Introduction
The International Council for Harmonisation (ICH) stands as a cornerstone in the landscape of global pharmaceutical regulation, bridging the gap between diverse regulatory authorities and the pharmaceutical industry. Established in 1990, ICH's mission revolves around harmonizing the technical requirements for drug registration, thereby streamlining the complex processes involved in clinical trials.
As the demand for safe and effective medications grows, the significance of ICH's guidelines becomes increasingly evident, impacting not only the efficiency of clinical research but also the economic vitality of regions where trials are conducted.
This article delves into the multifaceted role of ICH, examining its influence on clinical research practices, the challenges faced in implementing its guidelines, and the future directions that promise to reshape the pharmaceutical landscape in response to technological advancements and evolving healthcare needs.
Through a detailed exploration of these themes, the critical importance of ICH in fostering innovation and collaboration within the pharmaceutical sector is highlighted.
Understanding the International Council for Harmonisation (ICH)
The International Council for Harmonization (ICH), established in 1990, serves as a vital initiative uniting regulatory authorities and pharmaceutical industry representatives from Europe, Japan, and the United States. Its primary objective is to foster international harmonization of technical requirements for the registration of pharmaceuticals. By creating guidelines that simplify the drug development process, ICH significantly improves the efficiency of studies, ensuring that new medications are not only safe and effective but also meet high-quality standards.
This harmonization is especially vital as management services for studies include a variety of tasks, such as:
- Assessing feasibility and choosing study locations and main investigators (PI)
- Compliance evaluations of study documents
- Setup and approvals from ethics boards and health ministries
- Import permits for investigational devices
- Strong project management and reporting
These services are vital in supporting the goals of ICH, which ich stand for medical, by promoting adherence and efficiency in studies. For instance, recent statistics indicate that Greece and France exhibit the highest rates of antibiotic consumption, with 19 and 22 defined daily doses per 1,000 people per day, respectively.
This highlights the importance of Ich's influence on pharmaceutical practices in Europe. Additionally, research studies have been demonstrated to generate substantial economic advantages, aiding in job creation and local economic development, especially in areas where these studies are carried out. Recent discussions also point to the growing importance of evaluating new medicines against existing therapies, a concept gaining traction in Europe but yet to be fully integrated into U.S. practices.
As Corey Ford noted, amid lingering questions and uncertainty, pharmaceutical companies must prioritize incorporating the Inflation Reduction Act into their strategic planning processes, as it could reshape market dynamics and pricing strategies. This evolving landscape underscores the crucial role that ich stand for medical plays in shaping the future of pharmaceutical registration and international collaboration. Additionally, the World Medical Association (WMA), established in 1947, emphasizes the significance of ethical standards in drug development, as reflected in its Declaration of Helsinki, which aligns with Ich's guidelines and their impact on pharmaceutical registration.
The Role of ICH Guidelines in Clinical Research
The International Council for Harmonization (ICH) guidelines, especially the Good Clinical Practice (GCP) guidelines, are essential in medical studies, as ICH stood for medical standards. These guidelines create a thorough structure for the conduct of medical studies, ensuring the protection of human subjects, the integrity of data, and the credibility of findings. Our service capabilities align with these guidelines, encompassing:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Nationalization of investigational devices
Addressing all aspects of trials—from design and execution to monitoring, auditing, documentation, analysis, and reporting—GCP compliance is essential for securing regulatory approval and ensuring ethical and scientific integrity in practices. Recent discussions emphasize the need for improved research conduct through enhanced data safety monitoring and reducing disparities among patient populations, reflecting a broader commitment to ethical standards in medical investigations. Significantly, the accuracy of heart failure evaluation rose to 95% when heart failure was the main diagnosis, highlighting the significance of precise assessments in medical studies.
The Clinical Trials Transformation Initiative (CTTI) actively promotes practices that prioritize quality and efficiency, focusing on:
- Informed consent
- Patient recruitment
- Institutional Review Board (IRB) conduct
As Omar Sherif Askar from Tawam Hospital observes, 'Following GCP guidelines is crucial for guaranteeing that research studies are carried out ethically and that participant safety is prioritized.' By standardizing practices across borders, ICH guidelines promote the acceptance of research data by regulatory bodies globally, ultimately speeding up the development and availability of new therapies for patients.
Moreover, our project management and monitoring services guarantee that studies are conducted within these frameworks effectively, including comprehensive reporting on study status, inventory, and serious and non-serious adverse events. For example, the Trial 360 digital platform demonstrates innovation in study management, providing features that guarantee compliance with GCP guidelines. With its findings and deviations module, Trial 360 allows users to document, track, and resolve issues in real-time, thereby maintaining data integrity and protecting participant safety.
