Introduction
Title 21 CFR Part 11 is a crucial regulation that governs the use of electronic records and electronic signatures in FDA-regulated industries, including clinical trials. This regulation sets out the criteria for the acceptance of electronic systems in managing various tasks related to electronic records, ensuring their integrity and security. Compliance with 21 CFR Part 11 is essential for organizations involved in drug development and clinical trials, as it not only supports data integrity but also streamlines processes and enhances the delivery of healthcare services.
In this article, we will explore the scope and application of 21 CFR Part 11, its general provisions, requirements for electronic records and signatures, key compliance elements, benefits of implementation, entities required to comply, steps to achieve compliance, common challenges, and solutions. By understanding and adhering to these regulations, organizations can maintain the highest standards of quality and integrity in their work while ensuring the safety and efficacy of healthcare products and services.
What is 21 CFR Part 11?
'Title 21 CFR Part 11 is a crucial regulation that oversees the utilization of digital documentation and digital signatures within FDA-regulated industries, including the realm of clinical trials.'. This rule outlines the standards for the approval of digital systems to handle different activities like generation, alteration, upkeep, storage, retrieval, and transfer of digital documents. It also defines the criteria for the implementation of signatures, ensuring that they are as trustworthy, reliable, and generally equivalent to paper records and handwritten signatures.
This regulation is crucial for preserving the integrity and security of documentation and is especially significant in the context of orphan drugs—a category of pharmaceuticals targeting rare diseases or conditions. The FDA grants orphan-drug designation, providing market exclusivity for seven years post-approval for drugs that treat these rare conditions, provided the drug has not been previously approved for the same indication. This exclusivity is crucial for encouraging the development of treatments for diseases affecting smaller patient populations.
Furthermore, in the age of digital revolution, the FDA's dedication to public health is further demonstrated by simplifying processes such as the submission of documents online. As an example, the SSA-827 form that is transmitted digitally facilitates a more expedited processing time for disabilities, showcasing how digital documents and signatures can improve operational effectiveness and delivery of services while safeguarding the confidentiality of sensitive data. Such advancements affirm the FDA's role in not only safeguarding public health but also in fostering innovation and efficiency in healthcare administration and clinical research.
