Introduction
In the dynamic landscape of the medical device industry, the role of project managers has become increasingly vital to ensuring the successful development and market introduction of innovative products. These professionals serve as the central hub in a complex web of stakeholders, navigating the intricate processes of regulatory compliance, risk management, and resource allocation. As they orchestrate the myriad tasks involved—from feasibility studies to trial setup—they not only drive projects forward but also mitigate the challenges posed by stringent regulations and resource constraints.
With a growing emphasis on soft skills and effective stakeholder engagement, the impact of adept project management is evident in the enhanced success rates of medical device initiatives. This article delves into the critical responsibilities of project managers, the essential skills required, and the challenges they face, all while highlighting the significant influence of strategic project management on the advancement of healthcare solutions.
The Role of Project Managers in the Medical Device Industry
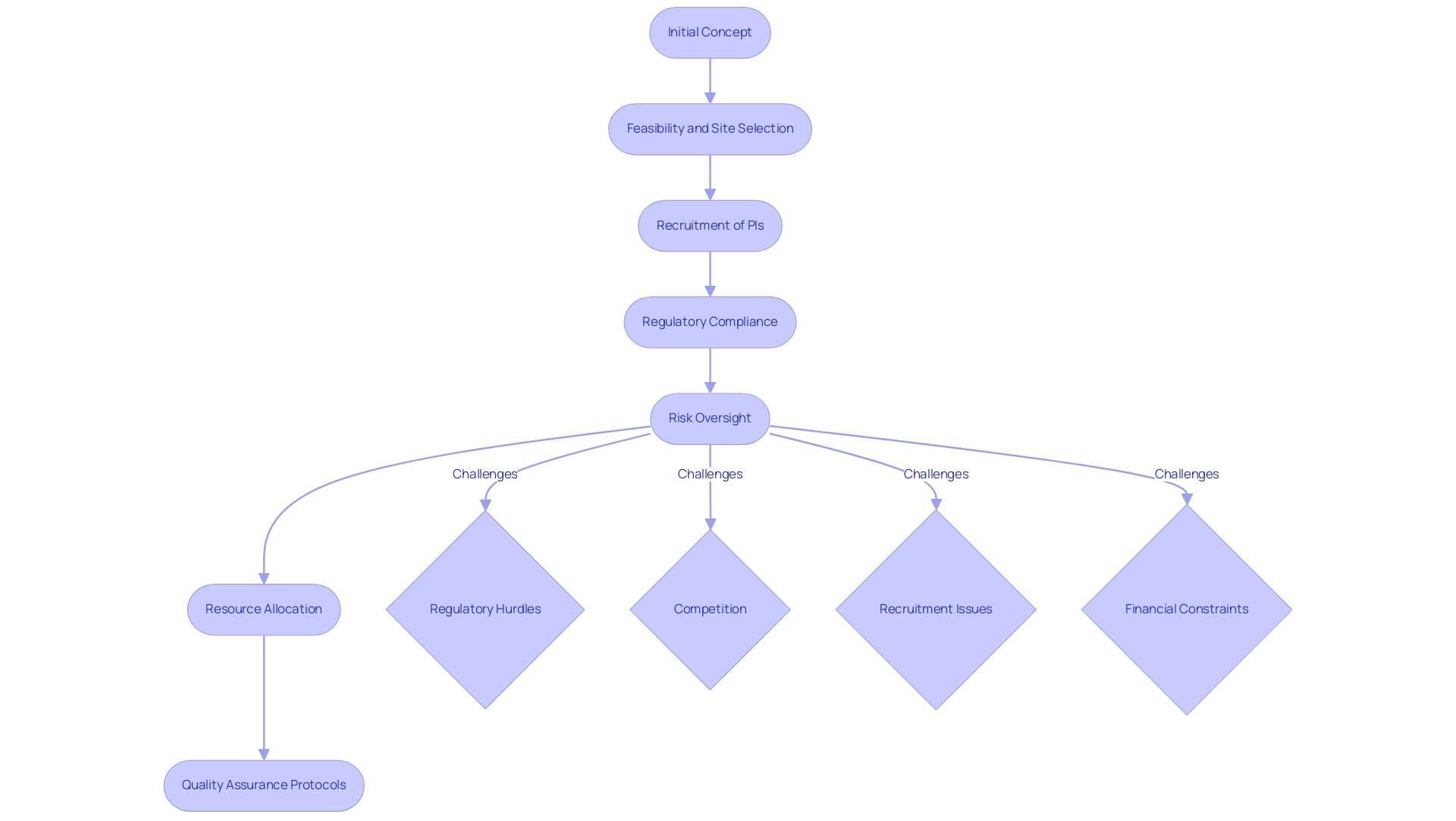
In the healthcare equipment sector, the role of a project manager medical device is essential in directing the creation and lifecycle of medical products from initial concept to market launch. Their responsibilities encompass a comprehensive process that includes:
- Feasibility and selection of research sites
- Recruitment of principal investigators (PIs)
- Strict adherence to regulatory compliance
Serving as the linchpin among stakeholders—including researchers, engineers, regulatory bodies, and marketing teams—coordinators ensure that all elements align with strategic objectives.
