Introduction
Navigating the regulatory landscape of medical devices is a critical task, with FDA clearance standing as a pivotal milestone. The U.S. Food and Drug Administration plays a pivotal role in ensuring public health by rigorously evaluating medical devices for safety, effectiveness, and security. The classification system of the FDA stratifies devices into three categories according to the level of risk they pose to patients.
For manufacturers to legally market their devices in the United States, obtaining an FDA clearance or approval is mandatory, depending on the device classification. Recent advancements, such as the Impella Connect System, underscore the FDA's commitment to adapting its regulatory oversight to innovative technologies. Understanding these pathways and the FDA’s classification system is not only a regulatory necessity but also a strategic business advantage.
Understanding FDA Clearance
Navigating the regulatory landscape of healthcare equipment is a critical task, with FDA clearance standing as a pivotal milestone. The U.S. Food and Drug Administration plays a crucial role in ensuring public health by rigorously evaluating medical equipment for safety, effectiveness, and security. The categorization system of the FDA classifies products into three categories based on the level of risk they pose to patients. In order for manufacturers to legally sell their products in the United States, obtaining an FDA clearance or approval is mandatory, based on the classification of the product.
Typically, Class I and II devices, which are categorized as low to moderate risk, frequently go through the 510(k) clearance procedure. This entails showing that the new apparatus is significantly comparable to a lawfully marketed precedent apparatus. On the other hand, Class III products, which pose significant risks and frequently assist or maintain human life, generally undergo the stricter Pre-Market Approval (PMA) procedure, necessitating evidence of safety and efficacy.
Recent advancements, such as the Impella Connect System, a critical care instrument facilitating remote monitoring of ventricular support systems, underscore the FDA's commitment to adapting its regulatory oversight to innovative technologies. These instruments, as determined by the FDA, meet the legal description of a healthcare tool and therefore necessitate premarket authorization due to their essential functions in patient care.
Furthermore, the FDA's role goes beyond the health field, encompassing the regulation of food, cosmetics, dietary supplements, products emitting radiation, and tobacco products. The agency's recent final rule on direct-to-consumer drug advertisements bolsters its efforts to ensure that information is delivered in a clear, conspicuous, and neutral manner, reflecting the FDA's overarching mission to protect public health.
The distinctions between FDA terms like 'Registered,' 'Cleared,' 'Approved,' and 'Granted' are not just semantic; they have significant implications for regulatory strategy and compliance. In the dynamic field of healthcare equipment manufacturing, comprehending these pathways and the FDA's classification system is not only a regulatory necessity but also a strategic business advantage.
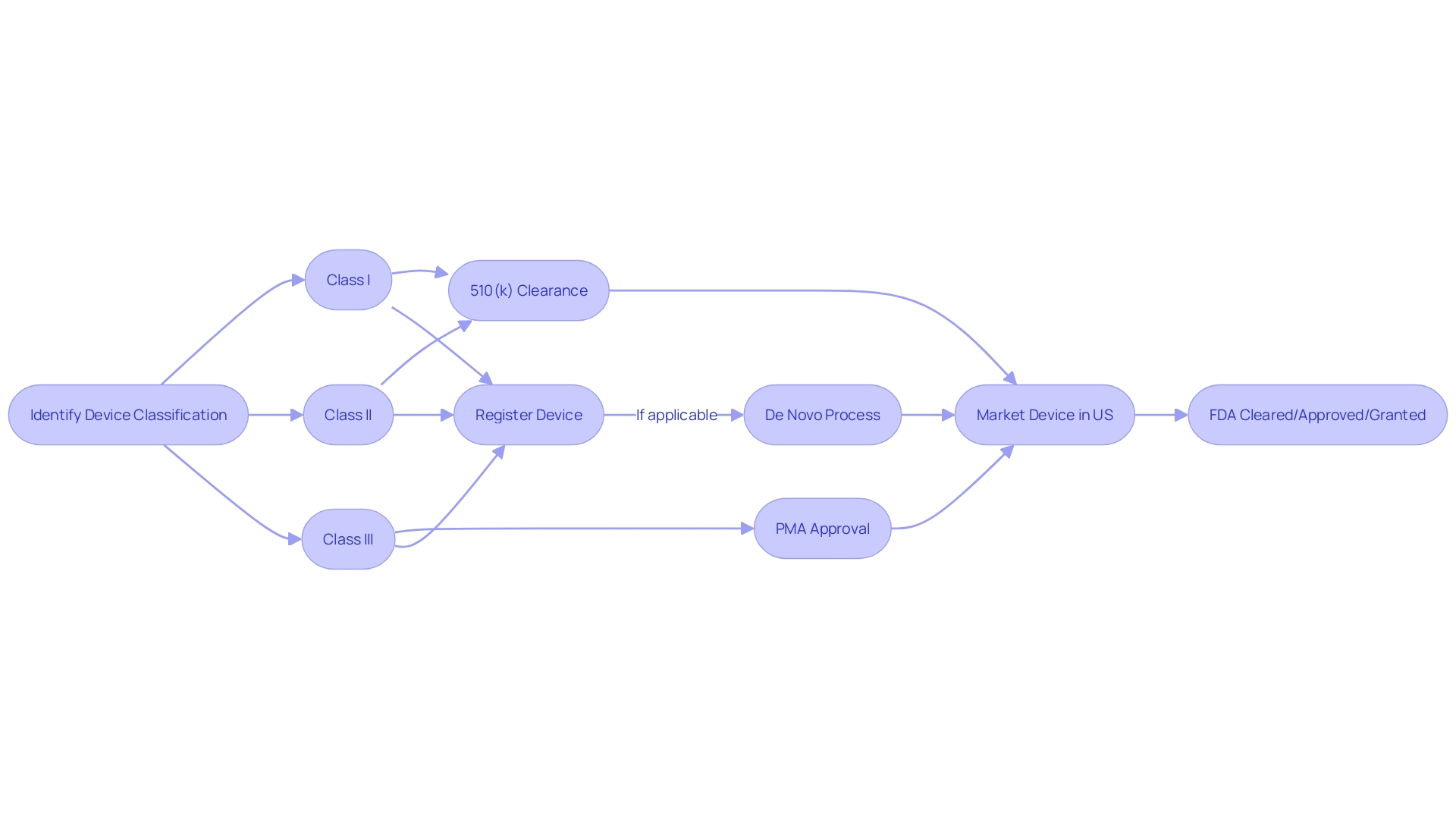
FDA Clearance vs. FDA Approval
Understanding the difference between FDA clearance, approval, and the De Novo pathway is crucial for successfully navigating the regulatory landscape of the FDA and bringing devices to the market. For most medical instruments, obtaining FDA clearance through the 510(k) procedure is the first stage. This involves showing that the new apparatus is essentially similar to a previously lawfully sold product. However, it's worth noting that clinical trials may not always be required for 510(k) clearance, which was highlighted in the 2018 documentary 'The Bleeding Edge' that raised concerns about patient safety for equipment fast-tracked via this pathway.