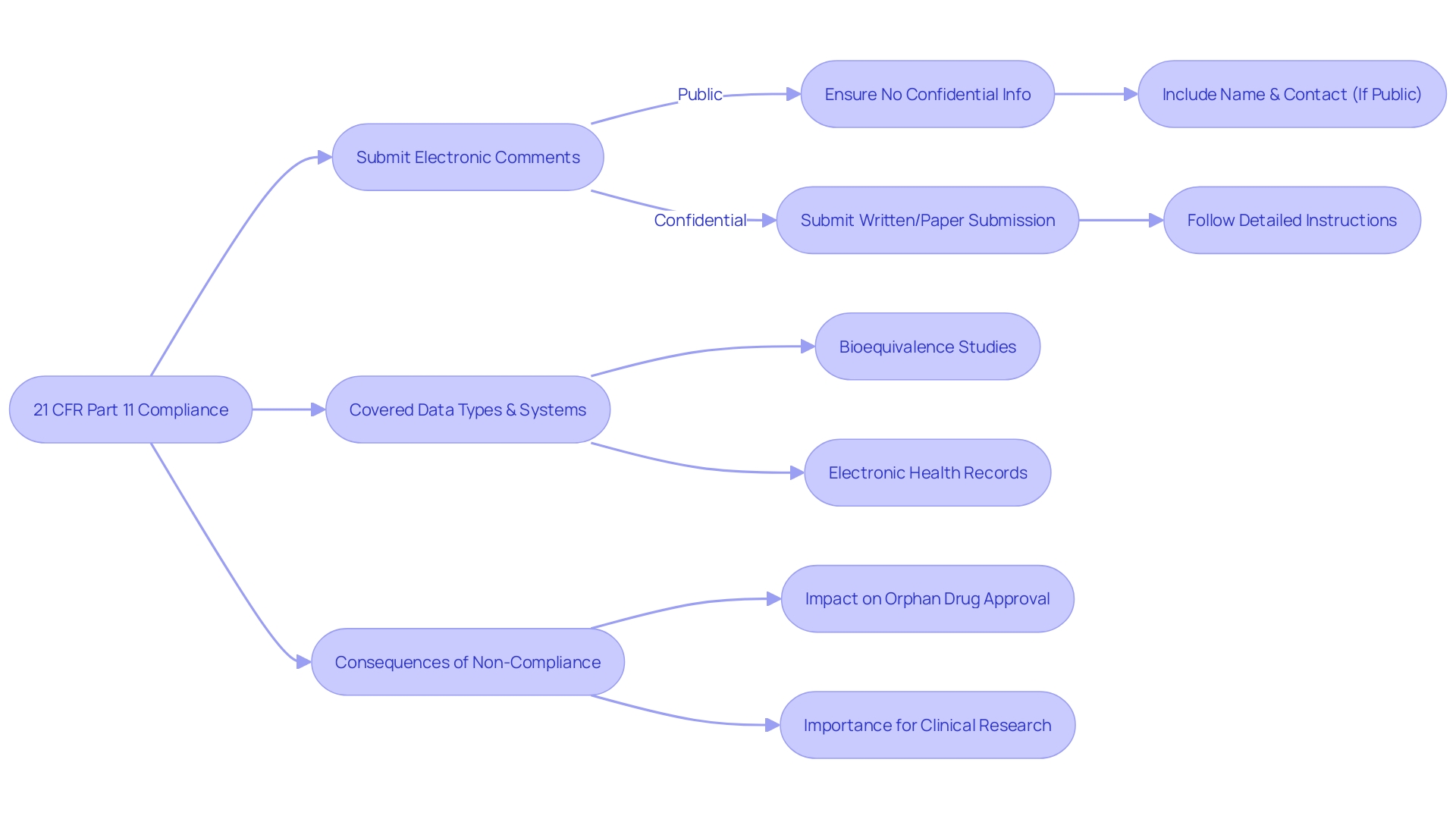
Scope and Application
21 CFR Part 11 outlines the standards by which digital documents and digital signatures are deemed trustworthy, dependable, and on par with physical documents. This regulation covers a broad array of data types and systems linked to clinical trials, including information involved in bioequivalence (BE) studies crucial for generic drug approval. For instance, BE studies, a pivotal part of regulatory submissions, often employ cross-over designs to determine whether a generic product performs similarly to its reference drug in terms of pharmacokinetic parameters. The regulation's implications extend to clinical trial management, where electronic health records (EHRs) serve as a critical tool for patient monitoring and data analysis. However, despite their importance, EHRs are not inherently analytical platforms, and integrating the review and management of trial data can be a challenge for clinical research teams, especially within high-volume patient settings such as oncology.
The consequences of non-compliance with 21 CFR Part 11 are significant. The FDA's authority to grant exclusive approval to orphan drugs, for example, hinges on adherence to stringent regulatory criteria. This exclusivity grants a seven-year market monopoly for drugs treating rare diseases, provided no other identical drug has been approved for the same indication. The designation of 'orphan subset' further complicates the regulatory landscape, emphasizing the need for adherence to regulations in drug development to ensure both the safety of therapies and the viability of their market potential.
Furthermore, the FDA's dedication to promoting clinical research through effective, well-planned studies emphasizes the significance of adhering to 21 CFR Part 11. The agency's recent initiatives highlight the need for reliable data and robust study designs, which are predicated on stringent regulatory adherence, to inform the FDA's decision-making processes. Entities responsible for compliance include drug manufacturers, clinical research organizations, and healthcare institutions involved in drug development and clinical trials. Adhering to 21 CFR Part 11 is not only a legal obligation but a crucial step in safeguarding the integrity of the clinical research process and the safety of patient data.
General Provisions (Subpart A)
Section A of Title 21 of the Code of Federal Regulations (CFR) Part 11 establishes the fundamental aspects for digital documents and digital signatures within the domain of FDA-regulated activities. It is intended to guarantee that digital documents are as dependable, reliable, and generally equivalent to paper documents and handwritten signatures executed on paper. This includes establishing clear criteria for the use of digital signatures, thereby allowing them to be legally equivalent to traditional handwritten signatures.