They navigate critical aspects such as:
- Risk oversight
- Resource allocation
- Enforcement of quality assurance protocols
While also addressing the complexities that a project manager medical device faces, including:
- Regulatory hurdles
- Competition
- Recruitment issues
- Financial constraints encountered by medical device startups
Moreover, the project manager medical device oversees trial setup, import permits, and compliance reviews, ensuring that all necessary steps are taken to advance clinical trials effectively. They also handle essential reporting tasks, including:
- Study status
- Inventory
- Serious and non-serious adverse events
As emphasized by the World Economic Forum, social abilities are becoming more crucial, and organizations that prioritize these soft skills report higher success metrics, including enhanced benefits realization and improved maturity in overseeing initiatives. A recent case analysis emphasizes this point, demonstrating that effective stakeholder involvement and risk oversight significantly enhance outcomes. Notably, 52% of respondents in a recent survey indicated experiencing delays due to difficulties in sourcing essential roles or skills, emphasizing the urgent need for effective talent acquisition and retention strategies.
In reality, 69% of CEOs consider these challenges their primary worry, further emphasizing the significance of strategic planning in the medical equipment sector. Investing in skills development for a project manager medical device has been demonstrated to greatly enhance performance, rendering those in charge essential in steering efforts toward successful completion and market readiness. To discuss how we can support you in advancing your medical equipment initiatives, SCHEDULE A MEETING.
Key Skills and Qualifications for Medical Device Project Managers
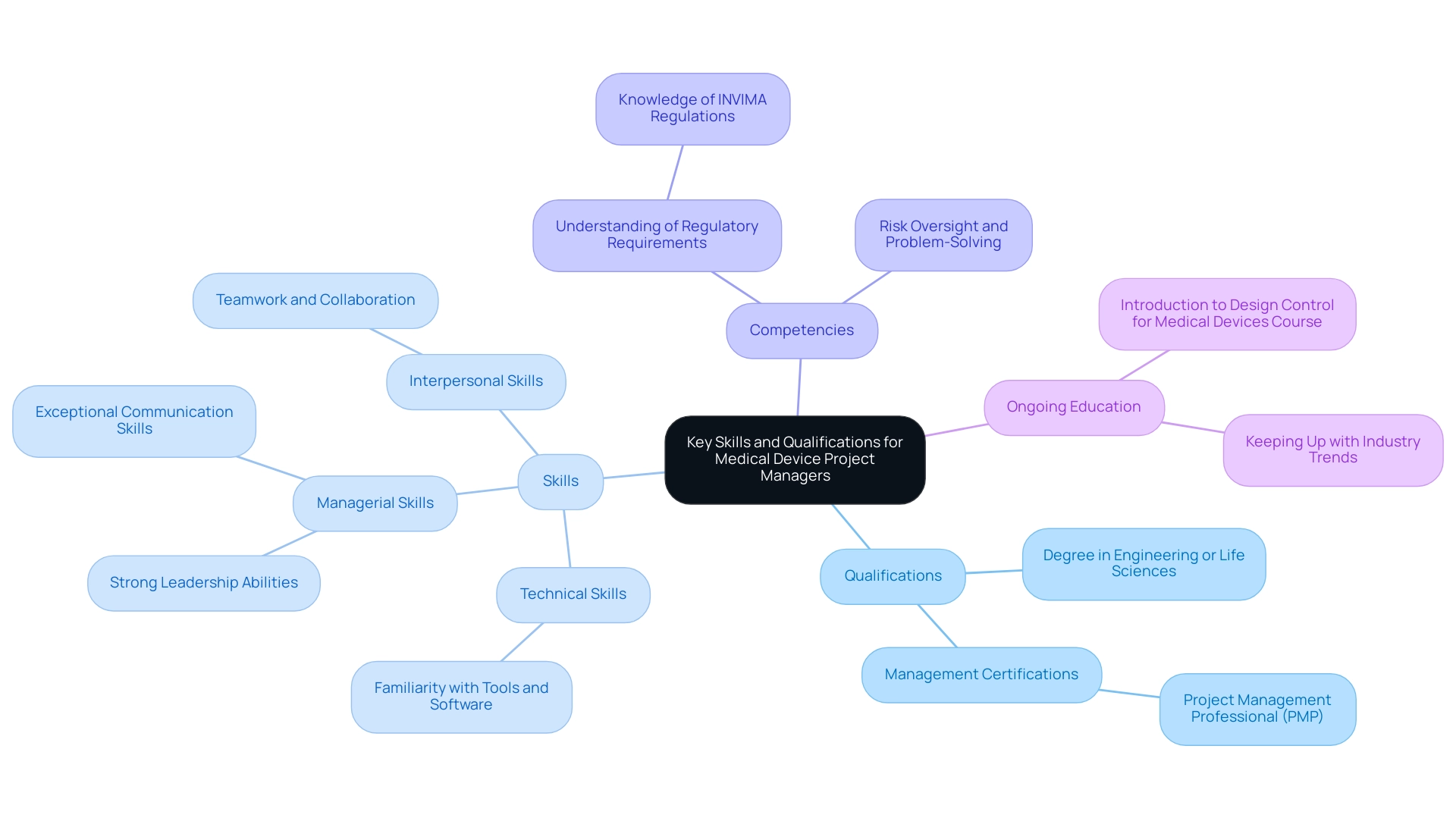
Effective leaders in the medical device industry, especially project managers in medical device roles, demonstrate a strong blend of technical, managerial, and interpersonal skills, which are vital for supervising comprehensive clinical trial oversight services. Their qualifications typically include:
