Introduction
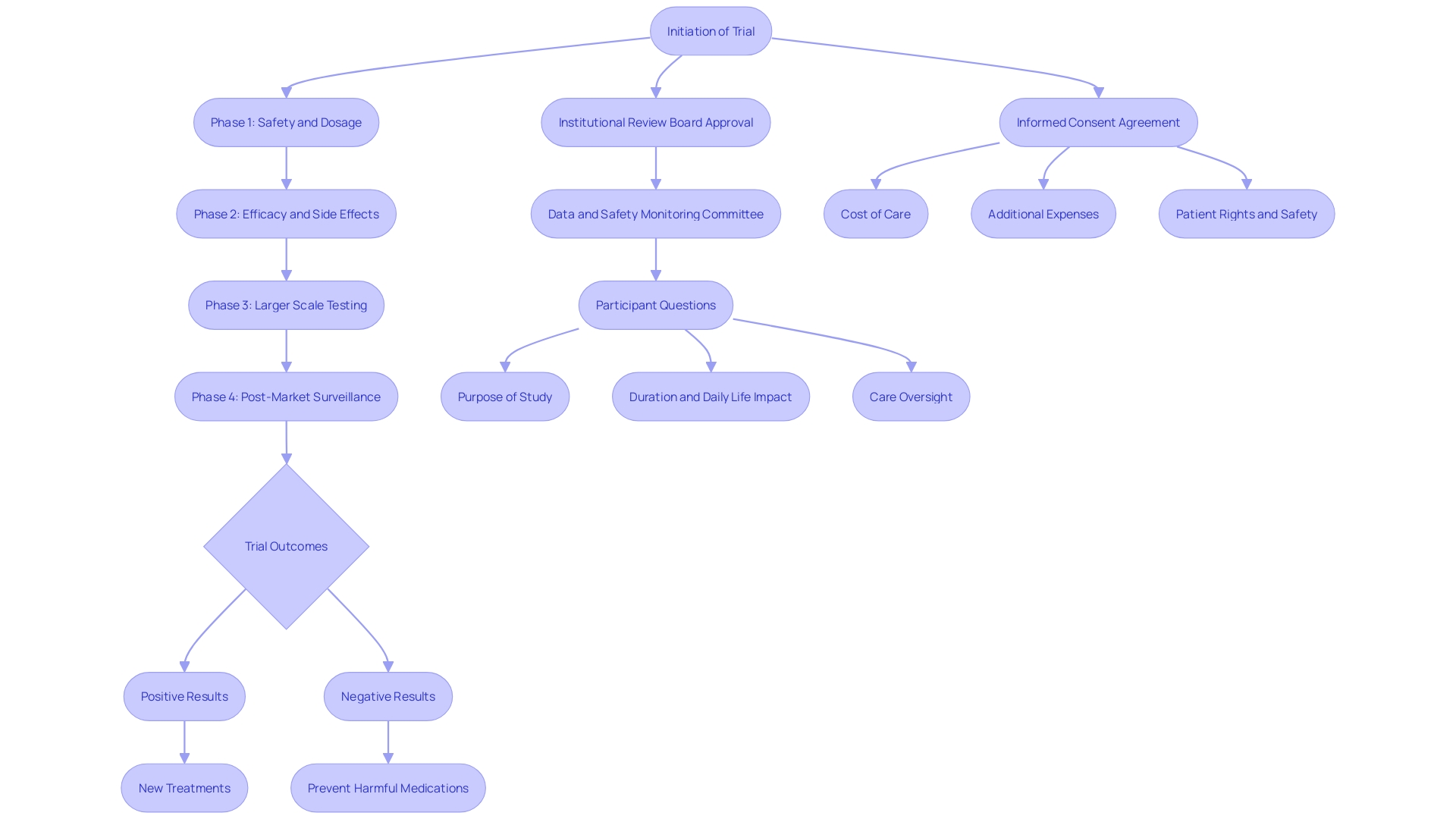
Pivotal clinical trials are integral to the development and approval of new drugs and treatments, providing essential evidence on their safety and efficacy. These meticulously designed studies, often guided by regulatory bodies such as the FDA's Center for Drug Evaluation and Research (CDER), compare new interventions against existing treatments or placebos. By doing so, they ensure a comprehensive evaluation of therapeutic benefits, which is critical for regulatory approval and public use.
