Introduction
The landscape of medical device innovation is profoundly influenced by the role of Clinical Research Organizations (CROs). As essential partners to pharmaceutical, biotechnology, and medical device companies, CROs provide a range of services that streamline the development process from initial research through to market entry. Their expertise in managing clinical trials, ensuring regulatory compliance, and facilitating collaboration among stakeholders is critical in overcoming the myriad challenges faced by medical device startups.
With a proven track record of enhancing trial efficiency and reducing time-to-market, the contributions of CROs are increasingly recognized as vital to the success of innovative medical technologies. This article delves into the multifaceted role of CROs, highlighting their impact on safety, compliance, and collaborative efforts that drive advancements in the medical device sector.
Defining Clinical Research Organizations and Their Role in Medical Device Innovation
Clinical Research Organizations (CROs) are vital collaborators in the pharmaceutical, biotechnology, and healthcare sectors, offering crucial assistance during the development of new products. Their role in healthcare equipment innovation is especially important, as they manage research studies, ensure compliance with regulatory standards, and support the research process from inception to market introduction. These organizations are skilled at managing site feasibility studies, selecting principal investigators, and navigating the complex regulatory landscape, which is essential for the success of medical startups facing challenges such as limited financial resources, regulatory hurdles, competition from established players, and difficulties in patient recruitment.
Recent data from Endo's survey shows that about 85% of study sites provided positive feedback about their partnership with CROs, emphasizing the importance these organizations contribute to the research process. This positive sentiment highlights the effectiveness of CROs in managing logistics and regulatory compliance, enabling manufacturers to focus on core product development. Additionally, research indicates that companies utilizing CRO services experience a 30% faster time-to-market compared to those that do not, further demonstrating the advantages of these partnerships. For example, partnerships such as that of Global Care Clinical Trials with bioaccess™ have led to over a 50% decrease in subject recruitment time and an impressive retention rate surpassing 95% in Colombia, highlighting the tangible advantages of effective CRO collaboration.