- A degree in engineering, life sciences, or a related discipline
- Certifications in recognized management methodologies, such as Management Professional (PMP)
Familiarity with specific tools and software that facilitate execution is crucial, as these technical skills enhance efficiency.
As the demand for skilled managers is projected to rise by 33% by 2027, creating nearly 22 million new job opportunities globally, possessing these qualifications is becoming increasingly essential. Key competencies for a project manager in medical devices include:
- Strong leadership abilities to effectively guide diverse teams
- Exceptional communication skills necessary for engaging stakeholders throughout the project lifecycle
- A thorough understanding of regulatory requirements, particularly those dictated by bodies such as INVIMA—the Colombia National Food and Drug Surveillance Institute, recognized as a Level 4 health authority by PAHO/WHO
This encompasses understanding how INVIMA's oversight influences trial setup, compliance reviews, and the handling of investigational devices. Proficiency in risk oversight and problem-solving is vital for a project manager in medical devices to navigate challenges that may arise in clinical trials.
Furthermore, it is advised to enroll in the 'Introduction to Design Control for Medical Devices' online course before the Project Administration course, as this foundational knowledge is advantageous for individuals pursuing leadership roles in this field.
As Julia Martins aptly notes, 'Teamwork ensures that everyone feels welcome, valued, and they are supported to contribute.' This underscores the significance of interpersonal skills in promoting a collaborative atmosphere, which is crucial for successful management. Ongoing education and keeping up with industry trends further enhance a leader's competitive advantage in this dynamic field.