In comparison, FDA endorsement is required for higher-risk class III instruments, such as life-supporting or implantable tools, which go through a more rigorous Pre-Market Approval (PMA) procedure. This procedure entails a comprehensive evaluation of the safety and efficacy of the equipment, encompassing significant supporting information, potentially derived from clinical trials. It's a common misconception that 'FDA Approved' is a blanket term for all medical instruments on the market, but this only applies to those that have successfully navigated the PMA procedure, which represents a minority of instruments.
The De Novo pathway provides an opportunity for low to moderate risk instruments that do not have a similar reference device, enabling them to be categorized as class I or II. This procedure offers a path to the market that may not necessitate the more intricate PMA procedure.
The classification system of the FDA plays a crucial role in the regulatory procedure, categorizing equipment into three classes based on the risk they present to patients. Class I devices have minimal risk and are subject to the least amount of regulatory oversight, whereas class II devices require specific measures to ensure safety and effectiveness, typically cleared through the 510(k) pathway. Class III products are the most risky and necessitate the PMA process, involving a thorough evaluation to guarantee they are safe and efficient for their intended application.
For industry professionals, it's imperative to recognize the nuances and select the appropriate registration pathway. As recent updates from the FDA suggest, the regulatory environment continues to evolve, emphasizing the need for clear and transparent communication of risks and benefits, such as the newly established standards for direct-to-consumer drug advertisements.
Innovation within the healthcare equipment industry is rapidly progressing, as demonstrated by recent FDA clearances like OxiWear's oxygen monitoring device. Such advancements underscore the importance of continuous monitoring and real-time data in enhancing patient care, reflecting the FDA's role in balancing innovation with patient safety.
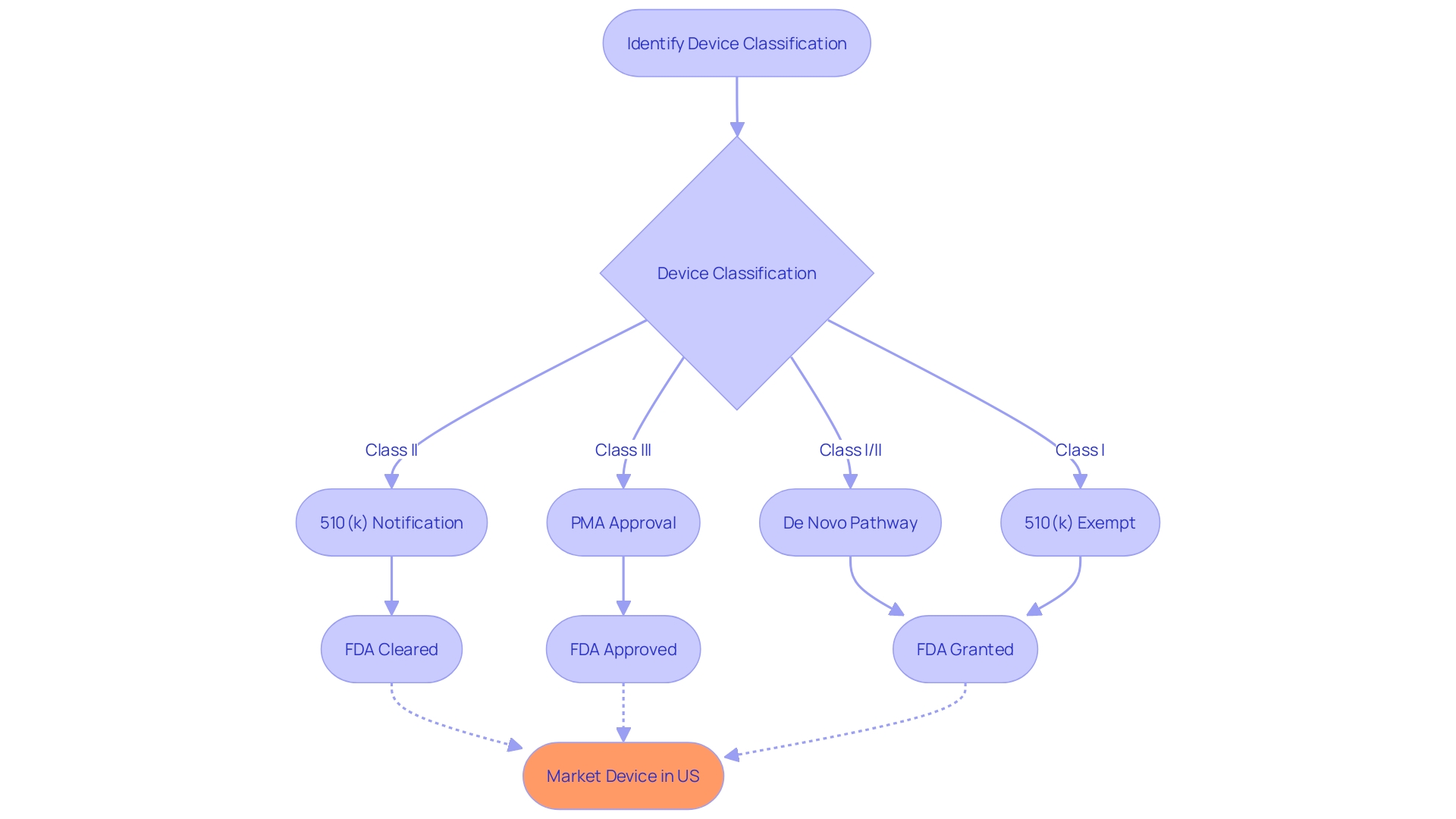
The 510(k) Premarket Notification Process
Navigating the FDA's regulatory landscape is crucial for medical instrument manufacturers aiming to market their products in the United States. The 510(k) premarket notification process is one such pathway that facilitates the introduction of new products to the market, provided they are substantially equivalent to existing legally marketed items, referred to as predicate items. To establish this substantial equivalence, manufacturers must meticulously compare their product's intended use and technological attributes with those of the predicate, ensuring adherence to the stringent regulatory standards set forth by the FDA.