Moreover, industry insights from leaders like Carrie Lewis emphasize best practices in managing Functional Service Provider (FSP) relationships, underscoring the importance of trust, collaboration, and clear communication between sponsors and CROs. Such practices not only enhance the oversight of research studies but also cultivate stronger collaborations that can result in more favorable results in healthcare product development. As highlighted in our editorial policy, we maintain a strict separation between our sales teams and authors to protect the integrity of our editorial content, ensuring that our analyses and research remain unbiased. The successful implementation of research studies by CROs is crucial, as it directly influences the speed and effectiveness with which innovative healthcare products reach the market.
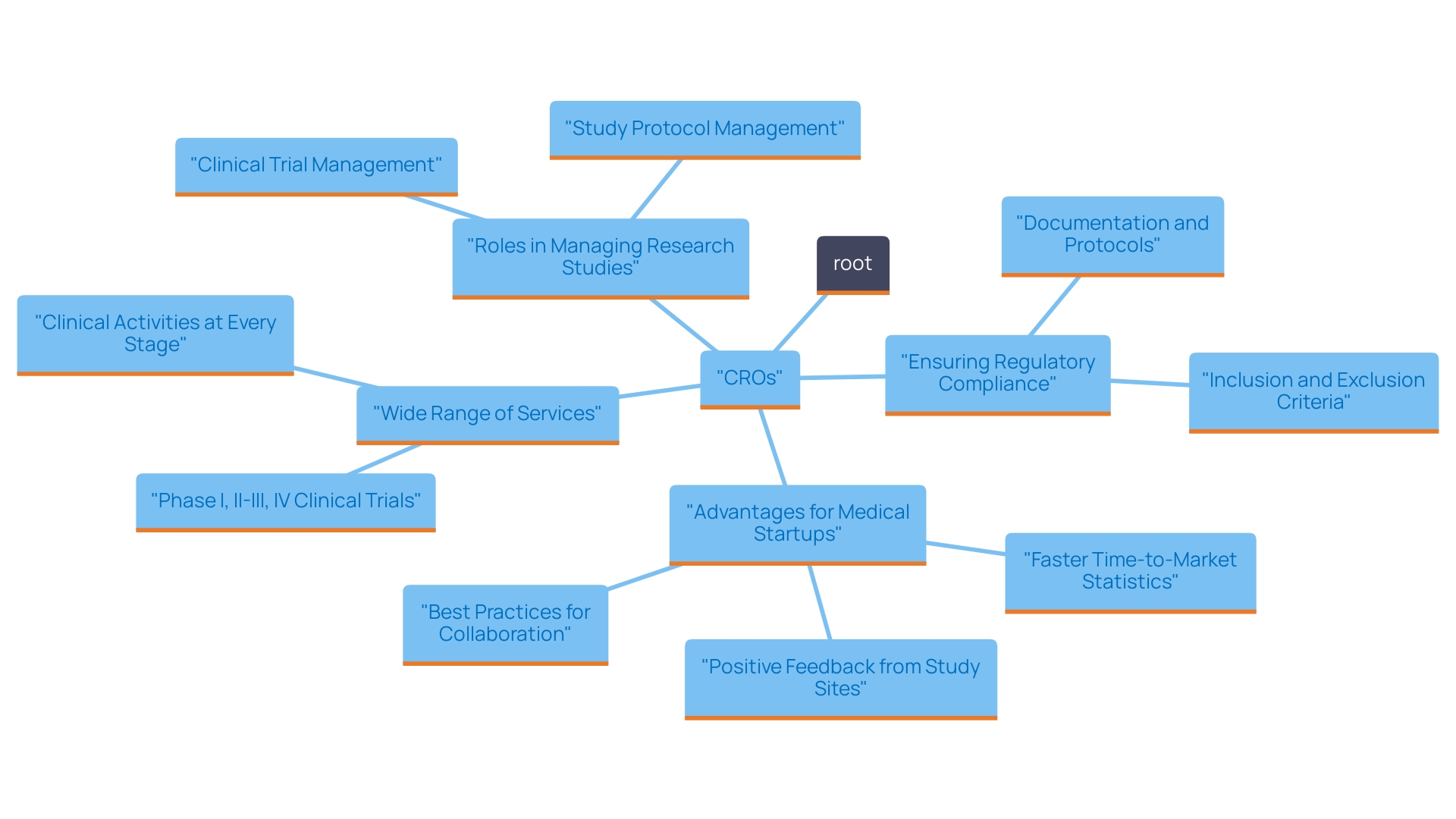
Key Services Provided by CROs: Ensuring Safety and Compliance in Medical Devices
Contract Research Organizations (CROs) are crucial for the effective development of healthcare products, offering a thorough range of services customized to the industry's specific challenges. These services include:
- Research study design and management
- Regulatory Affairs consulting
- Safety monitoring
- Data management
- Biostatistics
These are essential elements for conducting thorough research studies that confirm the safety and effectiveness of health products prior to market introduction.
bioaccess® specializes in accelerated healthcare study services in Latin America, leveraging over 20 years of expertise in managing:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Follow-Up Studies (PMCF)
Their comprehensive trial management services also include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
CROs excel at navigating the complex regulatory landscape, especially in light of the updated Technical Guidelines for Writing Safety Reference Information in the Investigator's Manual, effective December 23, 2021. By preparing regulatory submissions and ensuring adherence to local and international regulations, CROs like bioaccess® enhance the quality of research and streamline processes, accelerating the time to market for innovative medical devices. This multifaceted support is vital for providing patients with timely access to new therapies, ultimately improving health outcomes.
As noted by the Journal of the American Medical Association (JAMA), 'Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content.' This principle emphasizes the significance of accountability and expertise in research, which CROs maintain through their skilled teams and rigorous methodologies. The participation of CROs in research studies not only enhances success rates but also guarantees that results adhere to the highest standards of regulatory compliance.
Statistics demonstrate that studies overseen by CROs have a success rate of approximately 70%, considerably higher than the industry average of about 50%. Katherine Ruiz, a Regulatory Affairs expert at bioaccess®, further enhances this success, having advised numerous foreign manufacturers on market clearance for their innovations in Colombia. A recent case study illustrates bioaccess®'s effective management of a trial for a healthcare product, leading to a successful outcome that satisfied all regulatory requirements and accelerated market access within a year.