Challenges in Medical Device Project Management
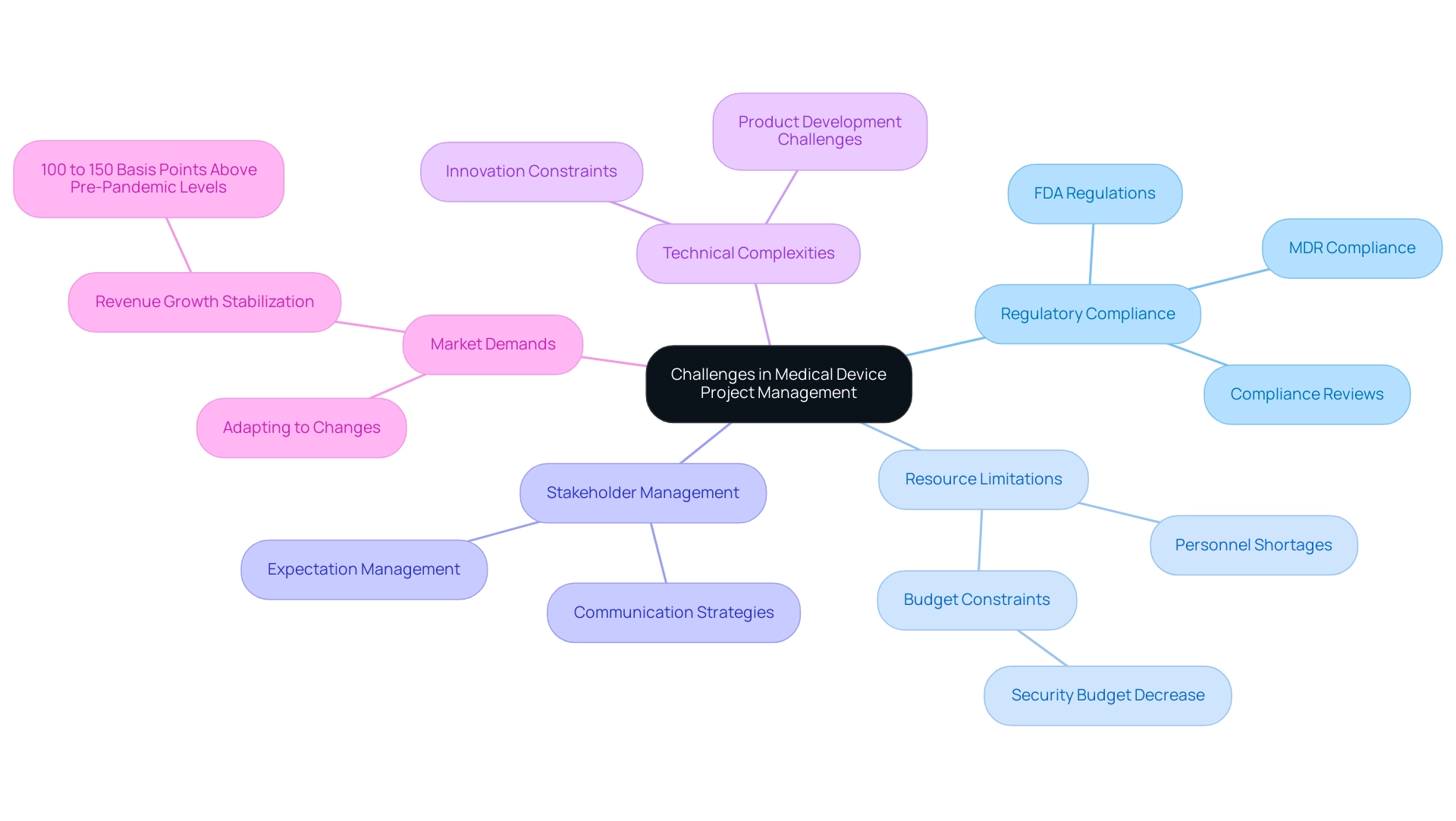
Project managers in the medical device sector oversee initiatives and face numerous challenges that can greatly affect timelines and results. A key difficulty lies in navigating the stringent regulatory landscape, which varies widely across different regions. Adherence to regulations like the FDA in the United States and the Medical Device Regulation (MDR) in Europe requires meticulous attention to detail and a current understanding of evolving guidelines, including recent updates on FDA regulations that could influence timelines and compliance strategies.
