Introduction
The Investigational New Drug (IND) application plays a vital role in the process of bringing new treatments to the public. It serves as a formal request to the U.S. Food and Drug Administration (FDA) to conduct human clinical trials and assess the safety and efficacy of a novel drug or biological substance. This article explores the definition and purpose of an IND, the types of INDs, key components of an IND application, preclinical testing and manufacturing information, clinical trial protocols and investigator information, emergency use and treatment INDs, expanded access and compassionate use, FDA review process and clinical holds, and labeling and informed consent requirements.
Understanding the IND application process is essential to navigate the complexities of drug development and ensure the advancement of safe and effective treatments.
Definition and Purpose of an IND
The Investigational New Drug (IND) application is a critical juncture in the path to bringing new treatments from the laboratory to the public. It serves as a formal request to the U.S. Food and Drug Administration (FDA) to permit human clinical trials to assess the safety and efficacy of a novel drug or biological substance. This application encompasses comprehensive details about the drug's composition, manufacturing processes, pharmacological and toxicological studies, and the proposed clinical trial protocols.
The IND's pivotal role is underscored by its requirements, which demand a meticulous presentation of the drug's components, even those not present in the final product, alongside a narrative of the manufacturing and packaging procedures. The specifications must be stringent enough to ascertain the drug's identity, quality, and potency, ensuring that it meets the necessary standards for sterility, dissolution rate, and bioavailability. Moreover, the application should include stability data to propose appropriate expiration dating.
As the FDA scrutinizes these applications, it's evident that the agency's mandate extends far beyond the approval of new drugs. It is tasked with safeguarding public health through the regulation of a wide array of products, from human and veterinary drugs to food supply and cosmetics. The FDA's recent publication of the final rule regarding direct-to-consumer prescription drug advertisements exemplifies its dedication to clear communication of drug information, mandating that side effects and contraindications are presented in a way that is understandable and accessible to consumers.
The evolution of the FDA's role is also reflected in the significant increase in IND applications over the past years, particularly those involving cannabis and cannabis-derived products. This surge is indicative of the changing societal and legal landscapes, as well as the agency's adaptability in evaluating a broadening spectrum of drug modalities and components.
Understanding the landscape of drug development is essential, and the rigorous process of IND application is a testament to the critical balance between innovation and patient safety. The context of a drug's effectiveness is inherently tied to its clinical trial design and indicated uses, which are paramount in driving the development plan. This intricate web of regulatory requirements, scientific evaluation, and commitment to public health forms the backbone of the IND process, ultimately guiding new treatments from conception to clinical application.
