Introduction
In the realm of medical device development, ensuring safety and efficacy is paramount, and accelerated aging tests serve as a critical component in this process. These assessments replicate the long-term effects of use within a condensed timeframe, allowing manufacturers to predict how devices will perform throughout their intended shelf life.
By subjecting devices to controlled conditions that simulate aging, stakeholders can identify potential failures and degradation pathways early in the product lifecycle. This proactive approach not only enhances patient safety but also aligns with stringent regulatory standards, reinforcing the importance of robust testing methodologies in a rapidly evolving healthcare landscape.
As the demand for reliable medical devices continues to grow, understanding the intricacies of accelerated aging tests becomes essential for manufacturers aiming to navigate the complexities of compliance and market expectations.
Understanding Accelerated Aging Tests: Definition and Importance
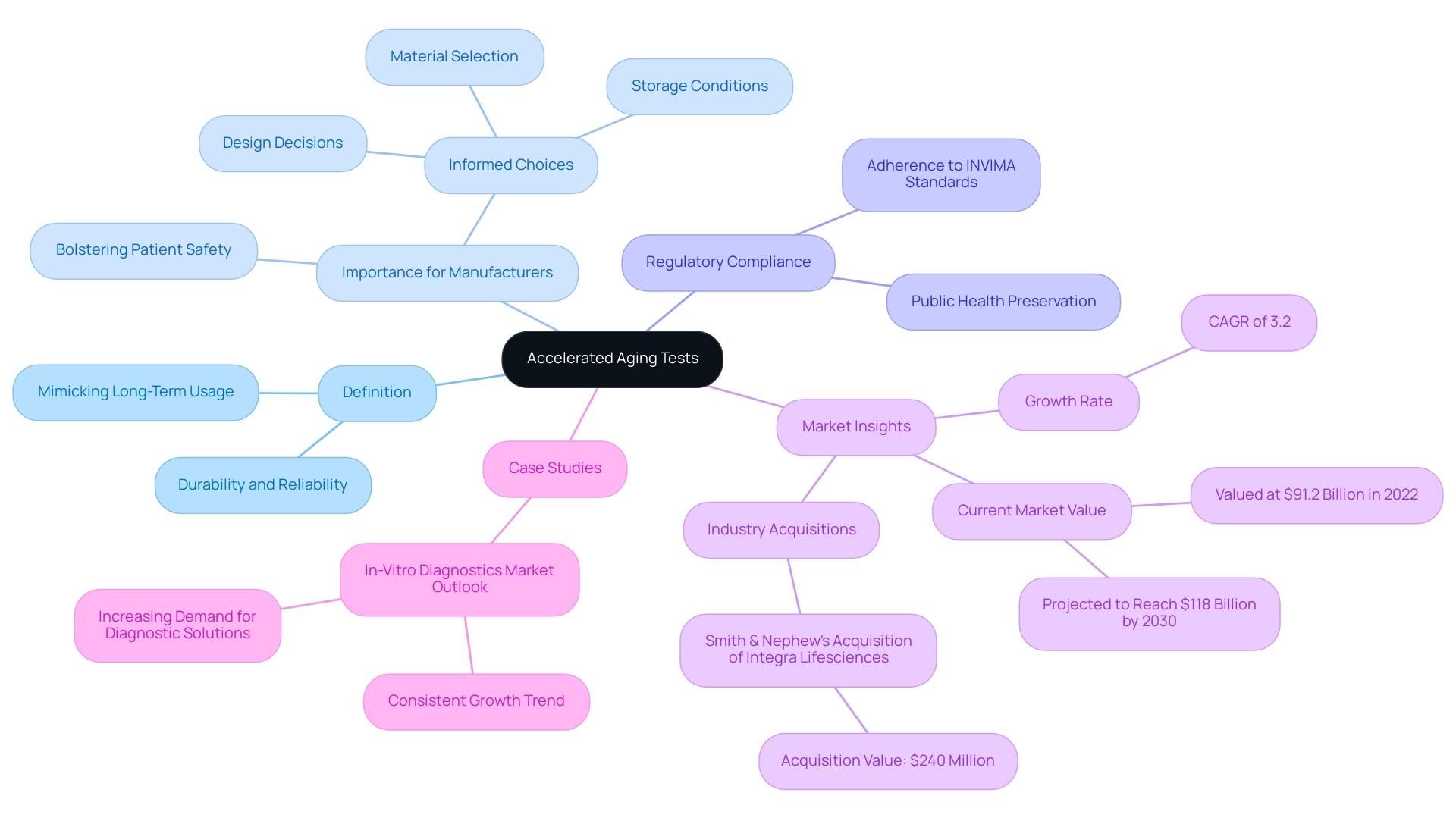
Accelerated lifespan evaluations serve a crucial function in the lifecycle management of medical equipment, as they are specifically crafted to mimic the long-term impacts of usage within a shortened period. These assessments rigorously evaluate the durability and reliability of equipment by subjecting them to heightened temperatures and humidity, thus mimicking the aging process that would occur over years. The primary objective of these tests is to accurately predict the performance and safety of an apparatus throughout its intended shelf life.
