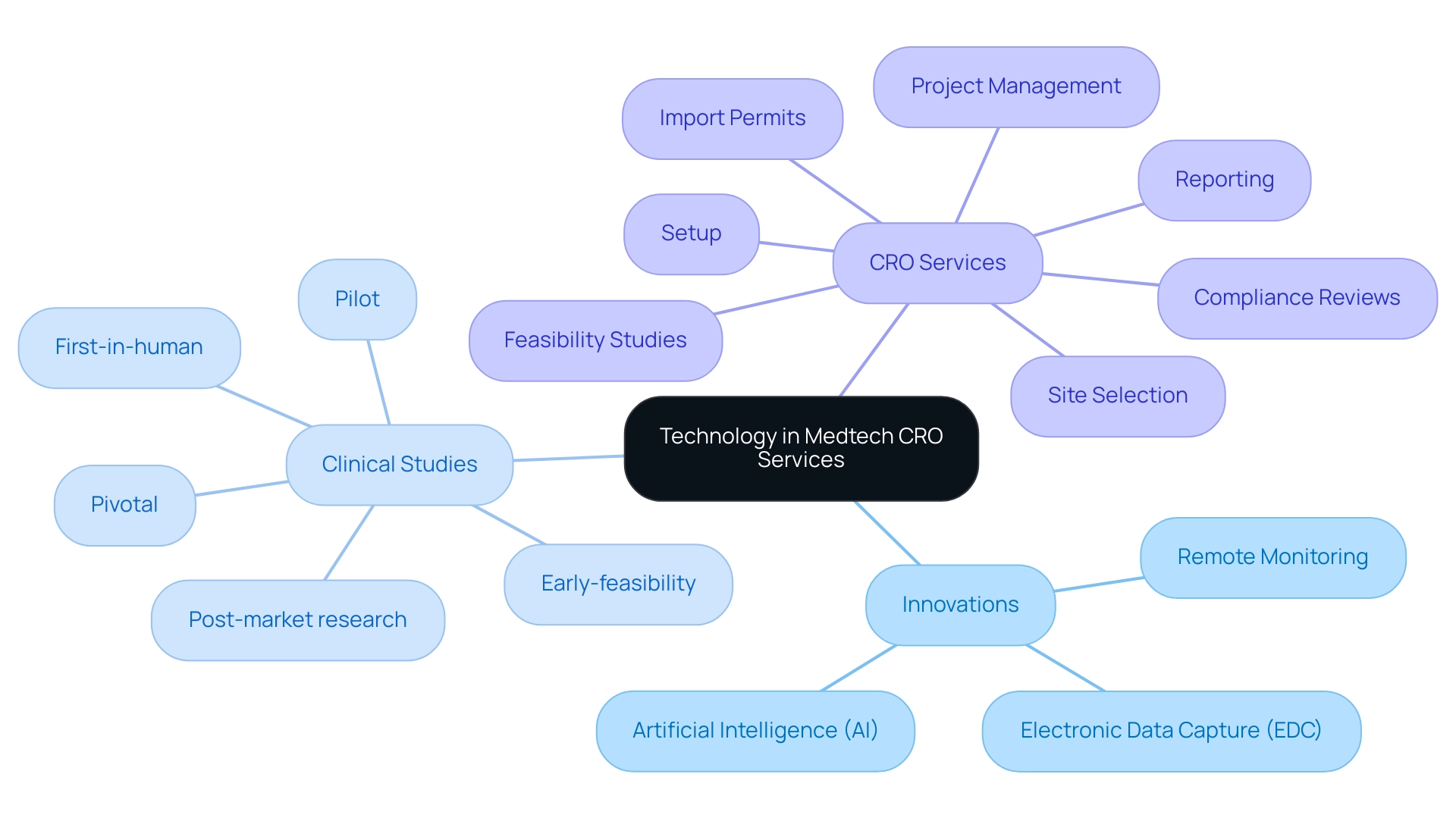
Technology plays a pivotal role in enhancing the efficiency and effectiveness of Medtech CRO Services Colombia, especially through the comprehensive management services offered by companies like bioaccess®. Innovations such as electronic data capture (EDC), remote monitoring, and artificial intelligence (AI) are revolutionizing how clinical studies, including:
- Early-feasibility
- First-in-human
- Pilot
- Pivotal
- Post-market research
are conducted. EDC systems streamline data collection and management, reducing the likelihood of errors while ensuring real-time access to information.
Remote monitoring technologies enable more adaptable study designs and enhance patient involvement, addressing specifically the needs of Medtech startups maneuvering through the intricacies of Latin American regulations, which can be supported by Medtech CRO Services Colombia. Additionally, bioaccess® provides essential Medtech CRO Services Colombia, which include:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting
all vital for successful execution. AI assists in data analysis and patient recruitment, further enhancing trial efficiency.
By incorporating these technological advancements and comprehensive services, CROs like bioaccess® can enhance operations, resulting in quicker and more dependable research outcomes, ultimately closing gaps in research and innovation across the region.
Future Trends in Medtech CRO Services in Colombia
The future of Medtech CRO Services Colombia is poised for growth, driven by advancements in technology, regulatory improvements enforced by INVIMA, and increasing demand for innovative healthcare solutions. As the medical technology sector continues to evolve, CROss like bioaccess® are likely to play an even more significant role in facilitating comprehensive research through their expertise in:
- Early-Feasibility
- First-In-Human
- Pilot
- Pivotal
- Post-Market Follow-Up Studies
Our service capabilities encompass feasibility and selection of research sites, project management, and compliance reviews, ensuring a thorough approach to trials.
Furthermore, trends such as personalized medicine, telehealth, and digital therapeutics are expected to gain traction, necessitating the use of Medtech CRO Services Colombia that are agile and responsive. With over 20 years of experience in medical technology, bioaccess® is a trusted partner in navigating the complexities of clinical research. Collaborations between local CROss and international partners may become more prevalent, enhancing the capabilities and reach of Colombian healthcare technology firms while creating jobs and promoting economic growth.
By staying attuned to these trends, stakeholders can position themselves to leverage the opportunities that lie ahead in Medtech CRO Services Colombia.
Conclusion
The Medtech sector in Colombia is undergoing a transformative phase, driven by the essential services provided by Contract Research Organizations (CROs). As highlighted throughout the article, CROs like bioaccess® are pivotal in navigating the complexities of clinical trials, regulatory compliance, and technological advancements, ultimately facilitating the successful development and commercialization of medical technologies.
The landscape of Medtech CRO services in Colombia presents a unique blend of opportunities and challenges. With a favorable regulatory environment and a growing pool of trained professionals, the country is emerging as a competitive destination for clinical research. However, the intricate regulatory framework requires careful navigation to ensure compliance and efficiency. The collaboration between local and international stakeholders is crucial in overcoming these hurdles and maximizing the potential of the Colombian Medtech sector.
Looking ahead, the integration of innovative technologies will further enhance the capabilities of CROs, enabling them to deliver faster and more reliable research outcomes. As trends such as personalized medicine and telehealth gain momentum, the demand for agile and responsive CRO services will only increase. By staying abreast of these developments and leveraging the existing strengths within the Colombian healthcare landscape, stakeholders can capitalize on emerging opportunities and contribute to the advancement of medical technology in the region.




