Introduction
In the realm of clinical research, Contract Research Organizations (CROs) have emerged as indispensable partners for the pharmaceutical, biotechnology, and medical device sectors. These specialized entities navigate the intricate landscape of clinical trials, offering a suite of services that streamline processes and enhance research quality.
With a particular focus on first-in-human trials in regions like Colombia, CROs leverage local advantages such as cost efficiency, rapid regulatory approvals, and a robust healthcare system to optimize trial outcomes.
As the industry evolves, understanding the multifaceted role of CROs, their key services, and the challenges they face becomes critical for stakeholders aiming to advance medical science and improve patient care.
This exploration delves into the operational significance of CROs, the future trends shaping their landscape, and the vital contributions they make to the drug development process.
Defining Contract Research Organizations (CROs)
Mexico Contract Research Organizations are specialized service providers that assist the pharmaceutical, biotechnology, and medical device sectors in carrying out studies and evaluations. bioaccess®, a leading Mexico Contract Research Organization in Latin America, offers a comprehensive range of services tailored specifically for medical device studies, including:
- Study design
- Site feasibility
- Investigator selection
- Regulatory compliance
- Project management
- Data analysis
Additionally, bioaccess® provides critical reporting services, including:
- Study status
- Inventory
- Serious and non-serious adverse events
- Assistance with import permits
- Nationalization of investigational devices
By outsourcing research activities to experienced Mexico Contract Research Organizations like bioaccess®, sponsors can leverage expertise to navigate the complexities of clinical research efficiently and effectively.
Colombia serves as a strategic location for first-in-human studies due to its cost efficiency—offering savings of over 30% compared to North America and Western Europe—rapid regulatory approval processes, and high-quality healthcare systems. Furthermore, the nation offers patient recruitment benefits, backed by universal healthcare coverage for approximately 95% of its population, along with R&D tax incentives that further improve the appeal of conducting studies there.
Mexico Contract Research Organizations play a crucial part in the drug development process, assisting in optimizing operations and lowering expenses for sponsors while improving the quality and integrity of research.
BOOK A MEETING to discuss how we can support your clinical study needs.

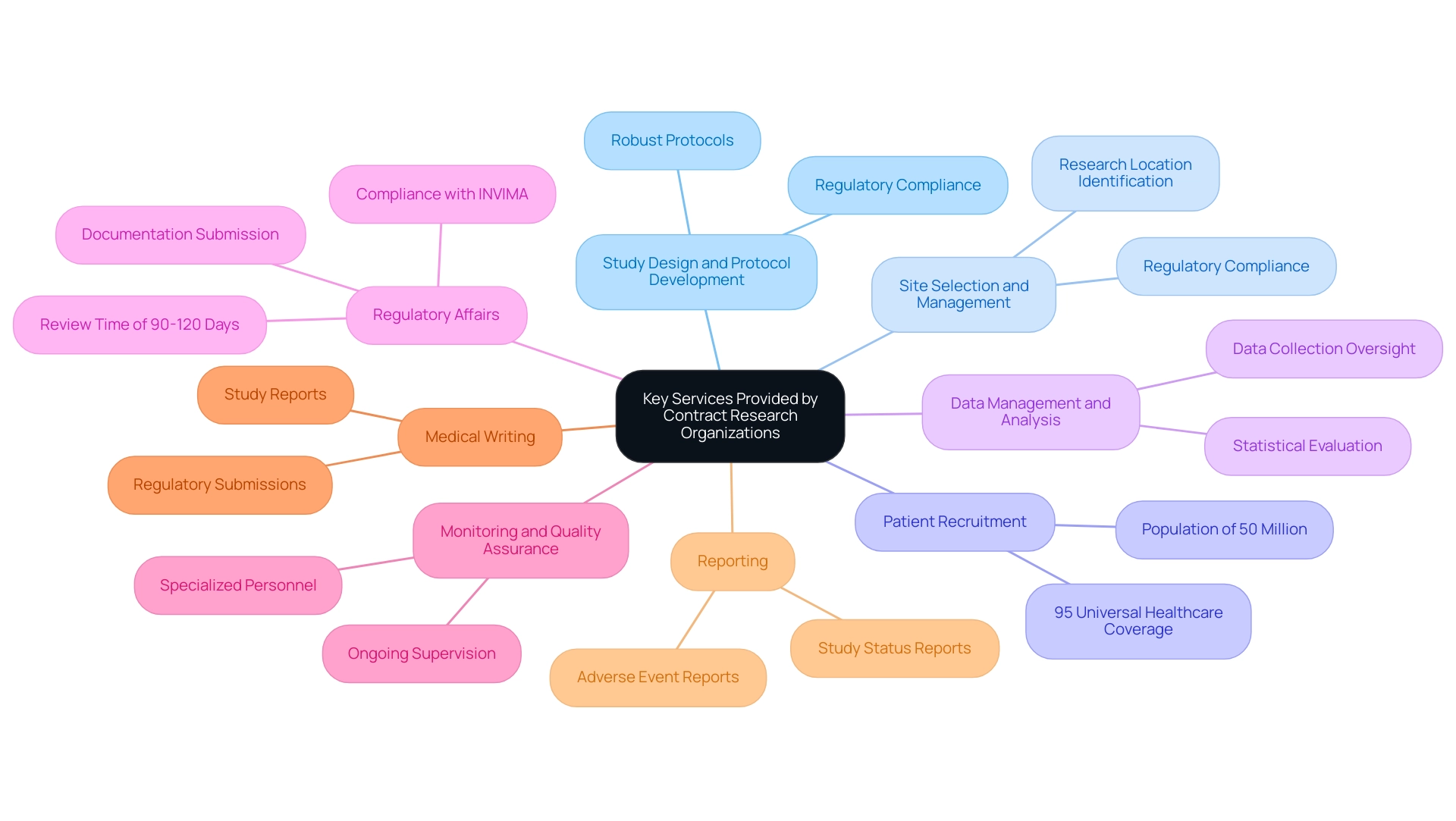
Key Services Provided by Contract Research Organizations
Contract research organizations provide a broad range of services that can be customized to address the unique requirements of their clients, especially regarding first-in-human medical device studies in Colombia. Key services include:
-
Study Design and Protocol Development: CROss aid in creating robust study protocols that adhere to regulatory requirements and scientific standards, ensuring that research is well-structured from the outset.