Additionally, managers often face resource limitations, including budget constraints and personnel shortages, which can impede progression. The typical rise in security budgets for 2024 is anticipated at 10.8%, down from 17% in 2023, suggesting restricted financial resources that could impact funding for initiatives.
Moreover, managing stakeholder expectations, addressing technical complexities in product development, and adapting to rapidly changing market demands are ongoing hurdles. As Karsten Dalgaard observes, "Entering 2024, we anticipate overall medtech revenue growth to level off at 100 to 150 basis points above pre pandemic levels," emphasizing the necessity for efficient coordination amid a variable market environment. To navigate these challenges successfully, a project manager in the medical device sector must employ robust strategies, leveraging strong leadership and communication skills to keep endeavors aligned with objectives.
Our extensive clinical trial coordination services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Oversight
- Reporting
All vital for addressing these challenges. For instance, feasibility studies involve assessing the suitability of potential sites and principal investigators, while site selection focuses on identifying locations that meet regulatory requirements and have the necessary resources. Compliance reviews ensure that all study documents align with country-specific regulations, and trial setup includes obtaining approvals from ethics committees and health ministries.
Reporting is critical, encompassing study status updates, inventory tracking, and documentation of serious and non-serious adverse events. Experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, and Katherine Ruiz, a specialist in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia, offer valuable insights and guidance, ensuring compliance and improving operational effectiveness. The StarFish Project Management Group exemplifies a structured approach focusing on four pillars that support technology commercialization, fostering innovation within the constraints of budget and time.
Through real-world examples and strategic planning, leaders can overcome regulatory challenges and drive successful outcomes in medical equipment initiatives, ultimately linking regulatory compliance to enhanced oversight effectiveness.
Understanding the Project Management Lifecycle in Medical Devices
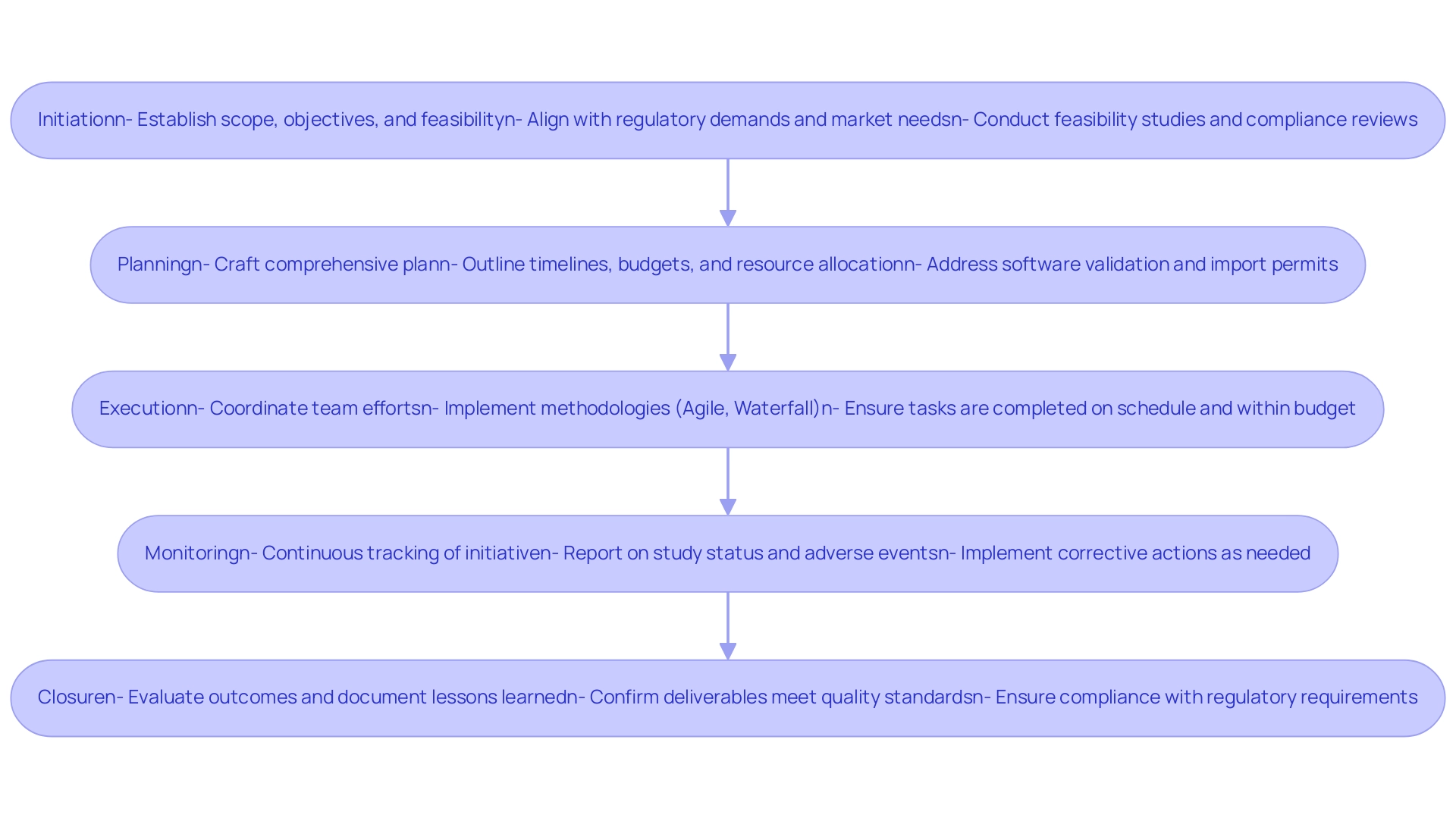