The significance of these trials is underscored by the approval of innovative treatments, such as ISM6331 for Mesothelioma, highlighting the urgent need for novel therapeutic options. The results from pivotal trials not only address safety concerns but also contribute to advancements in healthcare by offering new treatment possibilities for patients.
Definition and Purpose of Pivotal Clinical Trials
Crucial clinical studies are essential investigations aimed at producing strong proof regarding the effectiveness and safety of new medications or therapies. These trials meticulously compare the therapeutic benefits of new interventions against existing treatments or placebos, ensuring a comprehensive evaluation. The FDA’s Center for Evaluation and Research (CDER) plays a critical role in guiding developers on the necessary study design elements and data required for thorough assessment. This process leverages a deep understanding of the science behind new products, their testing and manufacturing, and the diseases they aim to treat.
Every year, CDER authorizes a varied array of new medications and biological products, many of which symbolize groundbreaking treatment choices that have never been applied in medical practice. For instance, the recent approval to initiate clinical evaluation of ISM6331 for Mesothelioma—a type of cancer primarily caused by asbestos exposure—highlights the urgent need for novel treatment options. Conventional treatments frequently do not deliver lasting advantages, highlighting the significance of these crucial evaluations in the medication approval process.
The outcomes from essential studies are vital for regulatory bodies to assess the appropriateness of a medication for public use. These evaluations address safety concerns and ensure compliance with rigorous standards, ultimately contributing to advancements in healthcare and providing new therapeutic options for patients.
Characteristics of Pivotal Clinical Trials
Crucial studies are characterized by their large sample sizes, accurate methodologies, and distinctly outlined endpoints. Frequently employing randomized controlled designs, these studies may also be multicenter, ensuring a wide range of participant demographics. Such studies must follow stringent protocols to uphold data integrity and reliability. Recent trends indicate an uptick in equity and inclusivity, with over 97% of clinical studies reporting on race and ethnicity, enhancing the robustness of study outcomes. Adaptive studies have introduced flexibility by allowing pre-specified changes to be made during the research, ensuring the study's validity and integrity are maintained. Furthermore, the participation of various demographics is essential, as demonstrated by studies such as the OPT-PACE investigation, where participant traits were evenly distributed among groups. These practices, combined with a rigorous adherence to statistical guidelines and power calculations, underscore the essential role of key studies in advancing medical research.
Importance of Pivotal Clinical Trials in Drug Approval
The importance of crucial clinical studies resides in their function as a foundation for regulatory submissions. These studies are essential for demonstrating the efficacy and safety of new treatments, which is crucial for obtaining approvals from agencies such as the FDA and EMA. For instance, the FDA's rigorous oversight of adverse events and therapeutic inequivalence highlights the significance of these studies in safeguarding public health. By examining these reports thoroughly, the FDA can mandate changes in medication usage or production practices to enhance safety.
Furthermore, pivotal trials offer strong proof that backs the bioequivalence of generic medications to their brand-name equivalents, an essential element in regulatory decision-making. According to the FDA, bioequivalence requires that there be no significant differences between test and reference medications in key pharmacokinetic parameters, such as time to maximum concentration and the area under the concentration-time curve. This rigorous evaluation process ensures that both new and existing medications meet high standards of quality, safety, and efficacy before they reach the market.
Furthermore, the FDA's Real-World Evidence (RWE) Program offers guidance to sponsors on using non-interventional studies to demonstrate substantial evidence of effectiveness and safety. This method demonstrates the increasing interest in using real-world information to enhance conventional study data, thus offering a more thorough evaluation of a drug's effect.
Overall, essential medical studies are invaluable in the regulatory environment, as they provide the necessary evidence to support the approval and ongoing safety monitoring of innovative and generic treatments alike.