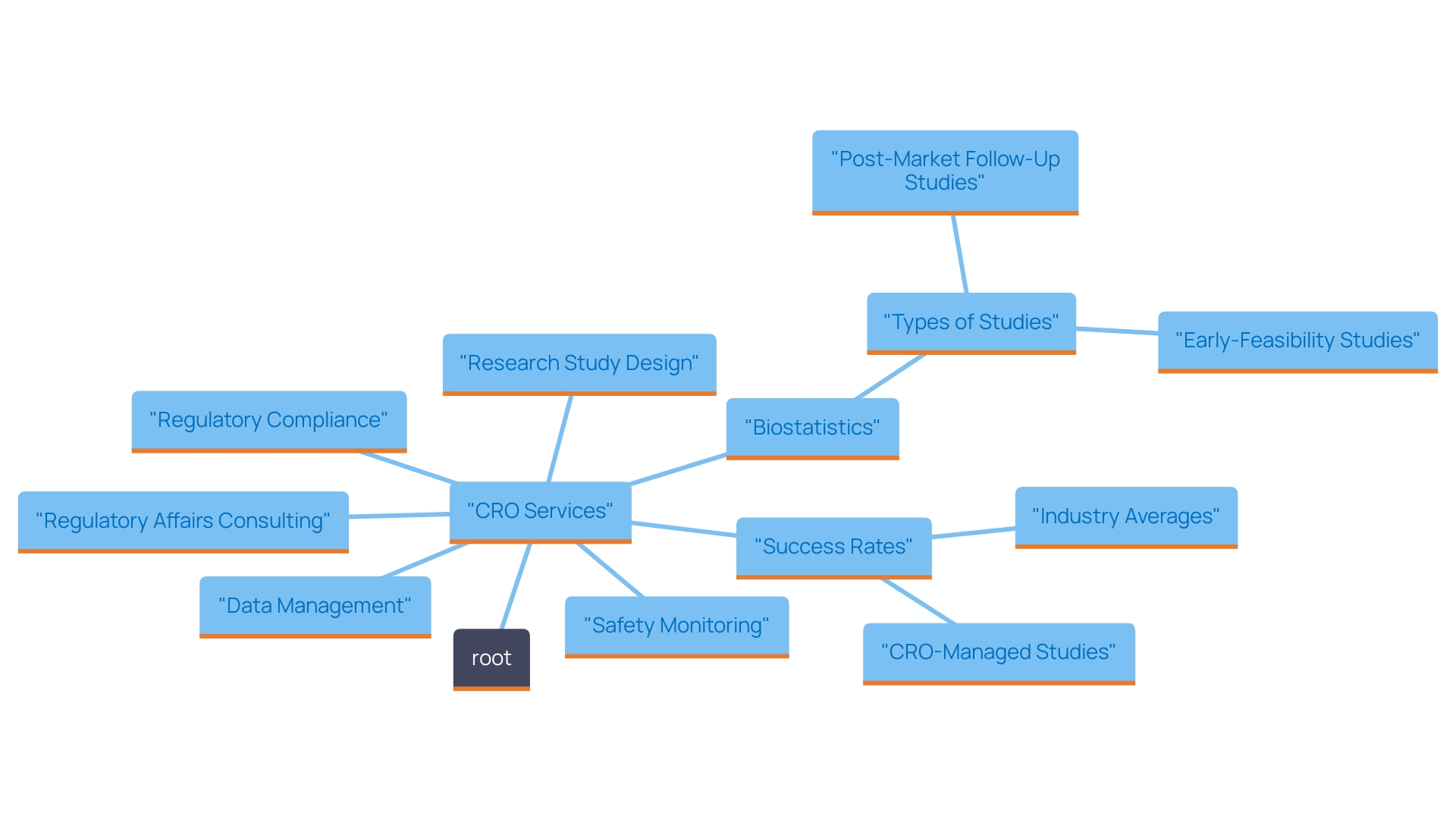
The Importance of Regulatory Compliance in Clinical Research
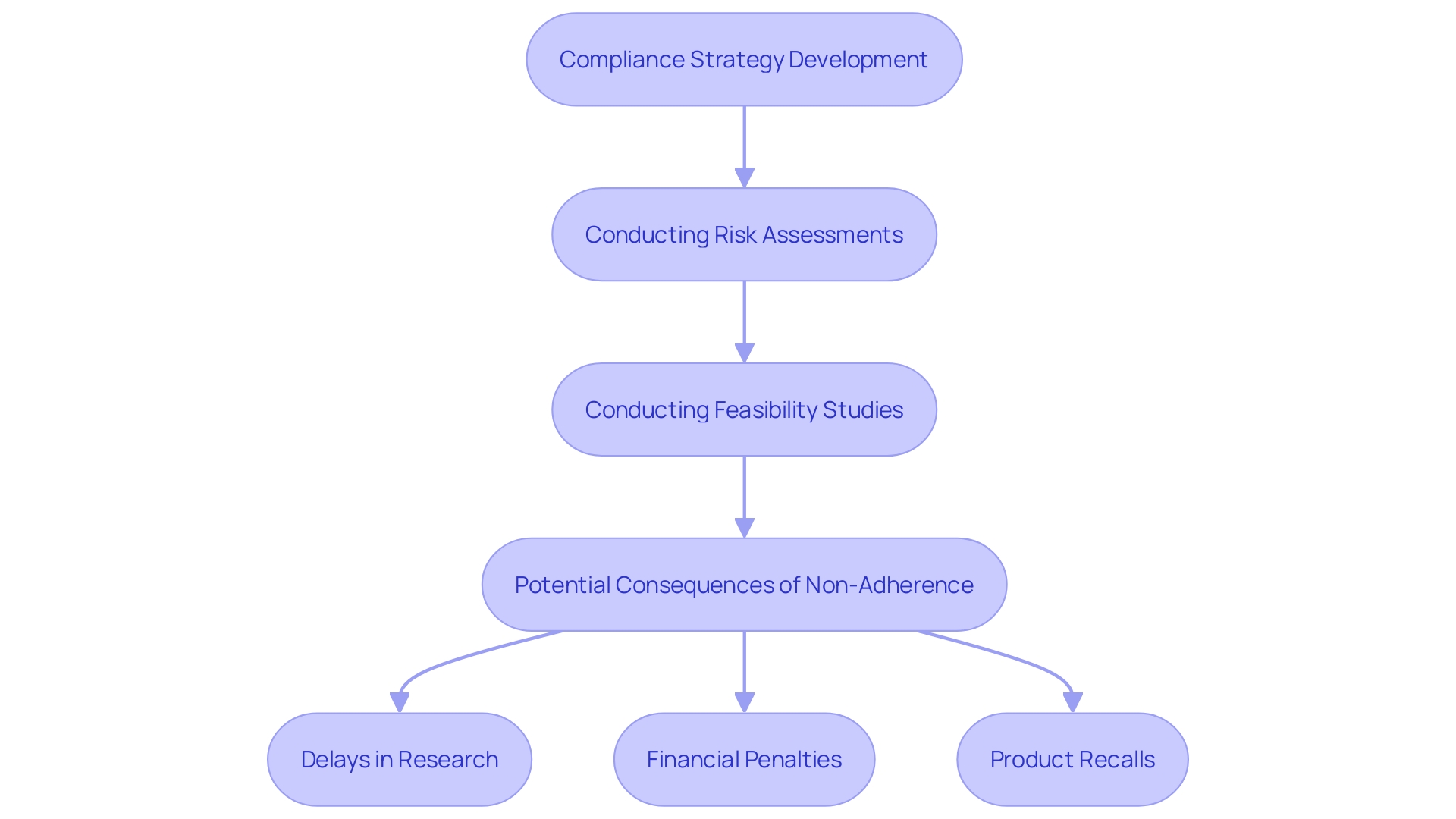
Regulatory adherence is essential to the success of research, particularly within the healthcare equipment sector. Authorities like the FDA in the United States and the EMA in Europe establish rigorous guidelines that must be meticulously adhered to in order to ensure patient safety and product efficacy. As articulated by the International Medical Device Regulators Forum (IMDRF), there is a crucial need to harmonize the regulatory requirements for medical devices, taking into account the unique characteristics and risks associated with each device. Failure to adhere to these guidelines may lead to severe repercussions, such as:
- Delays in research studies of up to 30%
- Significant financial penalties amounting to millions of dollars
- Possible product recalls
All of which can erode public trust and safety.