Before contemplating the 510(k) pathway, it is crucial to precisely categorize the product according to the FDA's three-tier system, which evaluates patient risk. This initial step determines the appropriate registration strategy, selecting between Premarket Notification (510(k)), Premarket Approval (PMA), or the De Novo process. Grasping the distinctions among phrases like Registered, Cleared, Approved, and Granted is crucial, as each indicates a varying degree of compliance and authorization.
The FDA's mission to protect public health includes thorough supervision of medical equipment, guaranteeing their safety and effectiveness. Recent updates, such as the clear and neutral presentation standards for direct-to-consumer prescription drug advertisements, underscore the agency's commitment to transparency and consumer understanding. These standards emphasize the importance of transparent communication within the 510(k) procedure, where precision in the comparative assessment between the new product and the precedent is crucial.
Industry professionals must immerse themselves in the intricacies of the subject product and its marketplace context. This includes a comprehensive review of user needs, detailed instructions for use, and a thorough examination of the competitive landscape. By means of this procedure, a comparative examination is created, emphasizing the resemblances and distinctions with potential predicate instruments, which is an essential element of the 510(k) submission.
Guidance documents issued by the FDA provide a roadmap for addressing the unique considerations of implant products within the 510(k) program. These documents emphasize the significance of thorough performance testing and patient experience data to back submissions, showcasing the agency's emphasis on patient safety and well-informed approval.
It's important to highlight that, as unveiled in a 2018 documentary, not all apparatus necessitate clinical trials for clearance through the 510(k) process, a fact that can lead to expedited market entry but also has raised concerns about patient safety in some cases. The FDA's Center for Drug Evaluation and Research (CDER) operates under a deep understanding of the science behind new products, emphasizing the necessity of appropriate study designs and data for a comprehensive assessment.
Considering these factors, manufacturers can use informed strategies to navigate the FDA's regulatory pathways and ensure that their products fulfill all necessary requirements for a successful market launch.
What is Substantial Equivalence in 510(k) Clearances
The notion of 'significant similarity' plays a crucial part in the 510(k) clearance pathway employed by the U.S. Food and Drug Administration (FDA) for healthcare instruments. It is based on the assumption that a new apparatus is equally secure and efficient as a previously approved 'predicate' object, and that there are no notable modifications in their intended utilization, structure, constituents, or performance standards. For a new medical instrument to navigate the 510(k) process successfully, manufacturers must rigorously demonstrate this equivalence.
Substantial equivalence requires a meticulous comparison between the new equipment and its predicate, often involving detailed tables that align the two products' features side by side. Manufacturers must familiarize themselves thoroughly with both the device and its competitive environment, including a comprehensive review of existing research literature, clinical studies, and marketing materials. This deep dive into the product landscape is essential not only to establish substantial equivalence but also to anticipate potential regulatory scrutiny and market reception.
Clinical data, while not always mandated for 510(k) submissions, becomes critical when there are discernible differences in indications for use, technological characteristics, or when non-clinical testing is insufficient to establish substantial equivalence. The FDA's recent draft guidance on the use of clinical data in 510(k) submissions highlights situations where such data may be necessary, clarifying expectations for manufacturers navigating this procedure.
Despite the strategic pathways laid out by the FDA, companies must remain cognizant of the inherent uncertainties and risks, as highlighted by the diverse experiences of firms like Vivos. The results of the 510(k) approval procedure, involving expected advantages, can deviate considerably from expectations due to a range of factors, from difficulties in implementation to unforeseen patient outcomes or the need for additional financing.
The importance of FDA clearance is underscored by the potential risks associated with distributing unapproved devices, as noted by Assistant Commissioner for Criminal Investigations at the FDA, Justin Green. The legal and ethical implications of ensuring proper clearance cannot be overstated, as patient safety and public trust hang in the balance.
In summary, achieving FDA clearance through the 510(k) pathway is a complex endeavor that necessitates a comprehensive understanding of the product, its market, and the regulatory landscape. It involves a combination of strategic planning, thorough testing, and an awareness of potential risks and uncertainties inherent in the procedure.