The rule thoroughly covers the establishment, alteration, upkeep, storage, retrieval, and transfer of digital documents. It mandates the implementation of strong systems that must be capable of producing precise and unaltered copies of records for inspection and review, thereby safeguarding the authenticity, integrity, and confidentiality of data.
Recent advancements in digital technology underscore the importance of these regulations. For example, the Social Security Administration's modernization of the SSA-827 form into a completely digital version demonstrates the shift towards efficient digital processes, reducing the need for physical documentation and expediting service delivery. Likewise, the Ministry of Health's decision to eliminate 30 paper forms in support of a centralized healthcare system that relies on modern technology mirrors the wider digitalization movement in the healthcare industry, underscoring the importance of secure record-keeping systems.
To further demonstrate the importance of CFR Part 11, take into account the healthcare sector's growing dependence on digital systems to store billions of medical documents. These systems ensure that medical information is centrally stored and protected, thus requiring compliance with strict regulatory standards for records.
Given these advancements, comprehending and abiding by the regulations of 21 CFR Part 11 is crucial for organizations to stay compliant and to build confidence in their documentation practices. The regulation not only supports the integrity of data but also facilitates the adoption of digital systems that can streamline processes and enhance the delivery of healthcare services.
Electronic Records (Subpart B)
Section B of Title 21 CFR Part 11 establishes the standards by which digital documents and digital signatures are deemed reliable, dependable, and comparable to physical documents. This covers principles for producing, adjusting, upkeeping, storing, finding, and sending digital documents. It requires the utilization of secure, computer-generated, time-stamped audit trails to independently capture the date and time of operator entries and actions that initiate, alter, or remove digital records. Record changes do not obscure the original entry, ensuring traceability.
Furthermore, it necessitates the execution of system validation measures to guarantee precision, dependability, and consistent intended functionality of digital documents. Access to these systems is restricted to authorized individuals through the use of secure, unique identifiers and passwords. The stipulation also states that digital documents must be easily accessible throughout their storage duration and accessible for agency examination and duplication.
To uphold compliance with both state and federal laws, practices concerning health information technology, such as those stipulated by 45 CFR 164.524(a)(1), must be consistent and non-discriminatory. In certain circumstances, exceptions are granted respecting an individual's request not to share information, provided specific conditions are met.
In line with maintaining up-to-date practices, the eCFR is presented in a user-friendly format, with paragraphs split and indented to reflect the hierarchy of the document. This format facilitates comprehension and adherence to the regulations without altering the intended meaning of the agency.
In the context of drug products, details such as the list of components, specifications, manufacturer details, and descriptions of manufacturing and packaging procedures are critical. The specifications must ensure the product's identity, strength, quality, purity, potency, and bioavailability. This includes acceptance criteria for various tests and analytical procedures.
The FDA also requires that each production aggregate of products, such as infant formula, be coded for traceability. This code identifies the product, the establishment where it was packed, and enables tracing of all stages of manufacture, including handling of raw materials. These rigorous standards underscore the importance of maintaining integrity and control over digital records in the pharmaceutical industry.
Electronic Signatures (Subpart C)
Subpart C of 21 CFR Part 11 is essential for ensuring the authenticity and integrity of signatures in the context of clinical trials and FDA regulations. This regulatory segment mandates that each signature must be unique to the individual using it, not to be reused by, or reassigned to, anyone else. To protect signatures, strong controls are necessary and must be put in place to verify the identity of the user and to prevent unauthorized access.
People who have the power to produce, change, uphold, or send digital documents hold important obligations. They must adhere to the stringent requirements set forth to maintain the security and integrity of these records. The utilization of signatures has been streamlined in recent changes, as witnessed in the Social Security Administration's adoption of the SSA-827, which permits signing and submission using digital means, enhancing disability processing times.
Moreover, the eCFR provides an updated and user-friendly version of these regulations with paragraph structure and indentation that reflect the hierarchy of the document, ensuring ease of understanding without altering the intent of the regulations. This method of document formatting is particularly advantageous for federal agencies aiming to uphold adherence to these intricate regulatory requirements.
The development of records and signatures is also apparent in the Social Security Administration's efforts to convert frequently used forms into online versions that can be completed and signed electronically, further reducing the risks associated with physical document handling.