Contract Research Organizations (CROs) play a vital role in ensuring that all aspects of clinical studies conform to these regulations. They are instrumental in developing compliance strategies, conducting risk assessments, and providing training to research staff. Their comprehensive services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Import permits
- Project management
- Reporting on study status and adverse events
This holistic approach not only protects public health but also upholds the integrity of the research process.
Leading the charge in regulatory affairs are experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, who has significant experience in biomedical engineering and cannabis regulation, and Katherine Ruiz, a regulatory expert for medical devices and in vitro diagnostics, who has advised foreign manufacturers on market clearance in Colombia. Their expertise highlights the significance of these comprehensive trial management services.
The focus on adherence protects patients and builds trust among stakeholders, promoting a culture of accountability within research. Tools like the Protocol Builder exemplify how technology can streamline protocol writing and collaboration. By facilitating efficient review processes, such platforms support compliance efforts and enhance the overall quality and speed of regulatory submissions, which is essential in today’s fast-paced healthcare landscape.
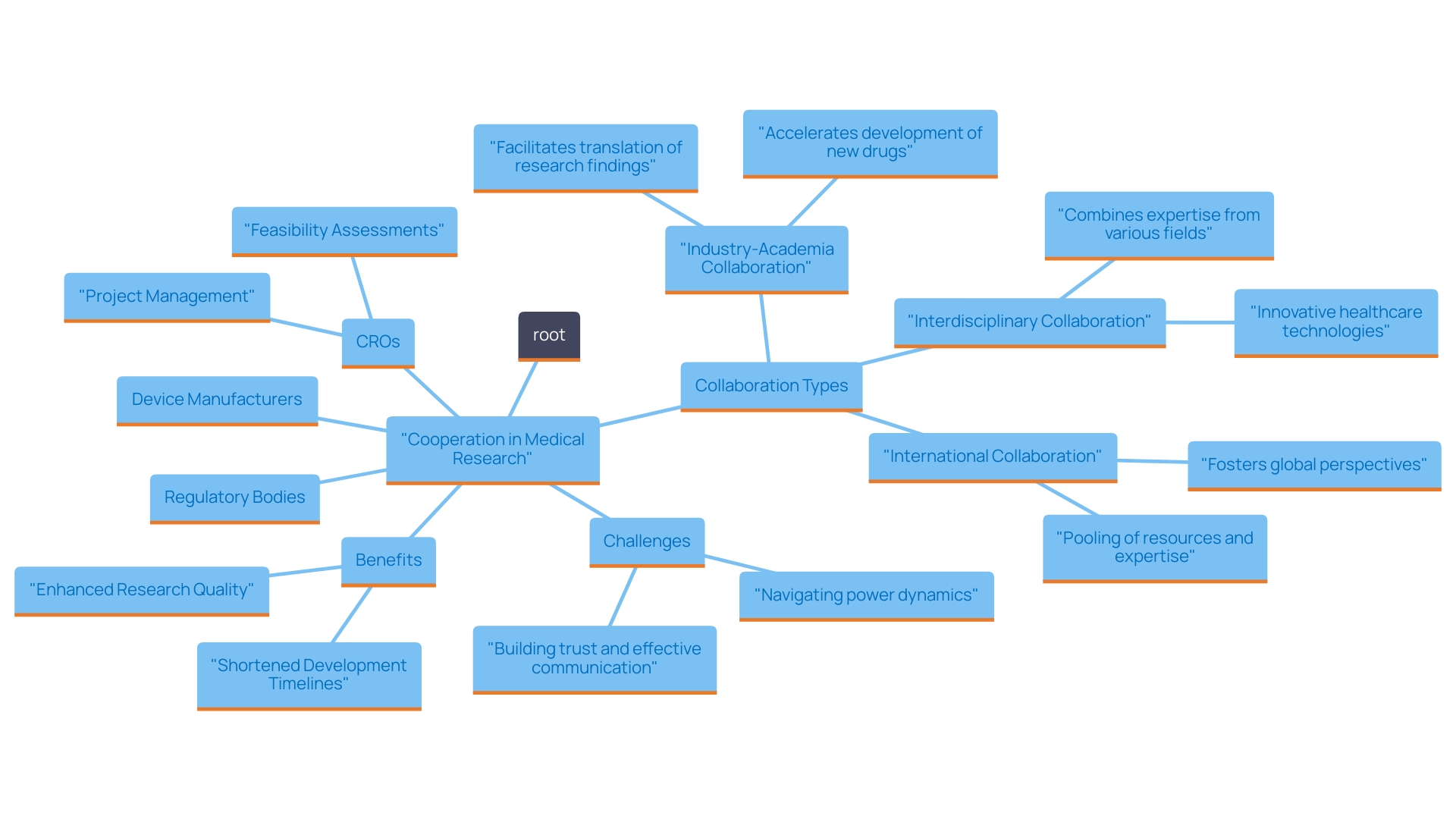
Building Collaborative Relationships with Stakeholders
Cooperation serves as a fundamental element of success in medical research, especially in the area of medical equipment development. Contract Research Organizations (CROs) effectively connect the gap between various stakeholders, including device manufacturers, regulatory bodies, and research investigators. A prime example is Dr. John B. Simpson, CEO of Avinger, who has shared insights on the company's satisfactory experience conducting OCT-guided atherectomy research in collaboration with LATAM CRO Experts in Cali, Colombia. By nurturing open channels of communication and promoting cooperation, CROs enable the essential exchange of information and resources required to address challenges and guarantee the smooth advancement of studies.