Challenges in Conducting Pivotal Clinical Trials
'Carrying out essential medical studies poses a variety of obstacles that can greatly influence their implementation and results.'. Recruitment remains one of the most time-consuming and costly stages, with difficulties in identifying sites in underserved areas and enrolling underrepresented patients. Socioeconomic factors, cultural differences, and geographic disparities further complicate this process, potentially leading to selection bias and limited participant diversity. According to a 2022 report, there were 27,133 studies in high-income nations compared to 24,791 in low- and middle-income nations, highlighting global inequities in clinical research.
The logistical complexities of managing data collection across multiple sites also pose significant hurdles. Traditional methods of data collection often rely on labor-intensive procedures, failing to integrate existing electronic health records and real-world data sources. This not only limits the scope of data captured but also affects the validity and generalizability of the findings. 'Improving inclusivity through data-driven study designs and utilizing digital technologies like social media for broader patient engagement can help mitigate these issues.'.
Furthermore, unforeseen variables such as changes in regulatory requirements and emerging safety concerns can disrupt the progress of the study. The recent WHO recommendations emphasize the need for robust infrastructure and streamlined bureaucratic processes to address these challenges. Additionally, external factors like the COVID-19 pandemic and geopolitical events—such as the war in Ukraine—have led to the withdrawal or relocation of participants, affecting the reliability of endpoint assessments and overall study power.
Interim evaluations to oversee participant representativeness and flexible study designs that include these factors are crucial for preserving the integrity of critical studies. By concentrating on these areas, research teams can ensure their studies are thorough, efficient, and representative of real-world situations, ultimately resulting in more effective and fair patient care.
Regulatory Considerations and Agency Roles
Regulatory bodies are crucial in supervising research studies to guarantee compliance with ethical and scientific standards. The FDA, for example, provides critical guidance on trial design, monitors compliance, and rigorously reviews data to make well-informed drug approval decisions. 'Their role not only protects participant welfare but also improves transparency in the research process.'. As stated by Anthony Keyes and a group from Johns Hopkins University, efficient and well-structured research is crucial for promoting the advancement of safe and effective medical products. This emphasizes the significance of regulatory supervision in upholding high standards in research studies.
Impact on Clinical Practice and Public Health
The results of crucial clinical studies hold substantial sway over medical practice and public health. 'Positive test results can pave the way for novel treatments that enhance patient outcomes and broaden therapeutic options.'. For instance, a recent study on Zilebesiran, an experimental medication aimed at lowering blood pressure by decreasing angiotensinogen production, exemplifies how successful experiments can lead to groundbreaking therapies. On the other hand, negative test results are just as important as they stop ineffective or unsafe medications from entering the market, thus protecting public health. This rigorous vetting process is essential, especially considering that up to 80% of clinical trials fail to finish on time, highlighting the importance of thorough and efficient trial execution to expedite the availability of effective treatments.
Conclusion
Pivotal clinical trials serve as a cornerstone in the development and approval of new drugs and treatments, providing essential evidence of their safety and efficacy. These meticulously designed studies, characterized by rigorous methodologies and extensive sample sizes, facilitate a comprehensive evaluation of new interventions against existing treatments or placebos. The role of regulatory bodies, particularly the FDA, is crucial in guiding these trials, ensuring adherence to stringent standards that ultimately protect public health.
The importance of these trials is further underscored by their impact on regulatory submissions, which are vital for obtaining necessary approvals. They provide robust evidence not only for new treatments but also for the bioequivalence of generic drugs, ensuring that all medications meet high standards of quality and safety. Additionally, the integration of real-world evidence represents a progressive approach to evaluating drug effectiveness, complementing traditional clinical trial data.
Despite their significance, conducting pivotal trials presents numerous challenges, including recruitment difficulties and logistical complexities. Addressing these issues through innovative strategies and inclusive trial designs is essential for maintaining the integrity and validity of the findings. The ongoing support and oversight from regulatory agencies play a critical role in navigating these challenges, fostering transparency and ethical conduct in clinical research.
In conclusion, pivotal clinical trials are indispensable for advancing medical research and enhancing patient care. Their outcomes not only influence clinical practice but also ensure that only safe and effective treatments reach the market, reinforcing the commitment to public health and the continuous improvement of healthcare practices.