The management lifecycle in the medical device industry is organized around several essential phases: initiation, planning, execution, monitoring, and closure. In the initiation phase, managers establish the scope, objectives, and feasibility, ensuring alignment with regulatory demands and market needs. Our service capabilities, including feasibility studies, the selection of research sites and principal investigators (PIs), and the trial set-up, start-up, and approval processes (including ethics committee and health ministry approvals), play a vital role in this phase, along with compliance reviews of study documents to meet country requirements.
The subsequent planning phase is crucial, as it involves crafting a comprehensive plan that outlines timelines, budgets, and resource allocation. This planning is particularly vital given the increasing complexity of software validation, especially as tightening regulations and advancing technologies present new challenges. Moreover, obtaining import permits and nationalizing investigational instruments are essential steps that must be addressed early in the planning process.
A robust Post Market Surveillance (PMS) plan is also necessary for newly developed devices to ensure ongoing compliance and safety.
During implementation, the manager plays a pivotal role in coordinating the team's efforts to execute the plan effectively, ensuring tasks are completed on schedule and within budget. This phase is where methodologies such as Agile or Waterfall come into play, influencing how tasks are managed and adapted to meet evolving requirements. Continuous monitoring of the initiative, including detailed reporting on study status, inventory, and serious and non-serious adverse events, is equally important, as it entails tracking progress against the established plan, identifying any deviations, and implementing corrective actions as needed to keep the initiative on track.
Finally, the closure phase encompasses evaluating outcomes, documenting lessons learned, and confirming that all deliverables adhere to quality standards. A pertinent example of these phases in action can be seen in the Initial Design and Development Plan, which involves defining the intended use of the medical instrument, analyzing market needs, and gathering user requirements to inform the design process. This design process includes multiple milestones such as prototyping, user feedback, and testing, which are essential for successful product development.
An effective manager understands that ultimately, the job of a good manager is to bring the product to the market by any ethical and legal means they can. This holistic approach ensures that medical products not only meet regulatory requirements, including those set forth by INVIMA, but also fulfill market needs, leading to a more efficient and profitable development process.
