Introduction
Pivotal studies play a crucial role in medical research, providing essential evidence for the approval of new treatments and drugs. These studies, often in the form of randomized controlled trials, are meticulously designed to generate reliable and statistically significant results. The impact of pivotal studies is exemplified by real-world cases, such as Barbara's story, where participation in a trial led to the detection and ongoing management of her cardiac condition.
Recent breakthrough studies have also highlighted the effectiveness of new interventions, such as a drug targeting angiotensinogen production for high blood pressure and the sequenced application of chemotherapy in cervical cancer patients. The foundation of these research endeavors lies in formulating precise research questions and conducting proper statistical analysis. With a focus on accuracy and collaboration, pivotal studies continue to advance medical knowledge and improve patient care.
Definition and Importance of Pivotal Studies in Medical Research
In the field of medical research, crucial investigations are vital in evaluating the safety and effectiveness of new medical interventions. These research projects are carefully planned to produce the significant evidence required for the regulatory endorsement of new therapies or medications, thus aiding their implementation in medical practice. Usually adopting the structure of randomized controlled trials (RCTs), pivotal investigations involve substantial participation to guarantee that outcomes are statistically significant and can be trusted.
The importance of crucial investigations is highlighted by real-life scenarios where involvement in exploration has had life-changing consequences for individuals. As an example, Barbara's narrative demonstrates the unforeseen discoveries about health that can result from such investigations. Initially unaware of her cardiac condition, Barbara's involvement in a trial she discovered through The New Normal, an online platform connecting people to health research opportunities, led to the crucial detection and ongoing management of her heart health.
In addition, breakthrough investigations have brought attention to recent progress, such as the examination of Zilebesiran, a medication designed to address high blood pressure by focusing on angiotensinogen production. In the same way, research on the sequenced application of chemotherapy before radiotherapy in cervical cancer patients demonstrated a noteworthy enhancement in survival rates, highlighting the importance of well-structured clinical trials.
In developing these investigative endeavors, the foundation lies in formulating a precise and addressable inquiry. This initial step is not about immediate solutions but about defining the problem with clarity, focusing on specific populations, interventions, comparisons, and outcomes. For example, establishing the connection between a factor, like smoking, and a result such as lung cancer, is crucial in observational research.
Statistical analysis remains the foundation of reliable medical investigations. Proper randomization and sample size are crucial to eliminate bias and infer results that may apply to the broader population. Authors and researchers are encouraged to embrace statistics not as a mere formality but as an essential tool in scientific exploration, often requiring collaboration with professional statisticians for the most accurate and meaningful outcomes.
Characteristics of Pivotal Studies
Crucial investigations are necessary in clinical research, formulated to evaluate the direct influence of a treatment or intervention on predetermined primary endpoints, which are crucial for assessing the efficacy or effectiveness of the intervention. These research projects are carried out with a high degree of accuracy and strictly adhere to established protocols and guidelines, guaranteeing that the gathered data is reliable and trustworthy.
Notably, crucial investigations necessitate a meticulously chosen research population that reflects the target patient group, enhancing the significance and applicability of the findings. For example, a recent trial studying the use of chemotherapy before radiotherapy in cervical cancer patients showed significant improvements in patient outcomes. The survival rate without cancer progression increased notably from 64% with standard radiotherapy to 73% when chemotherapy preceded radiotherapy.
Furthermore, the involvement in medical research frequently produces unforeseen advantages for people, as emphasized by Barbara's encounter. After joining 'The New Normal,' an online platform facilitating the recruitment of study participants, Barbara discovered a previously undetected heart condition requiring immediate attention, underscoring the dual benefits of progress in investigation and personal health insights.