By proactively identifying potential failures and degradation pathways during the product development phase, manufacturers are empowered to make informed choices about design, materials, and storage conditions. This strategic approach not only bolsters patient safety but also ensures adherence to regulatory standards set forth by authorities such as INVIMA, Colombia's National Food and Drug Surveillance Institute, which is recognized as a Level 4 health authority by PAHO/WHO. This adherence ultimately preserves public health.
Recent insights into the in-vitro diagnostics market, valued at $91.2 billion in 2022 and projected to grow at a CAGR of 3.2% to reach $118 billion by 2030, underscore the rising demand for dependable healthcare instruments. The consistent growth in this sector further emphasizes the necessity of robust testing methodologies, including the accelerated aging test for medical devices, to guarantee that products meet safety and performance expectations. Furthermore, Smith & Nephew’s purchase of Integra Lifesciences’ extremity orthopedics division for $240 million emphasizes the industry's emphasis on improving the dependability of healthcare products.
Additionally, the case study titled 'In-Vitro Diagnostics Market Outlook' illustrates the ongoing trends in the market, reinforcing the critical need for effective testing protocols to ensure safety and efficacy of the equipment. Leveraging the expertise of bioaccess®, which has over 20 years of experience in managing Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), can significantly enhance the success of clinical trials in this rapidly evolving field. Bioaccess® has consistently shown success in these areas, further reinforcing its position as a leader in clinical study services for healthcare.
Methodologies and Standards: The Role of ASTM F1980 in Accelerated Aging Tests