-
Site Selection and Management: They identify and manage research locations, ensuring that they are equipped and compliant with necessary regulations, leveraging Colombia's favorable landscape for site feasibility and researcher selection.
-
Patient Recruitment: With a population exceeding 50 million and 95% coverage under universal healthcare, Colombia offers distinct benefits in recruiting and retaining study participants, tackling one of the most difficult aspects of clinical research.
-
Data Management and Analysis: They manage the collection, oversight, and statistical evaluation of research data, ensuring accuracy and integrity.
-
Regulatory Affairs: Clinical research organizations navigate the complex regulatory landscape, assisting clients in submitting necessary documentation to regulatory bodies like INVIMA, including the import permit application and ensuring compliance with local and international guidelines. Notably, the total review time in Colombia is only 90-120 days, which improves the pace of proceedings.
-
Monitoring and Quality Assurance: Ongoing supervision of research studies is crucial for upholding standards and adherence; contract research organizations frequently supply specialized personnel to manage these activities.
-
Medical Writing: They produce essential documentation, including study reports, regulatory submissions, and scientific publications, ensuring clarity and compliance with industry standards.
-
Reporting: Clinical research organizations produce various reports throughout the study process, including study status, inventory, and adverse event reports, which are crucial for compliance with INVIMA and ethics committees.
Through these comprehensive services, including the importation and nationalization of investigational products, contract research organizations not only enhance the efficiency of medical studies but also contribute to the overall advancement of healthcare and patient support, backed by Colombia's competitive advantages such as significant cost savings and R&D tax incentives. For more information or to discuss how we can assist you, please BOOK A MEETING.
The Importance of CROs in Clinical Trials
Mexico Contract Research Organizations (CROs) are vital to the research ecosystem for several reasons. First, they provide specialized expertise that is often not available in-house, enabling sponsors to access a broader range of knowledge and skills. bioaccess®, with its extensive experience in managing trials across Latin America, excels in offering comprehensive services that include:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
This expertise is particularly vital for:
- Early-feasibility (EFS)
- First-in-human (FIH)
- Pilot
- Pivotal
- Post-market research follow-up studies
Second, contract research organizations assist in speeding up the drug development process by optimizing operations, which can significantly shorten the duration required to introduce a new drug to the market. Third, they improve the quality and reliability of clinical studies by implementing rigorous monitoring and quality assurance practices.