Such advancements emphasize the ongoing dedication to maintain the highest ethical standards in research studies while enhancing adherence to ICH GCP guidelines, which ich stand for medical.
Impact of ICH on Global Clinical Trials
The International Council for Harmonization (ICH) has profoundly changed the landscape of global research studies by promoting harmonization of regulatory requirements. This approach facilitates more efficient study designs, significantly reducing the duplication of efforts across various regions. Our extensive management services for studies include:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project oversight
- Reporting of both serious and non-serious adverse events
All of which contribute to improved efficiency and effectiveness in medical investigations.
According to the Technical Report Series, No. 850, Annex 3 (1995), the implementation of ICH guidelines has led to a notable increase in the speed of drug approvals, with statistics indicating that expedited processes can reduce timelines by up to 50% in certain cases. This enables pharmaceutical firms to carry out multi-regional research studies with enhanced efficiency, ensuring that the data gathered is both consistent and comparable.
The acceptance of ICH guidelines by diverse regulatory authorities has further expedited review processes, allowing new drugs to reach the market more swiftly. This acceleration is especially critical during public health emergencies, where rapid access to effective treatments can be life-saving. Furthermore, as highlighted in recent discussions on Bayesian Model Averaging (BMA) designs,
'the marginal likelihood of the data and the marginal posterior distributions for the [region-specific treatment effects](https://pmc.ncbi.nlm.nih.gov/articles/PMC10102881) have closed forms for the continuous-outcome situation described in this article, allowing for quick and efficient computations.'
This showcases the advantages of harmonization in addressing heterogeneous variability. The demand for a greater degree of country-independent standardization highlights the need to create minimum acceptable benchmarks for health studies worldwide, emphasizing that ICH stands for medical advancements and promoting global health through international cooperation and innovation in Medtech. Moreover, our services are vital in job creation and economic expansion by promoting local expertise and infrastructure development, which are necessary for successful research projects.
Challenges in Implementing ICH Guidelines
While the International Council for Harmonization (ICH), which ICH stood for medical, provides significant benefits for research, their execution poses a variety of challenges for organizations. Local regulatory variations can complicate adherence, as differing jurisdictions may impose unique requirements. Furthermore, cultural differences often influence compliance attitudes and practices, creating a need for tailored approaches to training and implementation.
Our service capabilities, including feasibility studies, site selection, compliance reviews, experimental setup, import permits, project management, and monitoring, are designed to navigate these complexities effectively. According to recent findings from a survey of Japanese stakeholders published in Ther Innov Regul Sci, the costs associated with training staff to ensure compliance with ich stand for medical can be substantial, necessitating a strategic allocation of resources. This survey also offered suggestions for effective practice renovation, highlighting the significance of managing these training expenses efficiently.
Data management and reporting complexities add layers of difficulty, particularly when navigating multiple regulatory environments. To effectively overcome these obstacles, organizations should prioritize fostering a culture of compliance through ongoing education and collaboration with regulatory bodies. Engaging in constructive dialogue with stakeholders is equally essential, as it can streamline the implementation process.
As noted by Elaine Eisenbeisz, an experienced statistician, continuous education is pivotal in addressing the unique challenges faced by quality functions in pharmaceutical and biotechnology sectors. The insights gained from initiatives such as the 'Statistical Elements of Implementing ICH Quality Guidelines' seminar highlight the importance of ich stand for medical, equipping teams with practical solutions and statistical methods to mitigate risks associated with poorly designed studies. Furthermore, with experts like Katherine Ruiz leading in regulatory affairs for medical devices and in vitro diagnostics in Colombia, organizations can leverage informed guidance.
Additionally, innovative training approaches, such as those offered by Simu Live through on-demand streaming, can effectively address the challenges of compliance and resource allocation. By applying these strategies and using extensive management services for studies, including strong project oversight and reporting, organizations can improve their adherence to ICH guidelines, ultimately resulting in more successful outcomes for investigations.