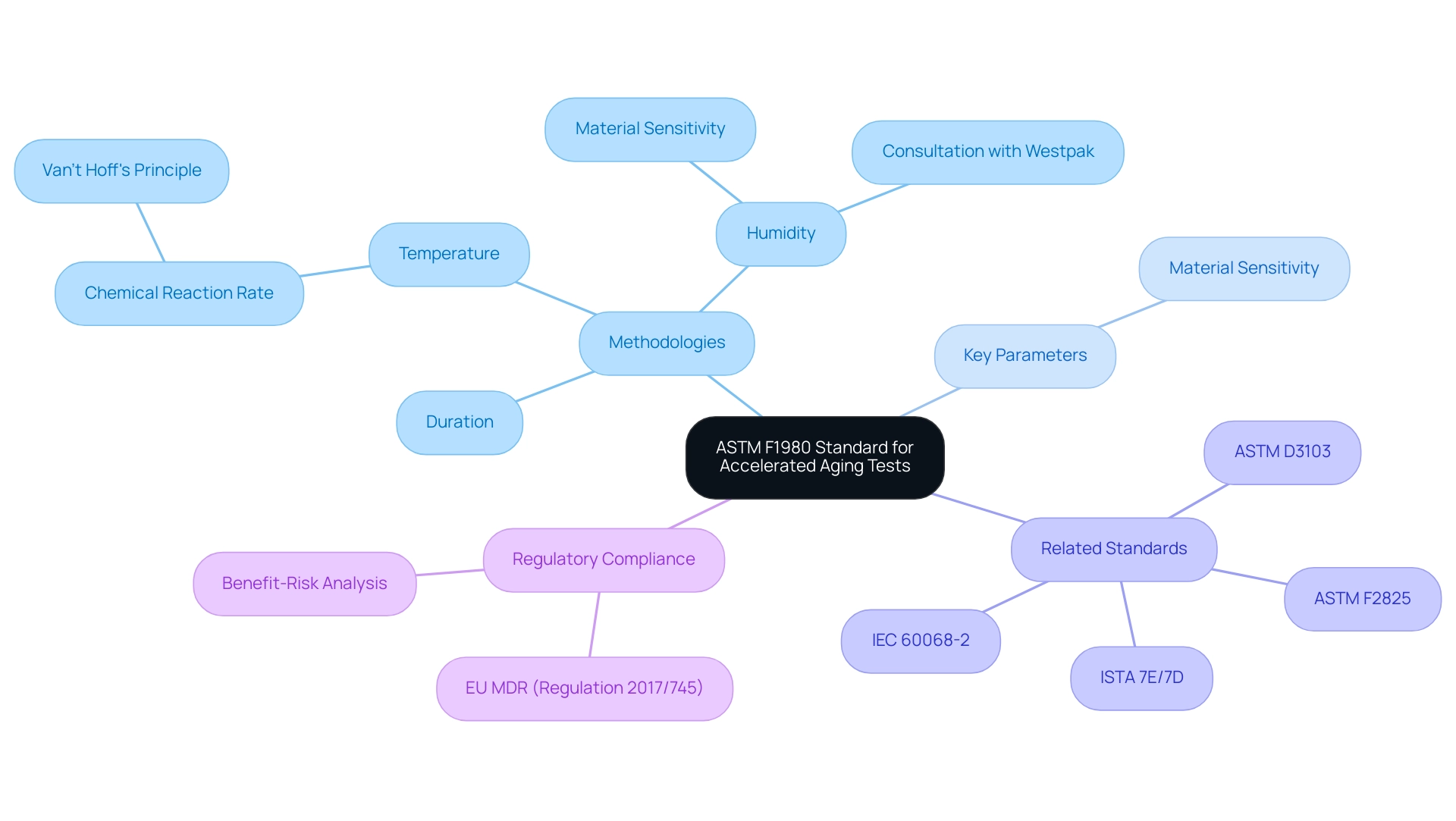
ASTM F1980 is an essential standard that outlines the methodologies for performing accelerated aging tests for medical devices. It stipulates the essential parameters for evaluation, including temperature, humidity, and duration, ensuring that these assessments are both consistent and scientifically robust. Other relevant test methods, such as ASTM F2825, ASTM D3103, IEC 60068-2, and ISTA 7E/7D, also play significant roles in establishing comprehensive testing protocols.
As articulated by J.H. Van’t Hoff, for every ten degree Celsius rise in temperature, the rate of chemical reaction will double, highlighting the critical role of temperature in understanding accelerated deterioration. The standard also emphasizes the importance of defining the materials used in healthcare instruments, as various materials display different reactions under the accelerated aging test for medical device conditions, with moisture sensitivity being a key factor that can significantly influence testing results.
For instance, the case study titled 'Recommended Humidity Levels for Accelerated Aging' illustrates how humidity levels depend on the materials used, emphasizing the need for controlled humidity to ensure accurate testing results. Westpac advises that the appropriate relative humidity levels should be determined in consultation with their sales team to ensure accuracy in testing. Moreover, adherence to the EU MDR (Regulation 2017/745) is crucial, as it mentions the term 'benefit-risk' concerning the evaluation and analysis of health products, connecting ASTM F1980 compliance to wider regulatory requirements.
Ultimately, adherence to ASTM F1980 fosters confidence in the longevity and safety of healthcare products, a paramount consideration in the sector.