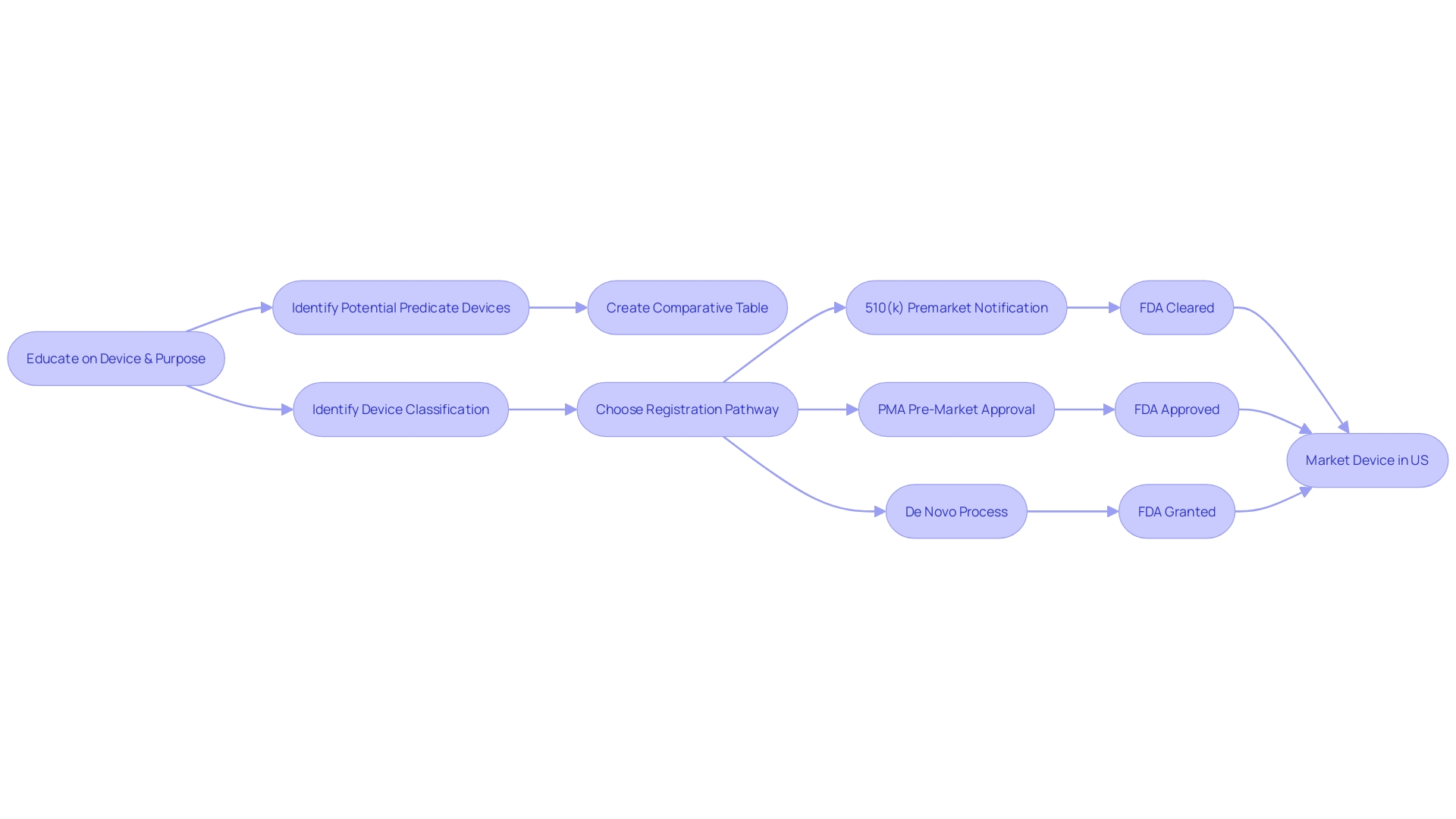
Regulatory Steps for FDA Clearance
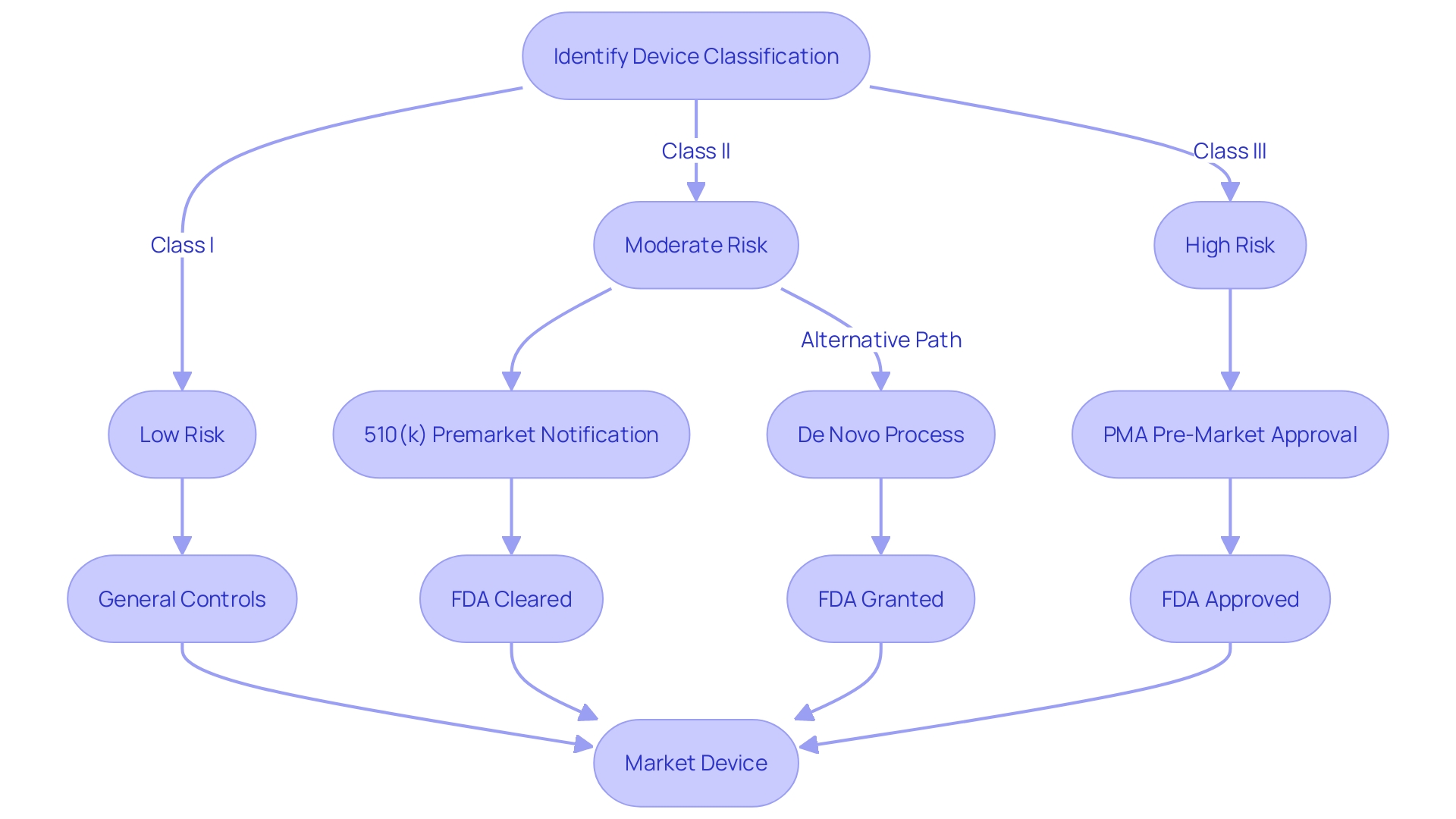
Understanding the FDA's classification system is the initial stage in the medical equipment clearance process. Medical equipment is classified into three categories based on the level of risk they pose to patients. Class I products pose the minimal risk and are subject to the least regulatory oversight, while Class III products are high-risk and require the most rigorous controls. After determining the appropriate class, manufacturers must choose the correct regulatory pathway. This could be the 510(k) Premarket Notification for objects substantially equivalent to one already on the market, the Pre-Market Approval (PMA) for high-risk items, or the De Novo procedure for new types of items without a comparable predecessor.