Additionally, Mexico Contract Research Organizations can provide geographic reach, enabling sponsors to carry out studies in various locations globally, which is essential for attracting diverse patient populations. Conducting trials in Latin America not only provides access to a rich and varied patient demographic but also benefits from the region's regulatory expertise and collaborative environment. As emphasized by Dr. John B. Simpson, CEO of Avinger, Inc., bioaccess® has demonstrated to be a valuable ally in performing OCT-guided atherectomy studies in Cali, Colombia.
Overall, the collaboration with organizations such as bioaccess® enables more efficient and effective trials, ultimately resulting in improved patient outcomes and progress in medical science.

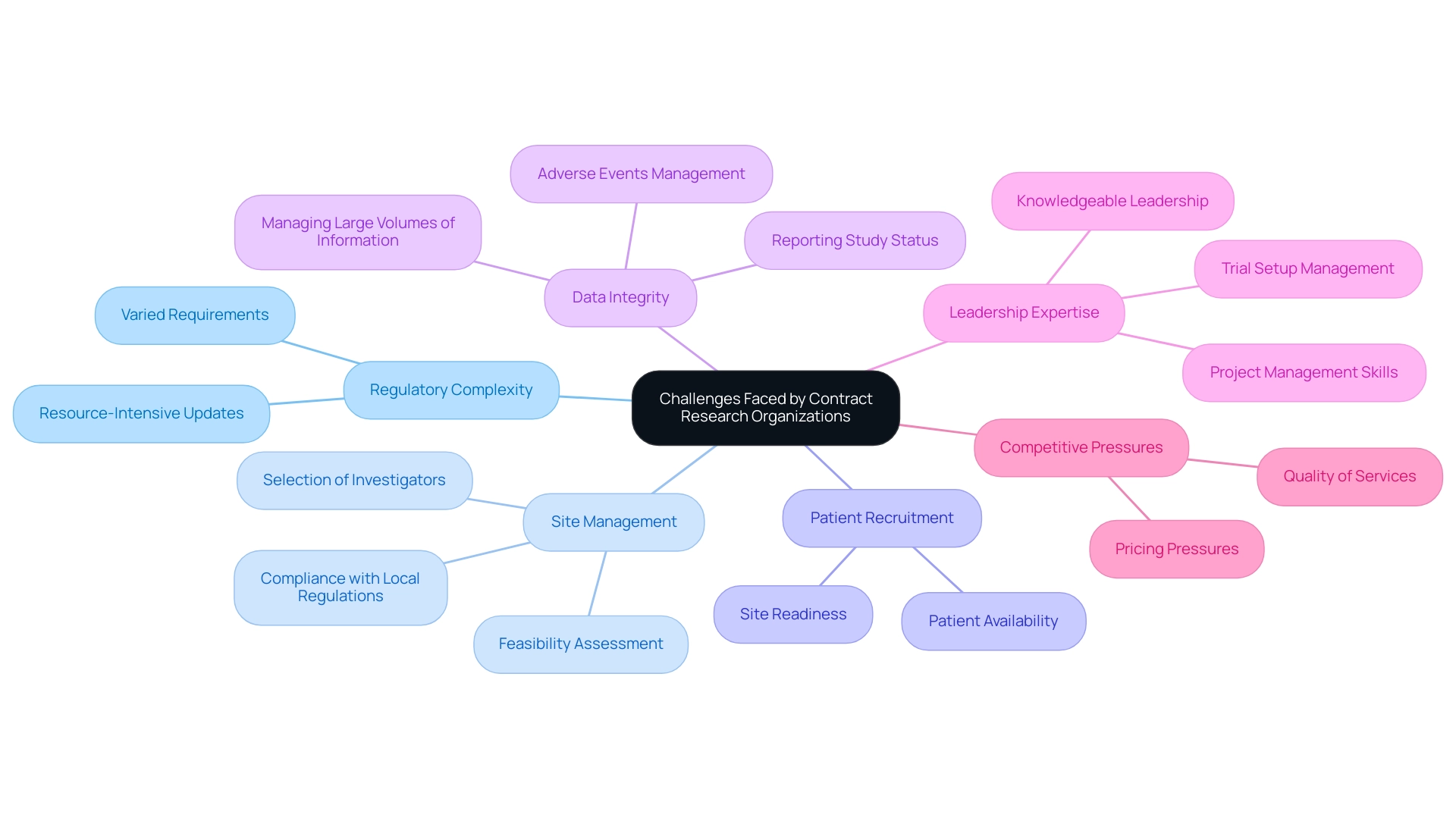
Challenges Faced by Contract Research Organizations
Despite their substantial contributions, clinical organizations encounter several challenges in the clinical landscape. One major challenge is the increasing complexity of regulatory requirements, which can vary significantly across different regions and countries, necessitating constant updates to protocols and compliance measures—a resource-intensive endeavor. Additionally, Mexico Contract Research Organizations must manage the feasibility and selection of research sites, principal investigators, and ensure compliance with local regulations, which adds layers of complexity.