Common Challenges in Conducting Accelerated Aging Tests
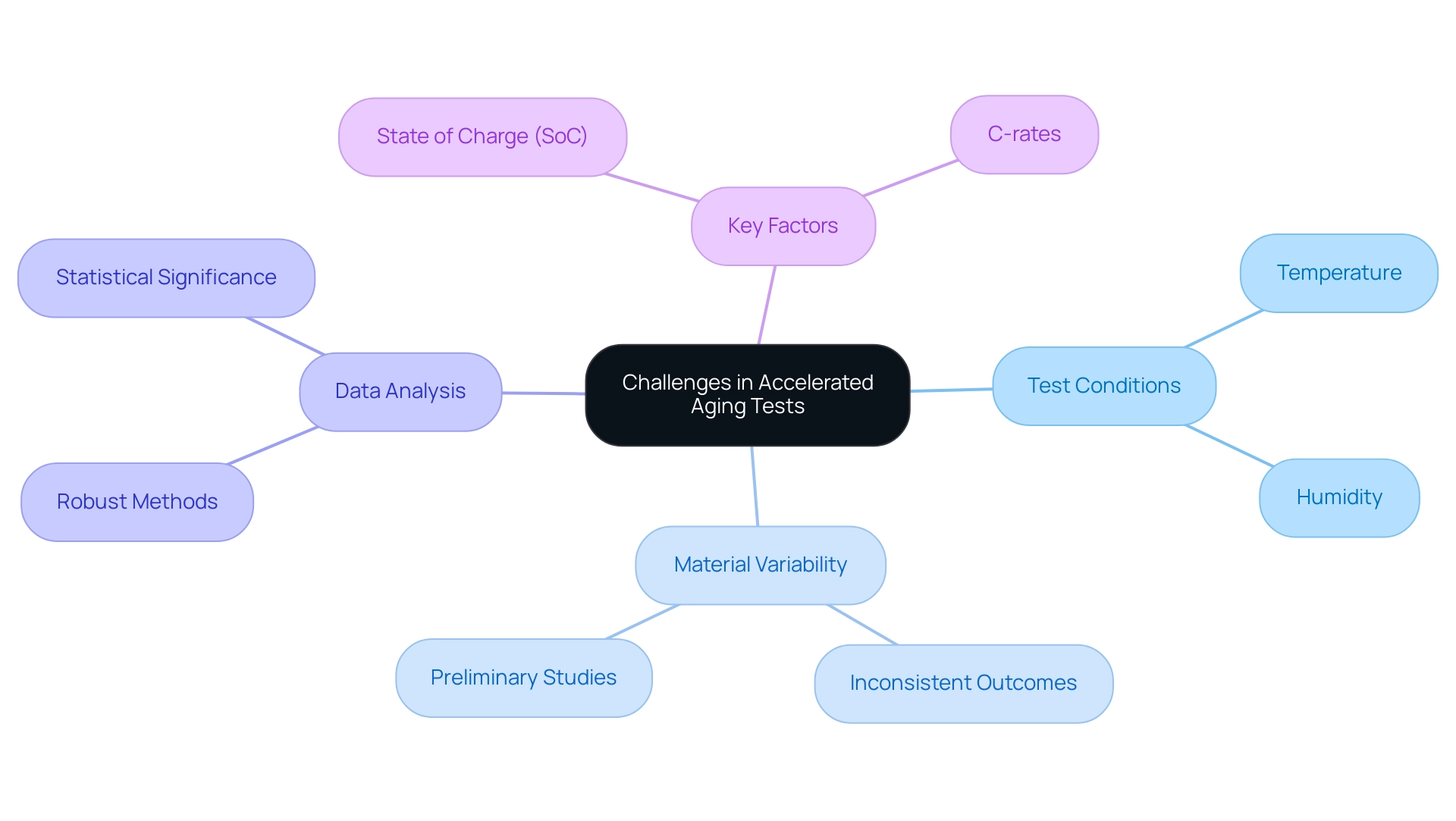
Carrying out an accelerated aging test for medical devices entails managing numerous challenges, especially in choosing the suitable test conditions and analyzing the resulting data. A primary concern is establishing temperature and humidity levels that effectively mimic long-term storage scenarios without introducing undue stress on the device. Variability in material properties can result in inconsistent outcomes, underscoring the importance of thorough preliminary studies to understand these characteristics.
Moreover, robust data analysis methods are essential to validate the statistical significance of the results. As highlighted by Trask et al., the use of innovative approaches, such as the coin cell setup, can identify improvement potentials for different areas of material properties. Recent research emphasizes that the accelerated aging test for medical devices must carefully consider factors such as state of charge (SoC), temperature, and C-rates to yield meaningful insights.
The typical time frame for application development is around 36 months, which underscores the urgency of addressing these challenges. Furthermore, the study titled 'Ageing Trends and Capacity Fade in Lithium-Ion Cells' reveals that cells stored at higher SoCs (above 60%) exhibit significant increases in deterioration, presenting a challenge to traditional t^0.5 dependency models. By proactively tackling these challenges and applying strategies for speeding up the characterization of time-related effects, manufacturers can enhance the reliability of their accelerated aging test for medical device assessments, ultimately aiding in the safety and effectiveness of their healthcare products.