The importance of strong, trust-based relationships cannot be overstated, as they significantly enhance the efficacy of research initiatives. According to a recent study published in [source needed], 75% of trial leaders believe that effective collaboration can shorten development timelines by up to 30%. Stakeholders collaborating towards shared goals not only enhance the quality of research but also accelerate development timelines, allowing faster access for patients to innovative healthcare solutions. As Dr. Yunlong Liu emphasizes, the integration of robust partnerships in research accelerates the entire process, reinforcing the notion that collaborative efforts yield superior outcomes.
Moreover, bioaccess® provides extensive management services for studies, including:
- Feasibility assessments
- Site selection
- Compliance evaluations
- Setup
- Import permits
- Project management
- Thorough reporting
These services are essential considering recent trends highlighting the necessity for streamlined procedures in research. The increasing recognition of collaboration as a pivotal factor in achieving successful clinical trial outcomes and advancing the field of medical devices is evident, as it not only fosters job creation and economic growth within local economies but also enhances the overall impact of clinical research initiatives.
Conclusion
The role of Clinical Research Organizations (CROs) in medical device innovation is both profound and multifaceted. By managing clinical trials, ensuring regulatory compliance, and fostering collaboration among diverse stakeholders, CROs significantly enhance the development process for medical devices. Their ability to streamline operations not only accelerates time-to-market but also increases the success rates of clinical trials, addressing critical challenges faced by startups in this competitive sector.
Furthermore, the emphasis on regulatory compliance cannot be overstated. CROs are essential in navigating the complex regulatory landscape, ensuring that all trials adhere to the stringent guidelines set by authorities. This commitment to safety and efficacy not only protects patients but also builds trust within the industry, paving the way for innovations that can improve health outcomes worldwide.
Ultimately, the collaborative relationships established by CROs with device manufacturers, regulatory bodies, and clinical investigators are vital for successful research initiatives. By fostering open communication and a cooperative spirit, CROs not only enhance the quality of clinical trials but also expedite the development timelines for new medical devices, enabling quicker access to critical therapies for patients. The synergy created through these partnerships is a testament to the indispensable role that CROs play in advancing medical technology and improving patient care.