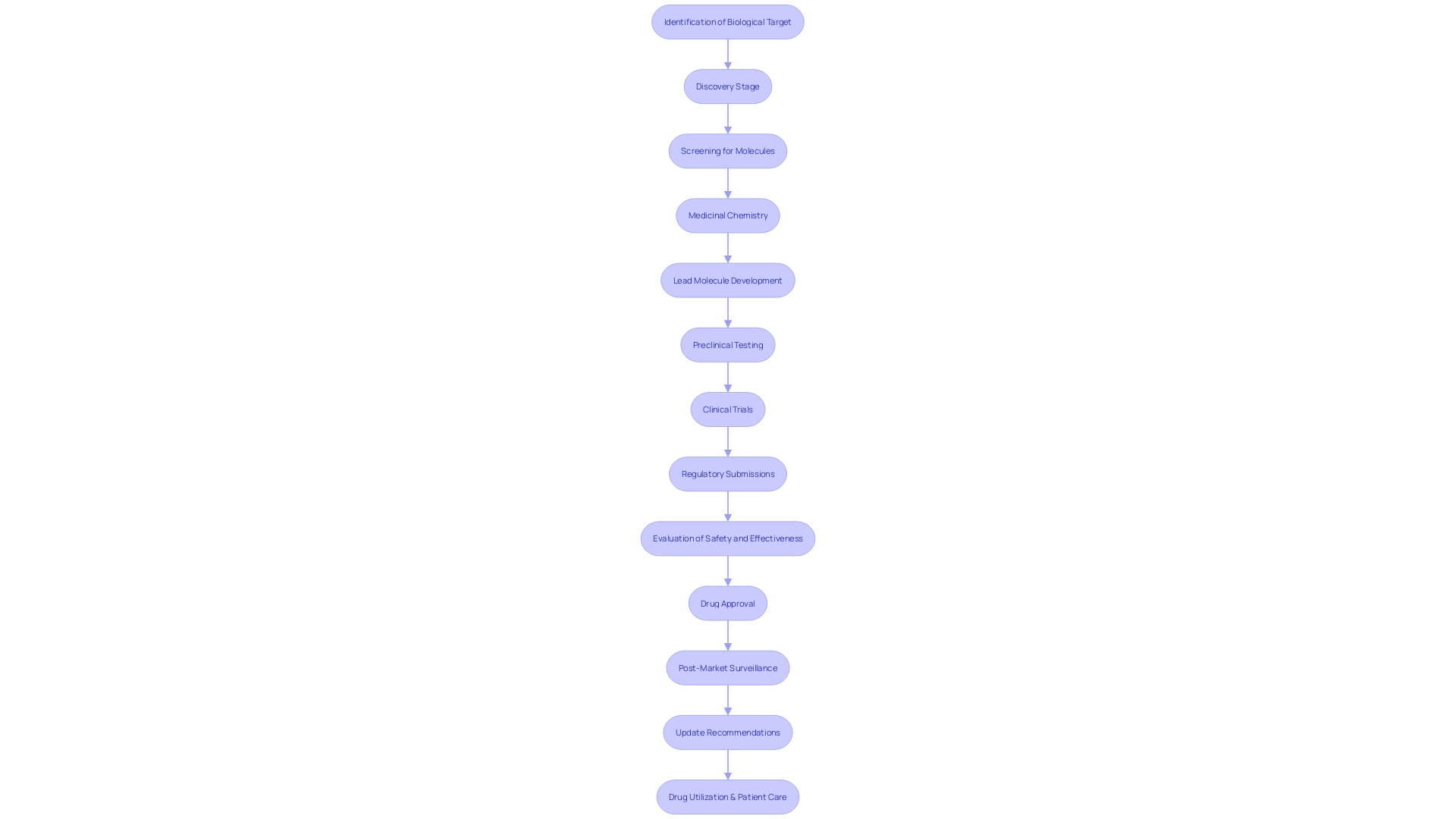
The progression of medical investigation from preclinical stages, through different phases of trials, to eventual implementation, is a testament to its transformative power in advancing medical knowledge and patient care. This process starts with fundamental investigation of diseases using cellular or animal models and extends to the implementation of confirmed interventions in clinical settings, thus impacting public health on a grand scale.
Clinical investigation aims to not only provide a deeper understanding of diseases but also to establish a solid foundation of evidence for regulatory approval of new treatments. This evidence-based approach is pivotal for decision-makers to acknowledge health issues and support interventions, as emphasized by the focus on description, prediction, causality, and measurement in formulating inquiries.
As the medical community continues to advocate for transparency and data sharing in clinical trials, it is recognized that more efforts are needed to ensure the availability of raw data and comprehensive trial protocols. This will further enhance the reproducibility and credibility of scientific inquiry, ultimately contributing to the betterment of patient care and public health outcomes.
Examples of Successful Pivotal Studies in Medical Research
Pivotal studies have been cornerstones in the evolution of medical practices, providing critical insights that lead to enhanced patient care. The Framingham Heart Study stands as a testament to the power of such investigations, revolutionizing our comprehension of cardiovascular disease risk factors and shaping prevention and treatment strategies. Similarly, the clinical trial for the human papillomavirus (HPV) vaccine exemplifies the potential of systematic investigation in combating HPV-related diseases through effective vaccination programs.
These pioneering research projects not only produce invaluable data but also highlight the unexpected benefits that participants may experience. As unveiled by the story of Barbara, who joined an investigation via The New Normal - an online platform created to link individuals with opportunities for examination - participants can uncover crucial health information that might otherwise go unnoticed. In her case, an investigation revealed a heart condition requiring immediate treatment, likely preventing a life-threatening event.