Interpreting Results: What Accelerated Aging Tests Reveal
Interpreting results from the accelerated aging test for medical devices is crucial for predicting the long-term performance of medical equipment. Key indicators of performance include changes in physical properties such as tensile strength, flexibility, and appearance, which can suggest potential failures. For instance, significant shifts in these properties may indicate a machine's diminished reliability over time.
In this context, it is essential to leverage the expertise of specialized services, such as those provided by bioaccess®, which boasts over 20 years of experience in managing clinical studies, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
Statistical analysis is vital in establishing the significance of observed changes, ensuring that conclusions drawn from the data are robust and valid. As Catarina I. Kiefe pointed out in 'A Comparison of 5 Measures of Accelerated Biological Aging and Their Association With Incident Cardiovascular Disease: The CARDIA Study', rigorous statistical methods are paramount in medical research.
The mediation analysis indicates that socioeconomic status accounts for 39.78% of the total effect on health outcomes, underscoring the relevance of statistical analysis in understanding performance across various contexts. Moreover, the relationship of CAA with other seniority measures such as EEAA, GrimAA, PhenoAA, and FRS underscores the interconnectedness of these metrics in evaluating equipment reliability. Manufacturers must contextualize their results, considering the intended application of the product and its exposure to various environmental factors.
By accurately interpreting these results, manufacturers can make informed decisions regarding design modifications, material selections, and shelf-life labeling. This method not only improves the safety and effectiveness of their products but also corresponds with current trends in data analysis, which highlight the significance of statistical relevance in the accelerated aging test for medical device results. Furthermore, comprehending the regulatory framework established by INVIMA, Colombia’s National Food and Drug Surveillance Institute, is essential as it supervises classification as a Level 4 health authority by PAHO/WHO.
This understanding can further enhance the conversation on analyzing results from the accelerated aging test for medical devices, ultimately resulting in better patient outcomes and improved risk management in healthcare product development. Bioaccess® employs a customized approach to navigate the complexities of clinical studies, ensuring that each trial is tailored to meet specific regulatory requirements and industry standards.
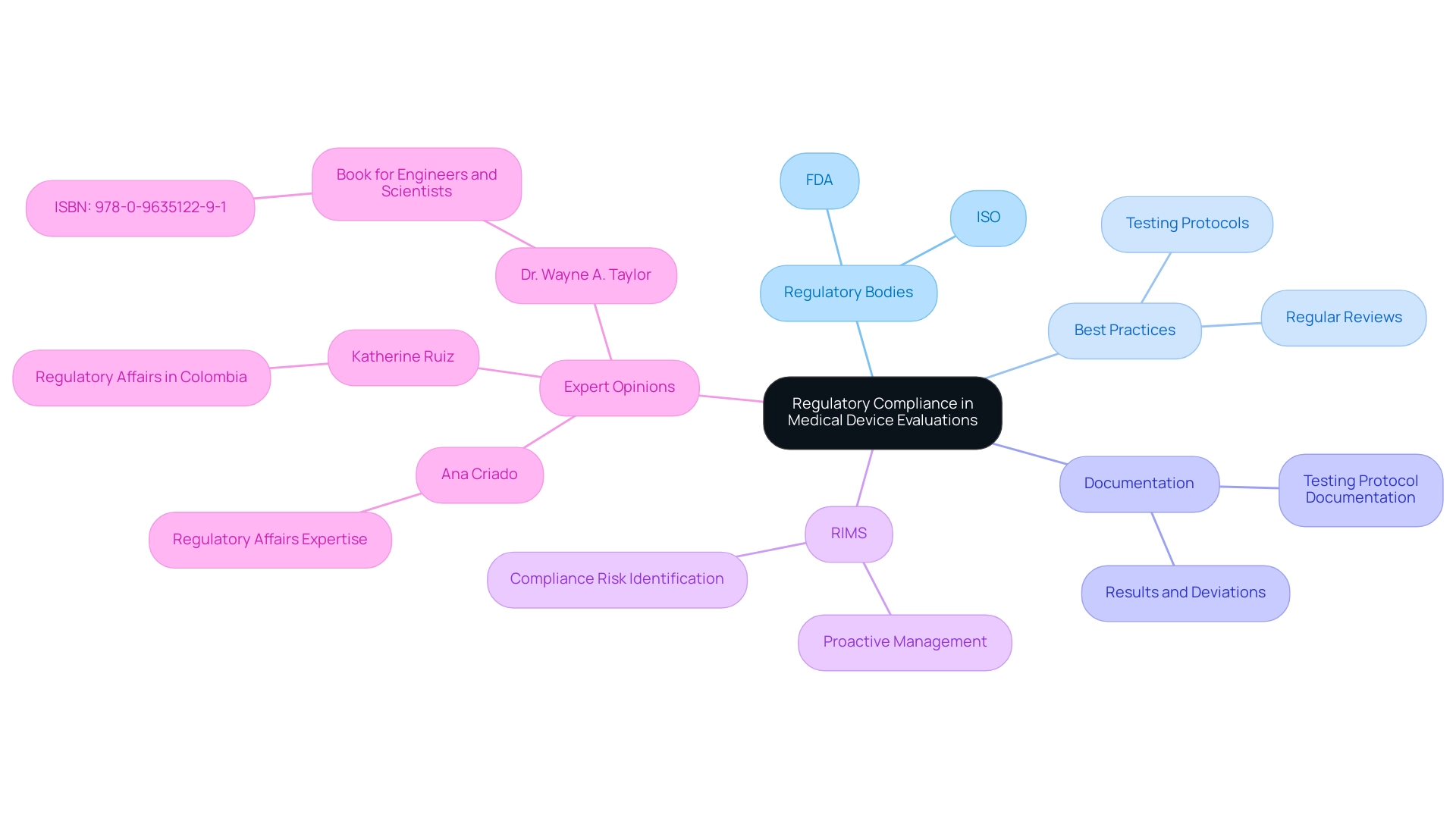
Regulatory Considerations: Compliance and Best Practices
Ensuring adherence to regulatory standards is crucial when conducting an accelerated aging test for medical device evaluations. Regulatory bodies, including the FDA and ISO, mandate that manufacturers demonstrate their products' safety and efficacy throughout their intended shelf life. Experts like Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, who holds a degree in chemical pharmacology, a master's in health economics & pharmacoeconomics, and certifications in clinical epidemiology, good clinical practices & study monitoring, and pharmacovigilance, emphasize the importance of adhering to best practices.