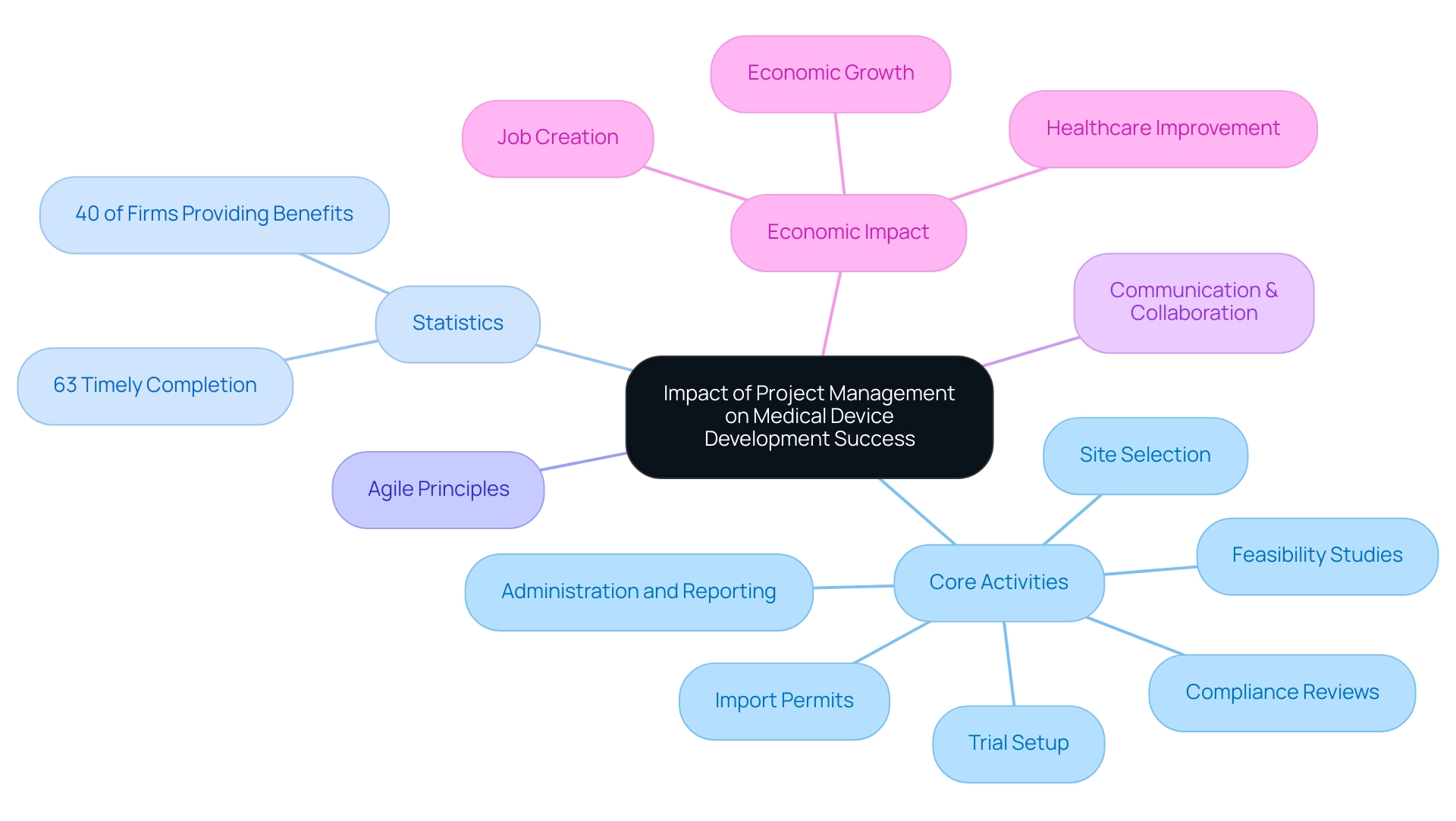
The Impact of Project Management on Medical Device Development Success
Effective oversight by the project manager medical device of initiatives is a cornerstone of success in medical device development, particularly when utilizing comprehensive clinical trial coordination services, which include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Thorough administration and reporting
By ensuring that initiatives are completed on schedule, within budget, and compliant with regulatory standards, the project manager medical device plays an essential role in the timely introduction of innovative medical solutions. Studies show that entities with robust program oversight abilities finish initiatives on schedule 63% of the time, highlighting the essential nature of efficient methods in this area.
As a project manager medical device, integrating agile principles in overseeing activities further enhances earned value and aids in reaching objectives. Such efficient oversight of tasks results in better product quality; careful planning and execution greatly minimize mistakes and improve results. Moreover, strong communication and stakeholder involvement are essential for a project manager medical device to promote collaboration, enabling teams to tackle challenges proactively and align objectives seamlessly.