The terms 'Registered,' 'Cleared,' 'Approved,' and 'Granted' are often used interchangeably in the industry, but each has a distinct meaning in the context of FDA processes. For example, 'Cleared' typically pertains to 510(k) products that FDA has determined to be as safe and effective as already available, legally marketed items. Understanding these terminologies is crucial for regulatory professionals as they navigate the complex FDA approval landscape.
Recent progress and the FDA's quick response are demonstrated by the approval of OxiWear's groundbreaking oxygen monitoring technology, which signifies a significant advancement in patient care for chronic illnesses. This demonstrates the FDA's dedication to guaranteeing the safety and effectiveness of healthcare equipment while fostering medical progress.
It's essential to be informed about unique issues specific to particular health fields when dealing with regulatory matters. As an instance, radiological health instruments may involve concerns such as radiation dose or the utilization of radiopharmaceutical drugs. These specialized considerations highlight the need for precise knowledge and careful regulatory planning.
From a statistical perspective, the FDA regulates approximately 10% of products classified as Class III, which are essential for sustaining or supporting life, including implants like pacemakers. These gadgets undergo a more thorough evaluation compared to the simplified 510(k) clearance, demonstrating their crucial significance. In addition, recent efforts seek to expedite the approval pathway for products that fulfill pressing healthcare requirements, emphasizing the ever-changing landscape of regulation.
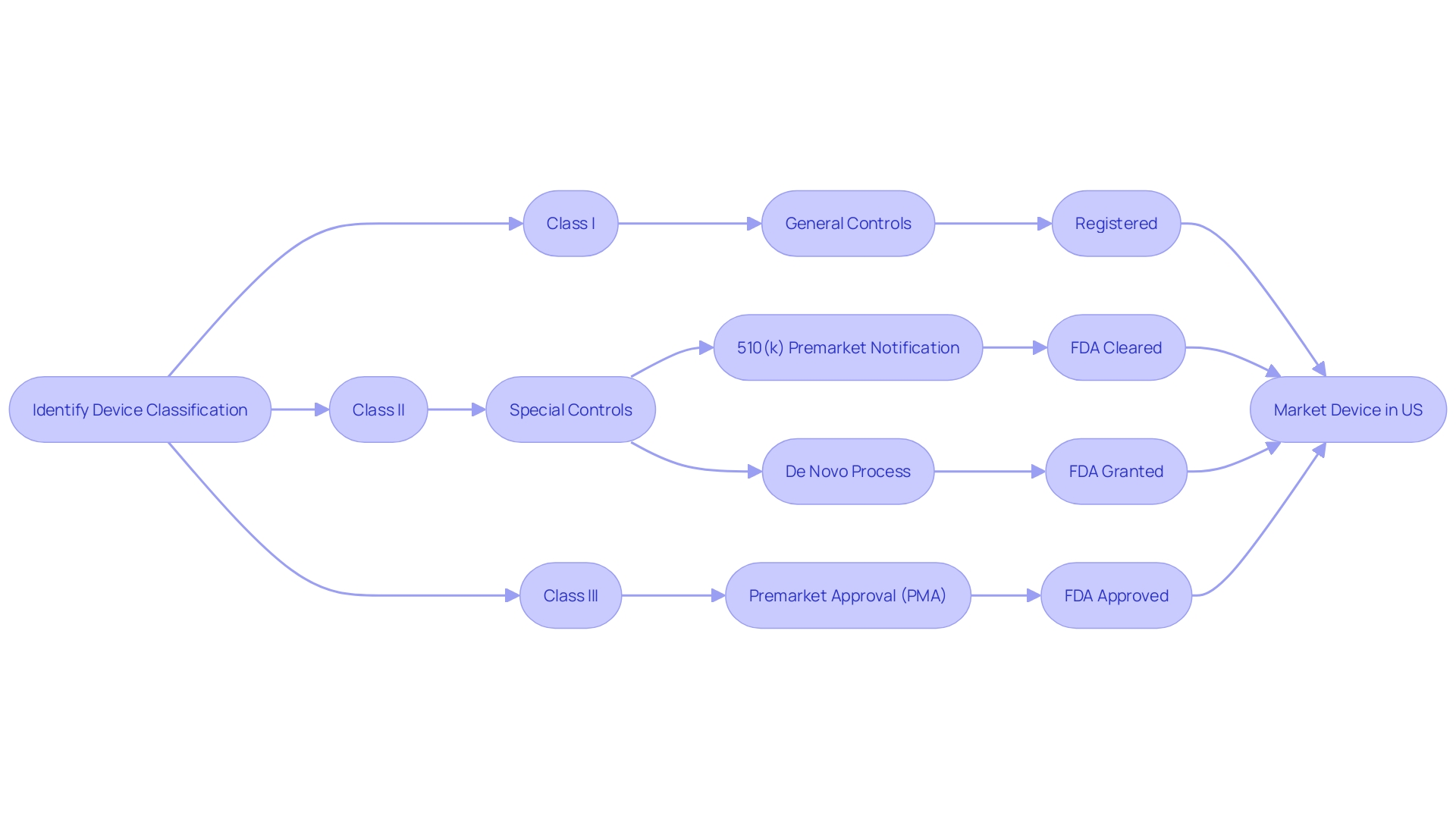
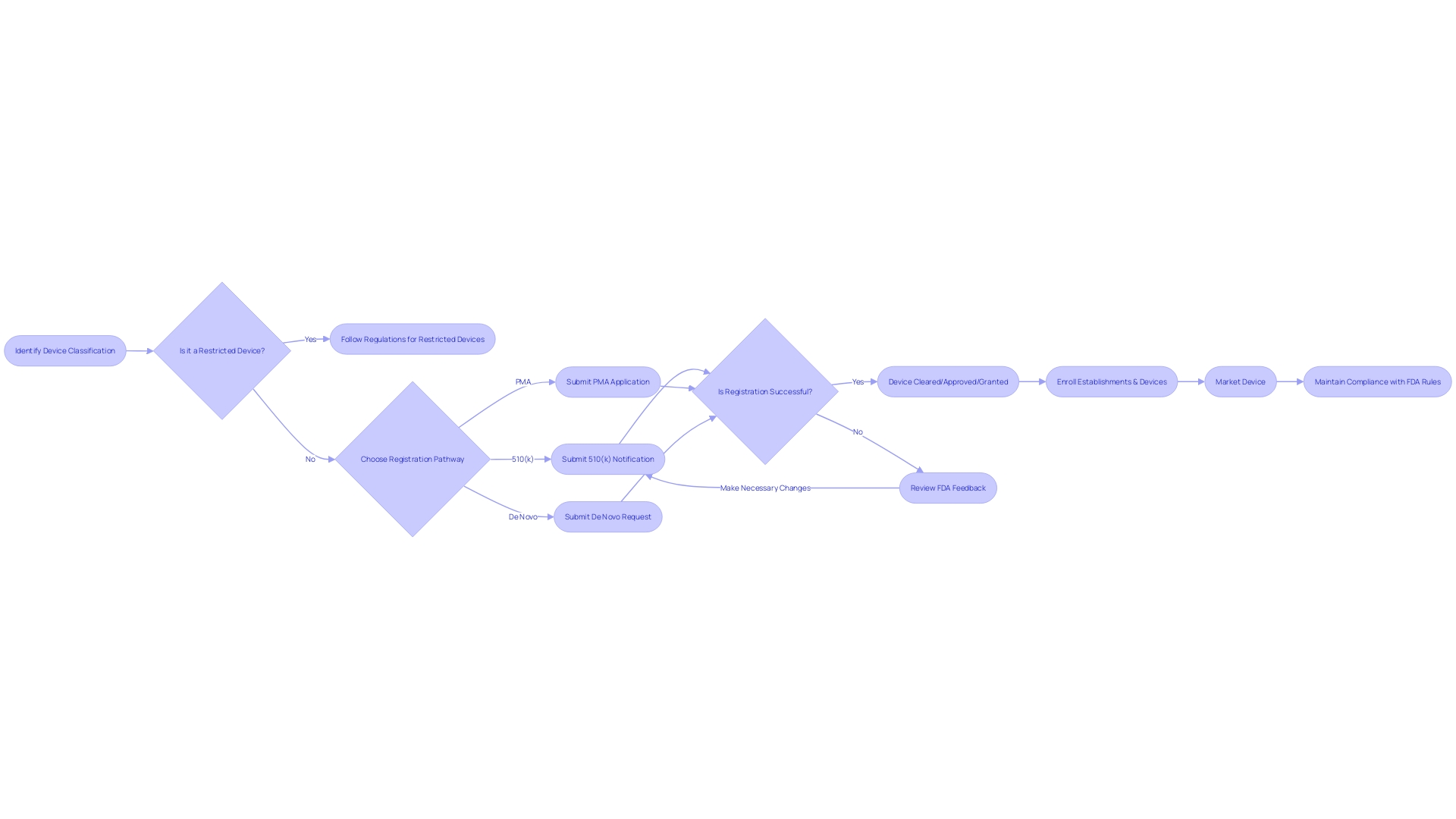
Establishment Registration and Device Listing