Rapid patient recruitment remains a critical hurdle, often impeded by patient availability and site readiness. Furthermore, maintaining data integrity and managing large volumes of information from multiple sources presents logistical challenges, particularly in reporting study status and adverse events. The expertise of professionals like Katherine Ruiz, a Regulatory Affairs expert, underscores the importance of knowledgeable leadership in navigating these issues, particularly in trial setup and project management.
Insights from Julio G. Martinez-Clark further highlight the need for adaptability and innovation in this competitive landscape. Finally, competition among Mexico Contract Research Organizations can exert pricing pressures, potentially affecting their ability to deliver high-quality services. Tackling these challenges necessitates robust project management abilities and a thorough comprehension of the operational difficulties encountered by Mexico Contract Research Organizations in the changing environment of clinical research.
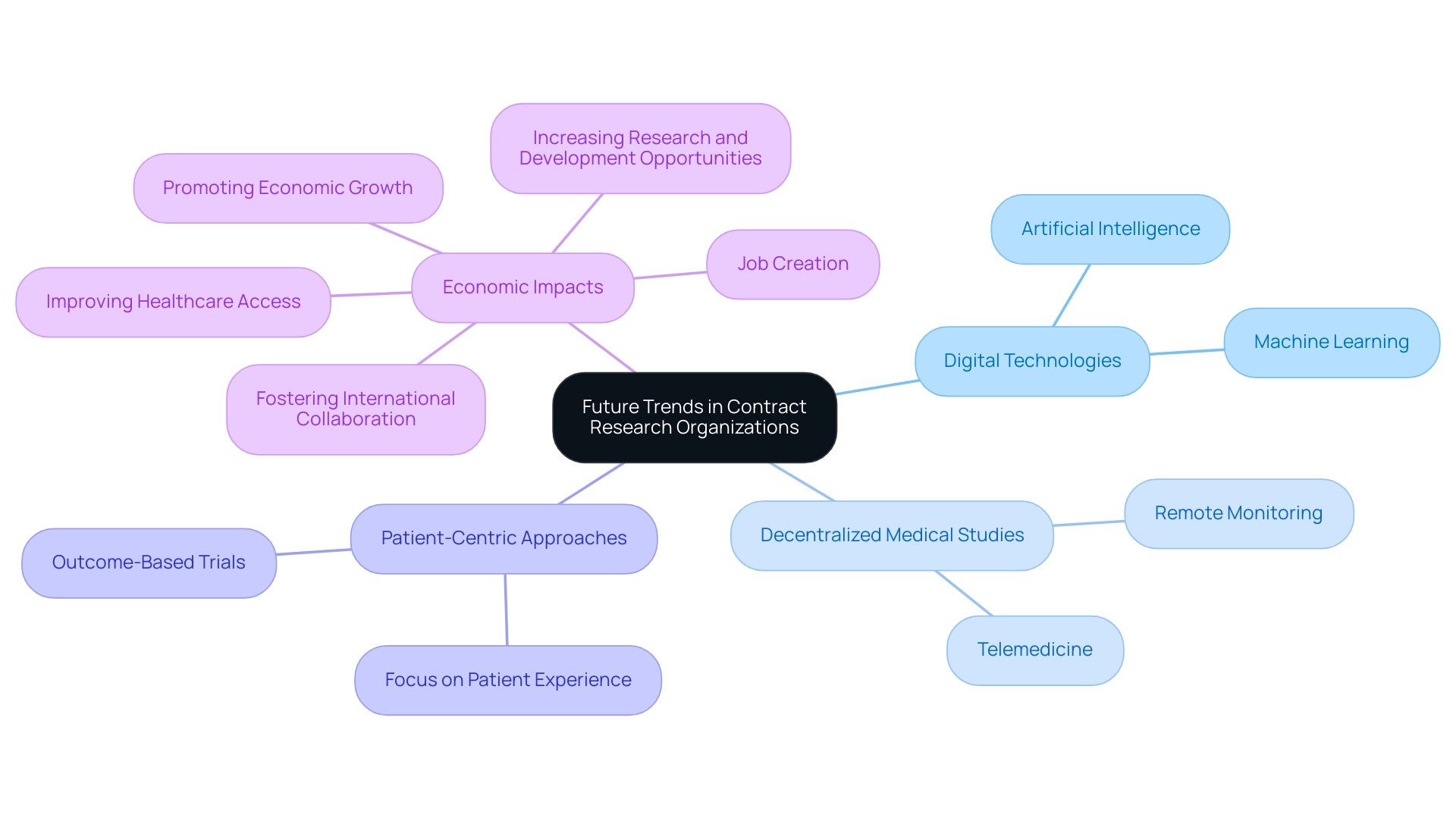
Future Trends in Contract Research Organizations
The landscape of Mexico Contract Research Organizations (CROs) is poised for significant transformation driven by technological advancements and changing industry demands. One prominent trend is the increasing reliance on digital technologies, such as artificial intelligence (AI) and machine learning, to improve data analysis and patient recruitment processes. These technologies can enhance the efficiency of experiments and provide deeper insights into patient responses.
Moreover, the implementation of decentralized medical studies, supported by telemedicine and remote monitoring, is gaining traction, allowing for greater flexibility and patient involvement. Furthermore, the Mexico Contract Research Organization, through their comprehensive clinical study management services—including feasibility studies, site selection, compliance reviews, study setup (including ethics committee approvals and import permits), project management, and thorough reporting—are making a significant impact on local economies. This includes:
- Job creation
- Promoting economic growth
- Improving healthcare access
- Increasing research and development opportunities
- Fostering international collaboration