A case study named 'Delivery Statistics' emphasizes that 40% of firms consistently provide complete benefits, with effective oversight relating to timely completion. This is particularly significant in the context of Medtech clinical studies, where the positive impact on local economies—through job creation, economic growth, and healthcare improvement—can be profound. As a demonstration of the importance of these practices, successful medical product launches frequently show the beneficial influence of a project manager medical device on addressing patient needs and advancing healthcare, ultimately improving patient outcomes and satisfaction.
In light of this, a recent survey indicated that 68% of organizations prioritize the development of management skills to enhance overall performance, highlighting a growing recognition of its significance in the industry. Additionally, comprehensive reporting on study status, inventory, and serious and non-serious adverse events, along with the trial setup and approval processes, including import permits and nationalization of investigational devices, are vital components of these services that further ensure compliance and the effectiveness of a project manager medical device.
Conclusion
Project managers play a critical role in the medical device industry, overseeing the journey from concept to market while ensuring compliance with regulatory standards. Their responsibilities include:
- Risk management
- Resource allocation
- Effective stakeholder coordination
All of which are essential for navigating the complexities of this fast-paced field.
As the demand for skilled project managers rises, the necessary skill set is becoming increasingly diverse, blending technical expertise with strong leadership and interpersonal skills. Continuous education and adaptability are crucial for addressing evolving regulatory requirements and market dynamics, allowing project managers to effectively manage challenges such as:
- Resource constraints
- Stakeholder expectations
Utilizing a structured project management lifecycle enhances efficiency, guiding projects from initiation to closure. By emphasizing communication and collaboration, project managers can significantly improve project success rates and ensure timely product introductions.
In conclusion, strategic project management is vital for the success of medical device development. Organizations that invest in project management skills and foster collaborative environments are more likely to achieve successful outcomes. This commitment not only improves the quality and timeliness of product launches but also drives advancements in healthcare, ultimately benefiting patients. As the industry evolves, the significance of skilled project managers will continue to grow, reinforcing their essential contribution to delivering safe and effective medical devices to the market.
Frequently Asked Questions
What is the role of a project manager in the medical device sector?
The project manager in the medical device sector directs the creation and lifecycle of medical products, overseeing processes from initial concept to market launch, including feasibility studies, recruitment of principal investigators, and regulatory compliance.
What are the key responsibilities of a project manager medical device?
Key responsibilities include feasibility and selection of research sites, recruitment of principal investigators, adherence to regulatory compliance, risk oversight, resource allocation, and enforcement of quality assurance protocols.
What challenges do project managers in the medical device field face?
They face challenges such as regulatory hurdles, competition, recruitment issues, and financial constraints, particularly those encountered by medical device startups.
What specific tasks does a project manager medical device oversee during clinical trials?
They oversee trial setup, import permits, compliance reviews, and essential reporting tasks, including study status, inventory, and serious and non-serious adverse events.
Why are social skills important for project managers in the medical device industry?
Social skills are crucial for effective stakeholder involvement and risk oversight, which significantly enhance project outcomes. Organizations that prioritize these skills report higher success metrics.
What qualifications are typically required for a project manager in medical devices?
A project manager usually needs a degree in engineering, life sciences, or a related discipline, along with certifications in recognized management methodologies, such as Management Professional (PMP).
How is the demand for project managers in the medical device industry expected to change?
The demand for skilled managers is projected to rise by 33% by 2027, creating nearly 22 million new job opportunities globally.
What key competencies are essential for a project manager in medical devices?
Essential competencies include strong leadership abilities, exceptional communication skills, a thorough understanding of regulatory requirements, and proficiency in risk oversight and problem-solving.
What educational recommendations are suggested for aspiring project managers in the medical device field?
It is advised to enroll in the 'Introduction to Design Control for Medical Devices' online course before taking the Project Administration course, as foundational knowledge is beneficial for leadership roles.
How does ongoing education benefit project managers in the medical device industry?
Ongoing education and keeping up with industry trends enhance a leader's competitive advantage in the dynamic medical device field.