Comprehending the FDA clearance procedure is crucial for manufacturers aspiring to enter the U.S. medical industry. At first, the apparatus must be accurately categorized based on the FDA's three-tier system, which evaluates the level of risk to patients. This categorization is vital as it directs the choice of the suitable registration pathway, like the 510(k) Premarket Notification, the Pre-Market Approval (PMA), or the De Novo procedure. Each pathway has unique requirements and implications for the journey of the product to market.
Enrollment of establishments and cataloging of equipment with the FDA is a compulsory measure in this procedure. By doing so, manufacturers contribute to a comprehensive FDA database, facilitating oversight and enabling swift communication regarding equipment safety and efficacy. This system of registration and listing is part of the FDA's broader mission to protect public health, which also includes the regulation of drugs, biologics, food, cosmetics, and tobacco products.
The importance of understanding the differences between terms like Registered, Cleared, Approved, and Granted cannot be overstated. These terms are not interchangeable, and their nuances carry legal and regulatory significance. For example, the 510(k) clearance pathway enables certain products to be fast-tracked to the market without requiring clinical trials, a procedure explored in the 2018 documentary 'The Bleeding Edge'. Nevertheless, it has been observed that this accelerated procedure has, in certain instances, resulted in products being released to the market that subsequently resulted in harm to patients.
As regulatory professionals navigate these pathways, it's essential to stay informed about the latest FDA rules and guidelines, such as the recent standards for direct-to-consumer prescription drug advertisements, which demand clear, conspicuous, and neutral presentation of a drug's major side effects in television and radio formats.
In the end, the objective is to guarantee that healthcare equipment is not only authorized or given approval by the FDA, but also that it is safe and efficient for public utilization. The FDA's watchfulness and the manufacturers' commitment to its rigorous process are crucial for upholding trust in innovations and equipment.