There is also a growing emphasis on patient-centric approaches, where the Mexico Contract Research Organization is focusing on the patient experience and outcomes, ensuring that trials are designed with participants' needs in mind. As regulatory environments evolve, contract research organizations will need to adapt to new compliance standards and expectations, further emphasizing the importance of agility and innovation in their operations. Overall, these trends indicate a promising future for CROs, with the potential to significantly enhance the effectiveness and efficiency of clinical research, exemplified by professionals like Dr. Sergio Alvarado, who is dedicated to innovative medical solutions in Latin America.
Conclusion
The role of Contract Research Organizations (CROs) in the clinical research landscape is undeniably significant, particularly in the context of first-in-human trials within regions like Colombia. By offering a comprehensive suite of services—from study design and regulatory compliance to patient recruitment and data management—CROs streamline the clinical trial process, enabling sponsors to navigate the complexities of drug development with greater efficiency. The advantages of conducting trials in Colombia, including cost savings, rapid regulatory approvals, and a robust healthcare system, further highlight the strategic importance of CROs in optimizing trial outcomes.
As the industry evolves, the challenges faced by CROs, such as navigating complex regulatory environments and ensuring data integrity, necessitate continuous innovation and adaptability. The integration of digital technologies and patient-centric approaches is transforming how trials are conducted, allowing for enhanced engagement and more meaningful outcomes. These advancements not only improve the efficiency of clinical research but also contribute to the overall advancement of medical science and patient care.
In conclusion, the partnership between sponsors and CROs is crucial for accelerating drug development and improving patient outcomes. By leveraging local advantages and specialized expertise, CROs are positioned to play an increasingly pivotal role in the future of clinical trials. Embracing these changes will ensure that the clinical research ecosystem continues to thrive, ultimately leading to breakthroughs that enhance healthcare and benefit society as a whole.