In order to keep up with the changing regulatory environment, it is crucial for clinical researchers to remain aware of the most recent advancements and enhancements in signature protocols to guarantee adherence and aid in the more effective and safeguarded handling of clinical trial information and accompanying paperwork.
Key Requirements for Compliance
Achieving compliance with 21 CFR Part 11 is a multi-faceted task that encompasses several critical elements. To comply with these regulations, companies must ensure that their systems are thoroughly validated to verify accuracy and reliability in their intended use. This verification procedure is an essential necessity, guaranteeing that any digital documents or signatures are deemed dependable, trustworthy, and comparable to hard copy documents. Furthermore, enforcing strong security measures is crucial to hinder unauthorized entry or modifications to digital documents. This includes maintaining strict access controls and the utilization of secure, computer-generated, time-stamped audit trails to independently document the date and time of operator entries and actions that generate, modify, or erase electronic records. Audit trails are essential for the reconstruction of events and for ensuring the integrity of the documents throughout their retention period. Moreover, companies must establish explicit document retention policies that specify the duration for which documents must be kept in a legible format. These policies must adhere to the relevant regulatory requirements and guarantee that documentation is easily accessible for examination by regulatory authorities when necessary. The importance of these components is underscored by the need for meticulous oversight and management to protect the integrity of clinical research and patient data within the healthcare industry.
Benefits of Implementing 21 CFR Part 11
Compliance with 21 CFR Part 11 is crucial for organizations conducting clinical trials, offering numerous operational and regulatory advantages. One such benefit is the assurance of data integrity, which is foundational to the credibility of clinical research. By adhering to these regulations, organizations are better equipped to safeguard the accuracy and consistency of their data throughout its lifecycle. Furthermore, effectiveness is greatly improved by utilizing digital documentation and signatures, simplifying the data gathering and administration procedures. This modern approach not only saves time but also reduces the likelihood of errors associated with manual data handling. Enhanced document control is another key advantage, providing organizations with the ability to trace and audit changes within their documentation easily. This level of control is essential for maintaining compliance with FDA standards and for the protection of sensitive data. Moreover, digital documents provide the additional benefit of facilitating more advanced analysis and reporting capabilities, allowing researchers to extract actionable insights more easily. These regulations, which include provisions such as the orphan-drug designation, underscore the FDA's commitment to promoting the development of therapies for rare diseases while ensuring rigorous safety and efficacy standards. The orphan-drug designation, in particular, provides market exclusivity for a period, incentivizing the development of drugs for smaller patient populations. Overall, the implementation of 21 CFR Part 11 is not just a regulatory requirement but a strategic asset that propels clinical research forward, ensuring that organizations can uphold the highest standards of quality and integrity in their work.
Who Must Comply with 21 CFR Part 11?
Organizations involved in the planning, execution, or supervision of clinical studies must comply with the Code of Federal Regulations (CFR) Title 21 Part 11, which establishes the standards for digital documentation and digital signatures. Understanding the extent of adherence is crucial, involving a variety of stakeholders like sponsors, contract research organizations (CROs), clinical investigators, and institutional review boards (IRBs). These regulations are integral to maintaining the integrity and reliability of electronic records, which is a cornerstone in the FDA's mission to safeguard public health by ensuring the safety and efficacy of human and veterinary products. Professionals in the field must stay informed about these regulatory requirements to ensure that their operations meet all the necessary standards for clarity and consumer accessibility, such as the recent final rule on direct-to-consumer prescription drug advertisements, as the FDA continues to update them.