Quality System Regulation and Good Manufacturing Practices
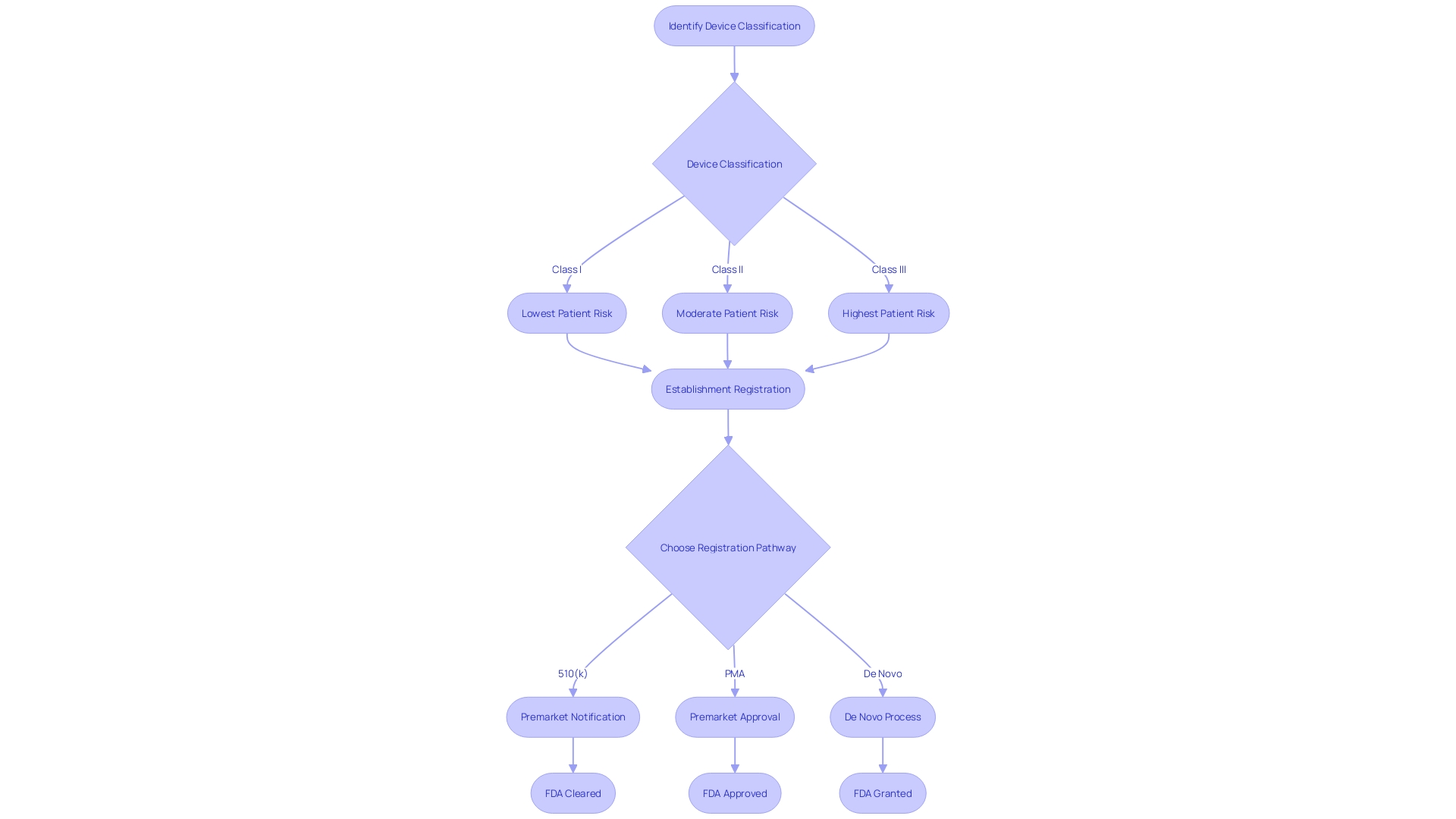
Ensuring that healthcare instruments meet quality standards is a crucial aspect of market approval. The FDA's Quality System Regulation (QSR) enforces a framework for the creation, production, and distribution of healthcare equipment, addressing the extensive variety in the healthcare equipment industry—from eyeglasses and dressings to advanced technologies like MRI machines and pacemakers. Adherence to Good Manufacturing Practices (GMP) is equally crucial, ensuring that products are consistently manufactured in a controlled environment that meets quality requirements.
With more than 10,000 varieties of medical equipment accessible, as indicated by the World Health Organization, manufacturers must navigate through a intricate classification system established by the FDA to determine the appropriate regulatory pathway, whether it is Premarket Notification (510(k)), Premarket Approval (PMA), or De Novo procedure. Based on the classification of the equipment, which indicates the level of risk for the patients involved, the suitable registration pathway must be selected. Only with FDA clearance, approval, or grant through the De Novo process can an instrument lawfully enter the U.S. market. The distinctions between these terms—Registered, Cleared, Approved, and Granted—are not only significant for regulatory professionals but also for manufacturers aiming to ensure compliance and protect public health.
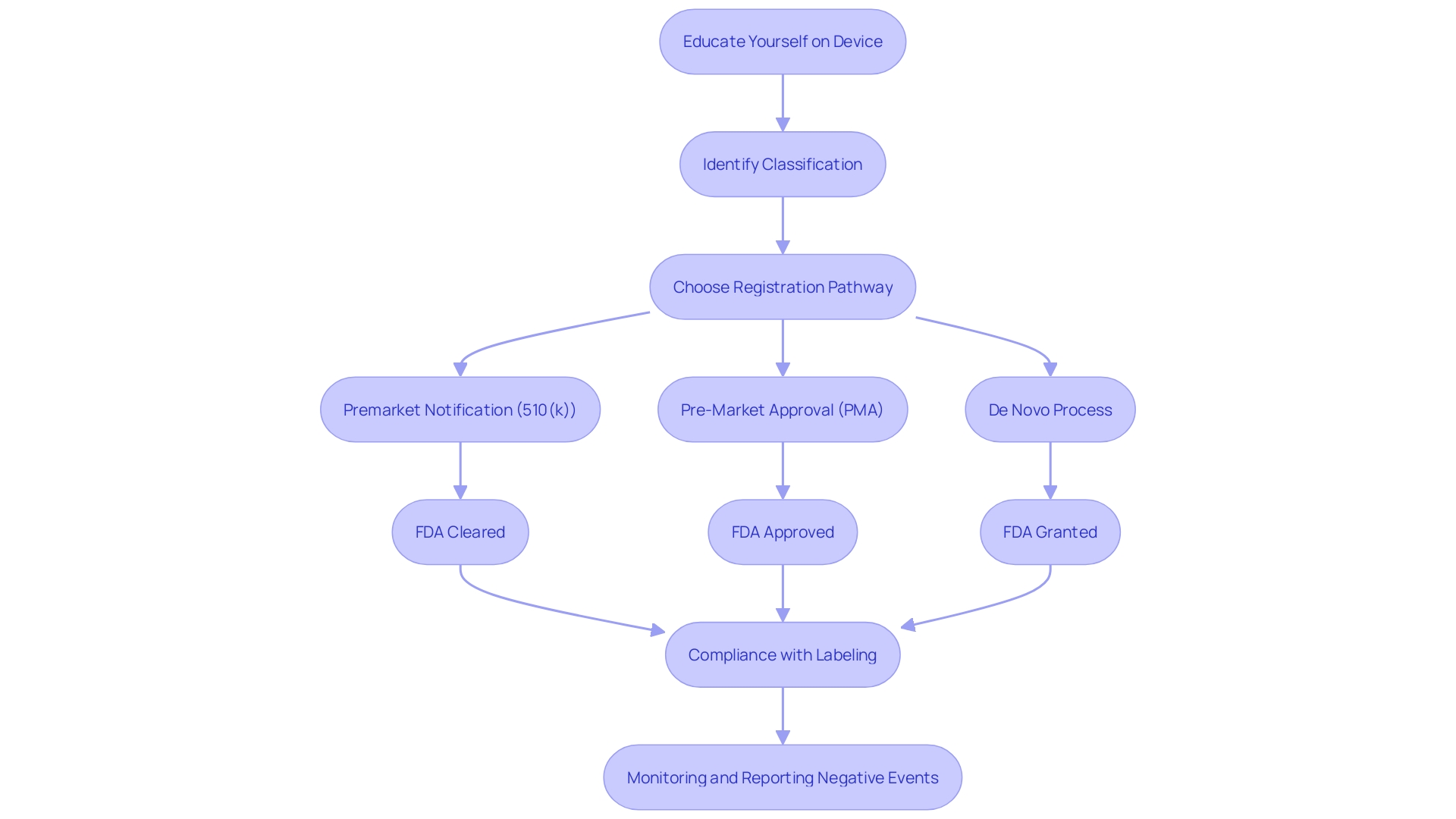
Labeling and Medical Device Reporting
In order for medical equipment to obtain FDA clearance, manufacturers must carefully comply with labeling requirements, guaranteeing that every apparatus is accompanied by detailed instructions for use, indications, contraindications, warnings, and precautions. The transparency and completeness of this information are crucial for both healthcare professionals and patients to comprehend the proper utilization of the equipment and potential risks.