The fortuitous nature of scientific investigation is further emphasized by the experience of Dr. Alexander Marneros, who undertook a ten-year investigation of aplasia cutis after an accidental observation. Such encounters highlight the unpredictable yet transformative impact of clinical investigation on individual lives and medical knowledge.
Recent advancements continue to demonstrate the dynamic landscape of medical research. For example, a new medication, Zilebesiran, shows potential in proactively managing high blood pressure, questioning the current approach. In another stride, a clinical trial revealed that administering chemotherapy before radiotherapy significantly improves survival outcomes in cervical cancer patients, potentially setting a new standard of care.
Moreover, a meta-analysis has linked Parkinson’s disease with an increased risk of autoimmune disorders, suggesting immune system aberrations may play a role in Parkinson’s. These findings not only contribute to a better understanding of the disease but also may pave the way for new treatment avenues.
Including these advancements and participant observations in the structure of pivotal investigations not only enhances our understanding of different medical conditions but also emphasizes the direct influence such exploration has on enhancing patient outcomes. It is a powerful reminder that behind every statistic and research outcome, there are individual stories and lives being influenced by the power of clinical research.
The Role of Pivotal Studies in Drug Development and Approval
Crucial research is vital to the drug development process, offering the necessary evidence required for regulatory submissions to agencies like the FDA. These research projects are carefully developed to evaluate the safety and effectiveness of new medications, establishing a strong basis of information that guides decisions regarding drug approval and usage recommendations. Through assessing ideal dosages, potential side effects, and long-term outcomes, pivotal studies impact the utilization of novel treatments, guaranteeing that they meet the rigorous standards for patient care and regulatory compliance.
Recent analysis by the UK's National Institute of Health and Care Excellence (NICE) highlights the critical need for high-quality evidence in drug approval processes. Only a fraction of the 400 appraisals examined was supported by what could be deemed 'good' evidence, emphasizing the importance of rigorous research. This strengthens the FDA's dedication to facilitating well-structured trials that produce dependable data to support the safety and effectiveness of medical products.
Ensuring that trial results are representative of the wider population and applicable to routine pathways is essential. It has been noted that frequently the comparators employed in trials are not appropriate for making informed decisions about rollouts. Moreover, more than two-thirds of technology appraisals have relied on indirect comparisons to gauge clinical effectiveness. Quality of life data, crucial for assessing a drug's impact on patients, frequently lacks the necessary quality and clarity, calling for improved methodology and transparency in reporting.
The FDA's Center for Drug Evaluation and Research (CDER) annually approves a variety of new drugs and biological products, many of which are innovative therapies. CDER's role is to provide clear guidance to developers on study design and data requirements for drug applications, ensuring a thorough assessment of new therapies. The pursuit of innovation in drug development is a testament to the strides being made in healthcare, offering new treatment possibilities and enhancing the quality of life for patients nationwide.
Conclusion
In conclusion, pivotal studies are essential for assessing the safety and effectiveness of new treatments in medical research. These studies generate reliable results through rigorous design and statistical analysis, shaping clinical practice and regulatory decisions.
Real-world cases, like Barbara's story, demonstrate the life-altering implications of participating in pivotal studies. Through her involvement, Barbara discovered and managed her previously undetected cardiac condition, highlighting the importance of research progress and personal health insights.
Recent breakthrough studies have shown the effectiveness of new interventions, such as a drug targeting angiotensinogen production for high blood pressure and the sequenced application of chemotherapy in cervical cancer patients. These advancements underscore the value of well-structured clinical trials in driving medical knowledge forward.
Pivotal studies are built on formulating precise research questions and conducting proper statistical analysis. By defining the problem with clarity and focusing on specific populations, interventions, comparisons, and outcomes, researchers can generate meaningful and applicable results.
These studies have had a transformative impact on medical practices and patient care. The Framingham Heart Study revolutionized our understanding of cardiovascular disease risk factors, while the clinical trial for the HPV vaccine has been instrumental in combating HPV-related diseases.
Efforts are being made to ensure transparency and data sharing in clinical trials, enhancing the reproducibility and credibility of research. This will contribute to better patient care and public health outcomes.
Pivotal studies are central to the drug development process, providing essential evidence for regulatory submissions. They influence the clinical application of new treatments by evaluating safety, efficacy, dosages, and long-term outcomes.
In conclusion, pivotal studies play a critical role in advancing medical knowledge, improving patient outcomes, and shaping the future of healthcare. By maintaining a focus on accuracy and collaboration, these studies will continue to drive innovation and enhance the quality of life for patients worldwide.