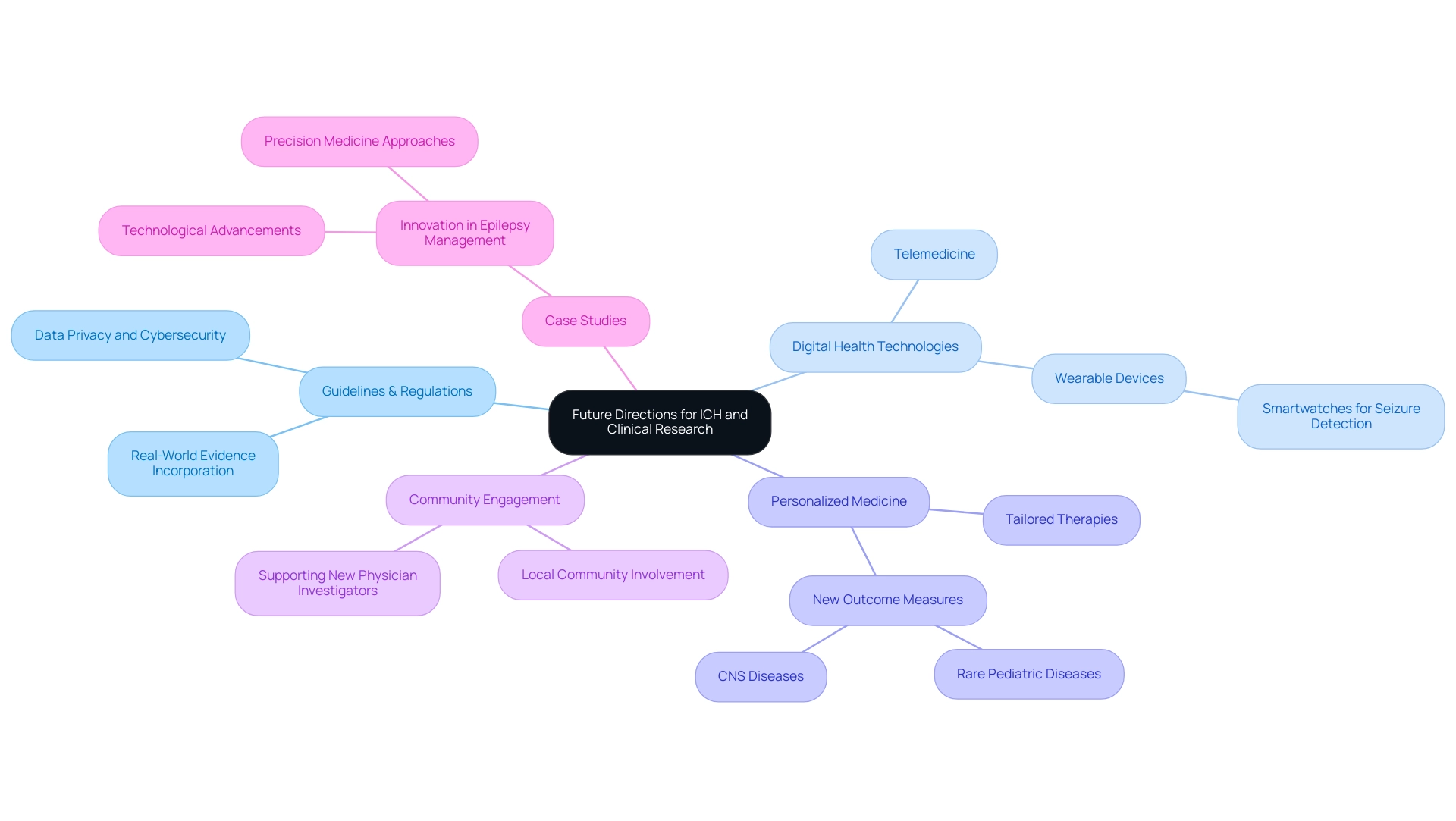
Future Directions for ICH and Clinical Research
As medical studies continue to evolve, ich stand for medical the International Council for Harmonization (ICH) is prepared to adjust its guidelines to reflect the rapid advancements in technology and methodologies. The proliferation of digital health technologies, including telemedicine and wearable devices, presents an array of opportunities and challenges within medical trials. These innovations require the creation of guidelines that not only tackle data privacy and cybersecurity but also promote the incorporation of real-world evidence into medical methodologies.
Furthermore, with the increasing emphasis on personalized medicine, the ICH is likely to highlight the need for frameworks that support the development of tailored therapies aimed at specific patient populations. In 2023, there is a significant emphasis on developing new outcome measures for rare pediatric and Central Nervous System (CNS) diseases, reflecting the ongoing evolution of medical priorities.
In this context, Sandra Smith, Senior Vice President of Clinical Solutions & Strategic Partnerships at WCG, emphasizes that identifying and supporting new physician investigators at study sites, especially those ingrained in the community setting, is critical to offsetting concerns and accelerating advancements. Furthermore, our extensive research management services—spanning from feasibility assessments and site selection to compliance evaluations, study preparation, and reporting on research status, inventory, and serious and non-serious adverse incidents—play a vital role in ensuring that studies are executed efficiently and ethically. The effective planning and execution of trials not only enhance the quality of studies but also contribute to local economies through job creation and improved healthcare outcomes.