This involves meticulous documentation of testing protocols, results, and any deviations from established standards. Regular review and updating of testing practices in accordance with the latest industry guidelines and scientific advancements are crucial. Notably, Standard Requirement Testing can eliminate the need for formal internal verification testing of redundant tests during certification, further emphasizing the importance of compliance.
As highlighted in the case study titled 'Boosted Risk Management,' employing a Regulatory Information Management System (RIMS) enhances the speed and accuracy of identifying compliance risks, allowing companies to address potential issues proactively. Additionally, spreadsheets are provided for executing the statistical techniques involved, with versions available for both validation and customization. By prioritizing regulatory compliance and implementing these best practices, manufacturers can streamline approval processes and ensure that their healthcare products remain safe for patient use.
Dr. Wayne A. Taylor aptly states,
This book is designed for engineers and scientists in the healthcare industry
(ISBN: 978-0-9635122-9-1), underscoring the importance of integrating regulatory knowledge into testing protocols. Katherine Ruiz, an expert in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, also highlights the critical role of regulatory compliance in this field.
Conclusion
Accelerated aging tests are an indispensable aspect of medical device development, providing a systematic approach to predict long-term performance and safety within a shortened timeframe. By simulating the aging process through controlled environmental conditions, manufacturers can identify potential failures early in the product lifecycle, thereby enhancing patient safety and adhering to stringent regulatory standards. The importance of standards such as ASTM F1980 cannot be overstated, as they provide a framework for conducting these tests while ensuring consistency and reliability in results.
Despite the benefits, conducting accelerated aging tests presents challenges that require careful consideration of various factors, including:
- Material properties
- Temperature
- Humidity levels
Addressing these challenges through thorough preliminary studies and robust data analysis methods is crucial for ensuring the validity of test outcomes. Moreover, interpreting the results effectively is vital for making informed decisions regarding device design and material selection, ultimately contributing to improved safety and efficacy in medical devices.
Compliance with regulatory standards is essential in this process, as it not only streamlines approval but also reinforces the commitment to patient safety and product reliability. By prioritizing regulatory adherence and best practices, manufacturers can navigate the complexities of the healthcare landscape, ensuring that their devices meet market expectations and regulatory requirements. As the demand for reliable medical devices continues to grow, the role of accelerated aging tests will only become more critical in safeguarding public health and advancing the medical device industry.