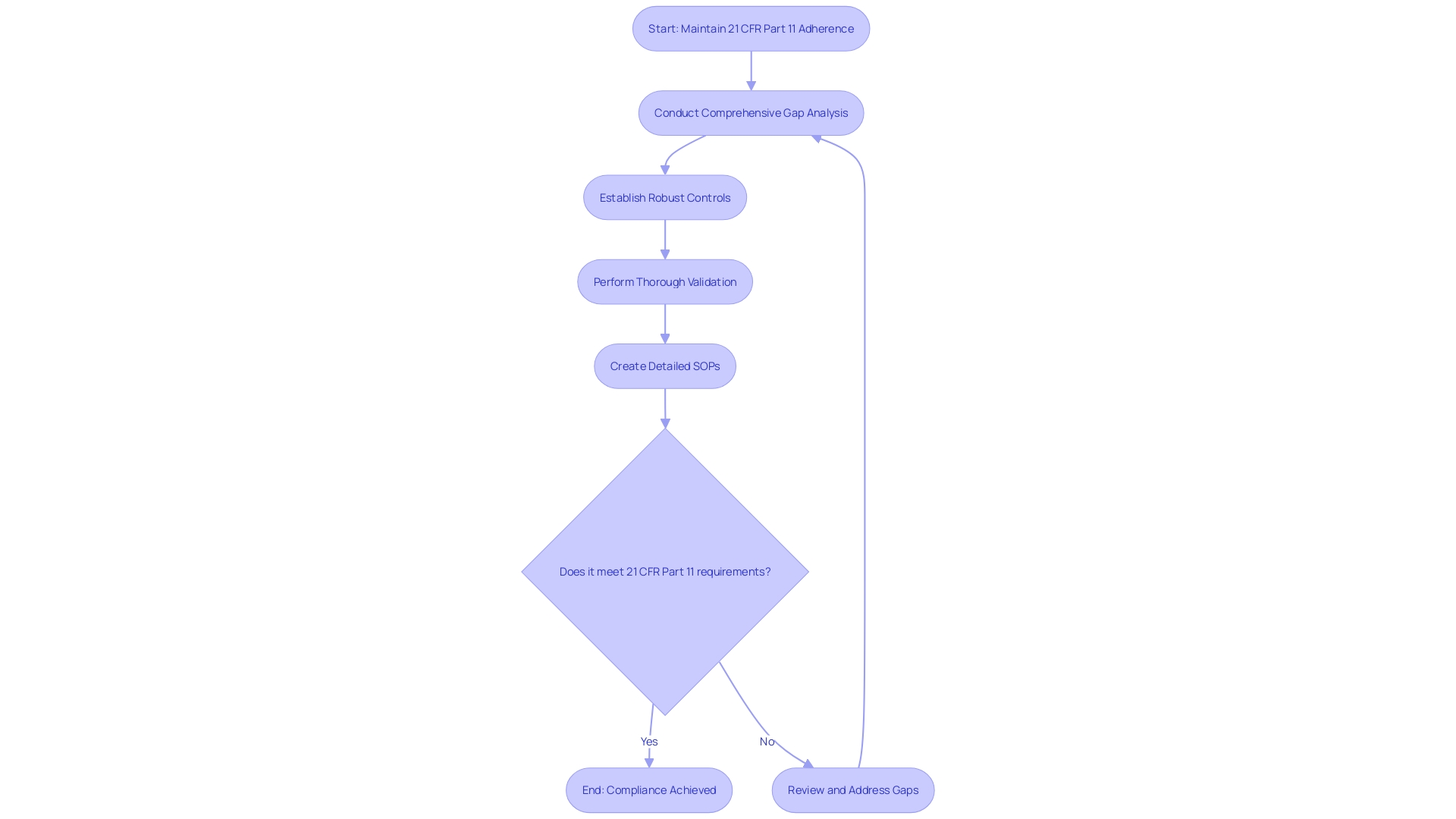
Steps to Achieve 21 CFR Part 11 Compliance
To maintain adherence to 21 CFR Part 11, organizations must adopt a thorough plan. Key steps include a comprehensive gap analysis to identify areas of non-compliance, the establishment of robust controls tailored to the organization's needs, thorough validation to ensure systems operate as intended, and the creation of detailed standard operating procedures (SOPs) to guide consistent, compliant operations. This structured approach is essential for mitigating risks associated with non-compliance, such as security breaches, financial penalties, and reputational harm, as evidenced by the digital transformation challenges faced by longstanding institutions like M&T Bank and healthcare systems like UHealth. These entities have had to rigorously enforce standards for software quality and performance to protect sensitive data and ensure seamless integration of services. Furthermore, as healthcare organizations utilize AI and data to improve patient care, as mentioned in recent industry webinars, the significance of adhering to federal regulations becomes even more evident to guarantee data completeness and interoperability. Ensuring adherence to the regulatory framework of the FDA involves understanding the definitions of restricted devices, classification names, product codes, and the implications of orphan-drug designation, as these directly impact the marketing and distribution of medical devices and drugs. Implementing effective internal controls, as mandated by the FCPA, is a critical component of a best practices program. Such measures are not just a precaution but a fundamental aspect of a robust governance strategy, defending against potential financial restructuring or legal actions, as faced by companies like Akumin following a ransomware attack. The eCFR's automated paragraph structure serves as a user-friendly guide to the official CFR formatting, assisting organizations in their efforts to align with regulatory expectations without altering the intent of the agency.