A pertinent example is the case study on innovation in epilepsy management, where over 30% of individuals with epilepsy do not respond to common antiseizure medications. This has resulted in technological progress, such as smartwatches for seizure detection, demonstrating how digital health technologies can improve medical study methods. By proactively addressing these emerging trends, including adherence to country requirements, ICH stood for medical integrity, ensuring that studies remain rigorous, ethical, and responsive to the evolving needs of patients and healthcare systems globally.
This proactive stance will not only enhance the quality of clinical trials but also foster trust and collaboration among stakeholders, paving the way for innovative solutions in medical research.
Conclusion
The International Council for Harmonisation (ICH) plays an indispensable role in the global pharmaceutical landscape, particularly in enhancing the efficiency and effectiveness of clinical trials. By harmonizing regulatory requirements across regions, ICH fosters a collaborative environment that not only accelerates the development of safe and effective medications but also promotes economic growth in areas where clinical research is conducted. The significance of ICH guidelines, particularly the Good Clinical Practice (GCP) standards, cannot be overstated; they ensure that clinical trials are conducted ethically and that participant safety is prioritized.
Despite the benefits, implementing ICH guidelines presents challenges, including local regulatory variations and cultural differences that can complicate compliance efforts. Organizations must navigate these complexities through ongoing education, strategic resource allocation, and engagement with regulatory bodies. The commitment to fostering a culture of compliance is essential for overcoming these obstacles and achieving the full potential of ICH's harmonized standards.
Looking ahead, the future of ICH will be shaped by technological advancements and the evolving needs of healthcare. The integration of digital health technologies and personalized medicine into clinical research practices will require adaptive guidelines that address emerging challenges, such as data privacy and the use of real-world evidence. By proactively embracing these changes, ICH can continue to ensure that clinical research remains rigorous, ethical, and responsive to patient needs, thereby reinforcing its critical role in the ongoing evolution of the pharmaceutical industry. Ultimately, the continued collaboration and innovation fostered by ICH will be vital in driving advancements in drug development and improving health outcomes worldwide.
Frequently Asked Questions
What is the International Council for Harmonization (ICH)?
The ICH, established in 1990, is an initiative that unites regulatory authorities and pharmaceutical industry representatives from Europe, Japan, and the United States to foster international harmonization of technical requirements for the registration of pharmaceuticals.
What are the main objectives of the ICH?
The primary objective of the ICH is to simplify the drug development process by creating guidelines that improve the efficiency of studies, ensuring that new medications are safe, effective, and meet high-quality standards.
What tasks are included in the management services for studies under ICH guidelines?
Management services include assessing feasibility, choosing study locations and principal investigators, compliance evaluations of study documents, obtaining setup and approvals from ethics boards and health ministries, securing import permits for investigational devices, and strong project management and reporting.
How does ICH influence pharmaceutical practices in Europe?
ICH's guidelines promote adherence and efficiency in studies, which is crucial for improving pharmaceutical practices. For example, statistics show high rates of antibiotic consumption in Greece and France, highlighting ICH's impact on medication practices.
What economic advantages do research studies generate?
Research studies create substantial economic advantages, aiding in job creation and local economic development, particularly in areas where these studies are conducted.
What is the significance of the Inflation Reduction Act for pharmaceutical companies?
Pharmaceutical companies must incorporate the Inflation Reduction Act into their strategic planning as it could reshape market dynamics and pricing strategies, emphasizing the evolving landscape of pharmaceutical registration.
What role does the World Medical Association (WMA) play in drug development?
The WMA emphasizes ethical standards in drug development through its Declaration of Helsinki, which aligns with ICH guidelines and their impact on pharmaceutical registration.
Why are ICH guidelines, particularly Good Clinical Practice (GCP) guidelines, important in medical studies?
ICH guidelines, especially GCP, provide a framework for conducting medical studies, ensuring the protection of human subjects, data integrity, and the credibility of findings, which are essential for regulatory approval and ethical integrity.
What practices does the Clinical Trials Transformation Initiative (CTTI) promote?
The CTTI promotes practices prioritizing quality and efficiency in clinical research, including informed consent, patient recruitment, and Institutional Review Board (IRB) conduct.
How does the Trial 360 digital platform support compliance with GCP guidelines?
The Trial 360 platform enhances study management by providing features that ensure compliance with GCP guidelines, including a findings and deviations module for real-time documentation, tracking, and resolution of issues, thus maintaining data integrity and participant safety.