In addition, manufacturers are required to actively monitor and promptly report any negative events or malfunctions linked to their equipment. These reports are important inputs for the FDA's reporting (MDR) system, which serves as a vigilant surveillance mechanism to safeguard public health. Data from the Manufacturer and User Facility Device Experience (MAUDE) database, maintained by the FDA, reveals the significance of such reporting. For instance, more than 100,000 reports were submitted concerning specific equipment, describing patient injuries, fatalities, and equipment malfunctions, which subsequently influenced regulatory supervision and manufacturer responsibility.
The FDA categorizes medical equipment into three classifications according to the danger they present to patients, which determines the regulatory route a manufacturer must followâwhether it is a Premarket Notification (510(k)), Premarket Approval (PMA), or the De Novo procedure. To lawfully promote a product in the United States, it must receive FDA Clearance, Approval, or Authorization through the De Novo process. The choice of the appropriate pathway is a crucial stage in ensuring that products meet the stringent criteria for safety and effectiveness established by the FDA.
In the context of public health emergencies, such as the COVID-19 pandemic, the FDA has issued Emergency Use Authorizations (EUAs) which come with specific adverse event reporting requirements. These requirements are designed to quickly gather safety information on products that are crucial for addressing the emergency, contributing to the FDA's ongoing evaluation of product performance and public health impact.
Examples of FDA-Cleared Medical Devices
The Food and Drug Administration (FDA) plays a critical role in protecting public health by ensuring the safety, effectiveness, and security of a broad range of products, including medical instruments. Healthcare equipment, which covers a wide range of health care applications, are thoroughly evaluated by the FDA before they can be made available to healthcare professionals and patients. Devices such as blood glucose monitors, ultrasound machines, pacemakers, artificial joints, and hearing aids have all undergone this stringent clearance process.
To understand the importance of FDA approval, it is crucial to acknowledge the rigorous pathway that products must navigate to obtain this status. The FDA categorizes medical equipment into three groups based on the risk they pose to patients, with each category requiring a different level of regulatory control. Once the classification of a product is determined, the manufacturer must select the suitable regulatory pathâPremarket Notification (510(k)), Pre-Market Approval (PMA), or De Novo processâto lawfully market their product in the United States.
An illustrative instance of the FDA's oversight can be seen in the case of the Impella Connect System, a sophisticated tool crafted to offer temporary ventricular support and enable remote monitoring of a patient's heart pump performance in critical care settings. This system, which includes both software and hardware components, went through a comprehensive FDA review process to ensure it met the requirements for a product categorized under section 201(h) of the Act. The Impella Connect System, with its capability to provide real-time, patient-specific information and generate time-critical alarms, is a reflection of the advanced technology the FDA assesses to certify the safety and effectiveness of the equipment.
In recent news, the FDA has continued to emphasize the importance of clear communication regarding healthcare products. For example, new standards were established to ensure direct-to-consumer prescription drug advertisements convey major side effects and contraindications in a manner that is clear, conspicuous, and neutral, further demonstrating the agency's commitment to public health.
Comprehending the distinctions among the terms 'Registered,' 'Cleared,' 'Approved,' and 'Granted' is vital for regulatory professionals and stakeholders in the healthcare instrument sector. Each term represents a different level of FDA evaluation and endorsement, with 'Cleared' indicating that an item has been found to be substantially equivalent to another legally marketed product.
The FDA clearance process is vital not only for compliance but also for the confidence it instills in consumers and healthcare providers, assuring them that the medical devices they rely on have been subject to rigorous evaluation for safety and performance.
Conclusion
In conclusion, navigating the FDA clearance process is crucial for medical device manufacturers entering the U.S. market. Understanding the classification system and selecting the appropriate registration pathway is essential. Demonstrating substantial equivalence and adhering to quality standards are vital for market approval.
Clear communication is key throughout the process, as recent standards for direct-to-consumer drug advertisements demonstrate. The FDA's commitment to balancing innovation with patient safety is evident in recent advancements, such as the Impella Connect System.
Manufacturers must understand the differences between terms like Registered, Cleared, Approved, and Granted, as they represent different levels of compliance and authorization. By effectively navigating the FDA clearance process, manufacturers can ensure compliance, protect public health, and instill confidence in consumers and healthcare providers.
In summary, manufacturers must navigate the FDA's regulatory landscape, demonstrate substantial equivalence, adhere to quality standards, and communicate clearly. By doing so, they can successfully bring their medical devices to market, ensuring their safety and effectiveness.