Common Challenges and Solutions
Navigating the intricacies of 21 CFR Part 11 can present significant hurdles for organizations, with obstacles ranging from limited resources to ensuring thorough staff training. To overcome these challenges, it is crucial to adopt a strategic approach. For example, the Ford Foundation recognized the need to evolve their digital engagement platform to better serve their audience's needs, leading to the implementation of new systems to keep up with the increased content output. Likewise, addressing the requirements of 21 CFR Part 11 may involve modernizing systems and processes for efficiency.
In the domain of adherence, the significance of strong internal controls cannot be emphasized enough. As Tom Fox, the Voice of Compliance, emphasizes, not only must appropriate internal controls be in place, but they must also be actively functioning. This principle is applicable when deploying technology solutions to streamline efforts in ensuring adherence to regulations. It is imperative to regularly review and update these controls to stay aligned with best practices and regulatory expectations.
The case of Austin Morgenroth at Somerset Academies of Texas illustrates the transformative impact of integrating technology solutions. By consolidating different software systems, the district improved its operations, emphasizing the potential benefits of technology in simplifying intricate regulatory processes. In the context of 21 CFR Part 11, utilizing integrated technology platforms can facilitate management of adherence, reduce errors, and enhance workflow efficiency.
Moreover, statistics emphasize the critical importance of cybersecurity in protecting sensitive data, a fundamental facet of adhering to 21 CFR Part 11 regulations. Given that a large number of organizations lack confidence in their cybersecurity measures, it is clear that there is a pressing requirement for enhanced security protocols to safeguard against cyber attacks—an issue that directly impacts adherence to regulations that mandate the safeguarding of electronic records and signatures.
In the end, organizations must acknowledge that while the journey to 21 CFR Part 11 adherence may be difficult, it is manageable with thoughtful preparation, the appropriate resources, and the implementation of efficient technology solutions. This proactive stance not only ensures regulatory compliance but also supports the organization's broader mission to advance in its respective field.
Conclusion
In conclusion, 21 CFR Part 11 is a crucial regulation that governs the use of electronic records and signatures in FDA-regulated industries, including clinical trials. Compliance with this regulation is essential for organizations involved in drug development and clinical trials as it supports data integrity, streamlines processes, and enhances healthcare service delivery.
The scope of 21 CFR Part 11 encompasses various tasks related to electronic records in clinical trials and bioequivalence studies. Non-compliance can jeopardize exclusive approval for orphan drugs, making compliance crucial for drug manufacturers, clinical research organizations, and healthcare institutions.
Implementing 21 CFR Part 11 brings several benefits to organizations conducting clinical trials. It ensures data integrity, enhances operational efficiency through electronic records and signatures, and provides better document control and analysis capabilities. The regulation also supports the development of therapies for rare diseases through provisions like the orphan-drug designation.
Entities involved in the design, conduct, or oversight of clinical trials must comply with 21 CFR Part 11. This includes sponsors, contract research organizations, clinical investigators, and institutional review boards.
To achieve compliance, organizations must undertake steps such as gap analysis, validation, and the establishment of robust controls and standard operating procedures. Overcoming common challenges, such as limited resources and staff training, requires a strategic approach and the adoption of technology solutions.
In summary, adherence to 21 CFR Part 11 is critical for organizations involved in drug development and clinical trials. It supports data integrity, streamlines processes, and enhances healthcare service delivery. By understanding and adhering to these regulations, organizations can maintain the highest standards of quality and integrity in their work, ensuring the safety and efficacy of healthcare products and services.
Learn more about how implementing 21 CFR Part 11 can benefit your organization's clinical trials.