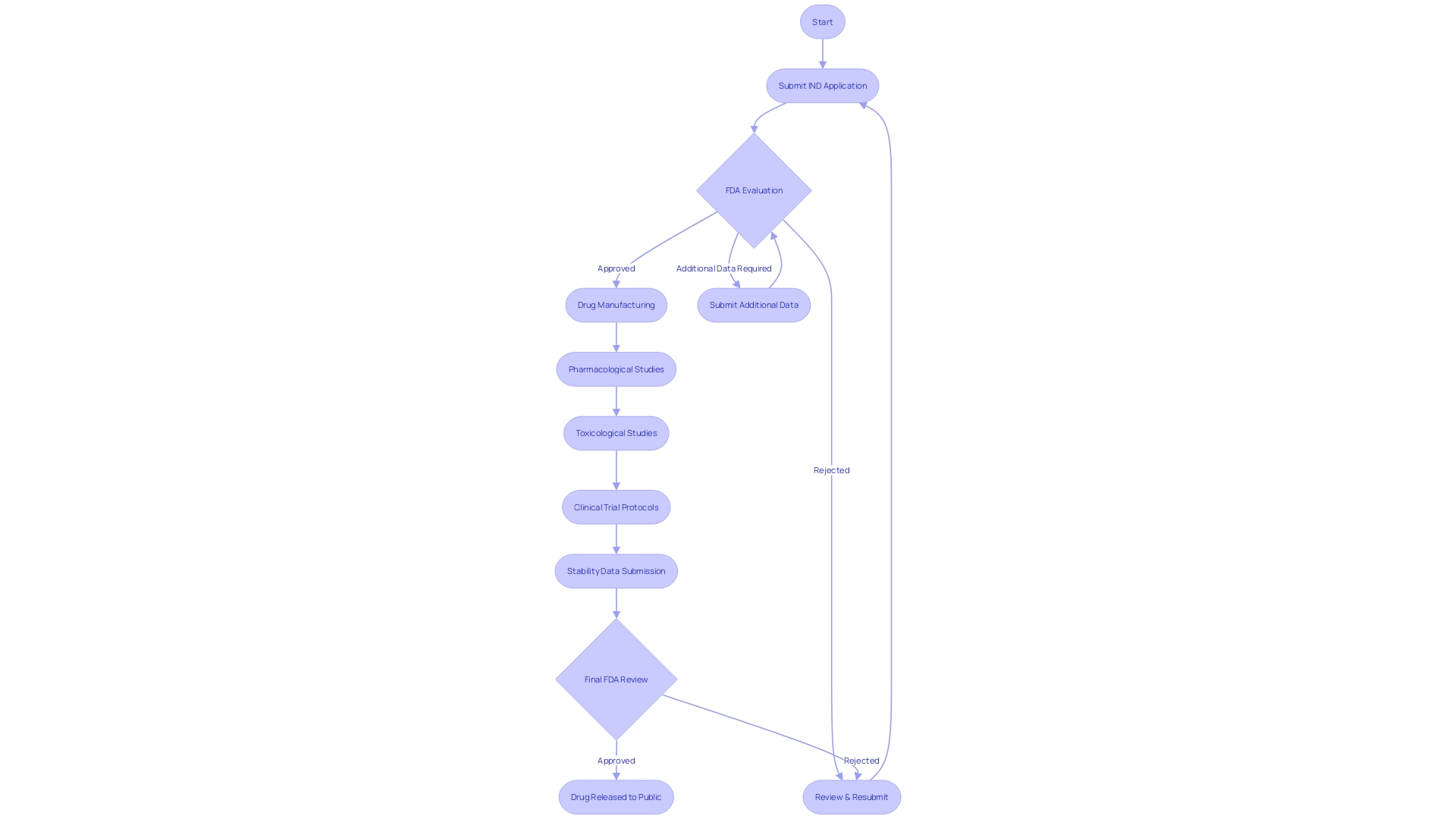
Types of INDs
Navigating the complexities of Investigational New Drug (IND) applications is a critical aspect for researchers and pharmaceutical companies aiming to bring new therapies to the market. The IND application is the foundation for conducting clinical trials and is classified into three primary types:
- Commercial IND: Primarily submitted by pharmaceutical companies or sponsors looking to eventually market the drug commercially post-approval.
- Research IND: This application is for non-commercial research, often filed by academic researchers or institutions to collect data on the drug's safety and efficacy.
- Treatment IN: A crucial pathway for life-threatening conditions where no alternative treatment exists, this IN allows the use of investigational drugs for serious or immediately life-threatening conditions.
Qualifying for an IND involves stringent criteria and regulations. For instance, the FDA's orphan-drug designation is granted to drugs intended for rare diseases or conditions, offering market exclusivity upon approval. This exclusivity means that for seven years post-approval, no similar drug for the same indication can be approved, unless it is proven to be clinically superior.
Moreover, the drug must not have been previously approved for the same use.
The terminology around INDs is precise. For example, an 'orphan subset' of a non-rare disease implies that the drug is appropriate for a subset of patients within a broader condition, but not for the whole population due to properties like toxicity or mechanism of action. Moreover, the distinction between drugs composed of small molecules versus large molecules (macromolecules) is crucial as it affects the approval process.
In the dynamic field of medical research, advancements such as the integration of artificial intelligence in cardiology are transforming the landscape, illustrating the potential of technology to enhance diagnostic accuracy and reduce the burden on healthcare professionals.
As the medical field continues to evolve with new technologies and regulations, staying informed and compliant with FDA guidelines is imperative for those involved in the development and approval of new therapeutic options.
Key Components of an IND Application
The Investigational New Drug (IND) application is a crucial step in the journey of a drug from conception to market, encapsulating detailed and comprehensive data to demonstrate the drug's potential for safe human use. Central to the IND application is the Investigator's Brochure, a pivotal document that synthesizes the drug's pharmacology, toxicology, and clinical trial history. Equally vital is the Chemistry, Manufacturing, and Control (CMC) Information, documenting the drug's composition, production, and quality assurance measures.
Moreover, the submission must present robust Preclinical Data, including animal study results that lay the groundwork for the drug's safety profile and therapeutic promise. This is supported by meticulously designed Clinical Trial Protocols, outlining the scope of clinical evaluations, from participant numbers to dosing strategies and targeted study outcomes.
Lastly, the inclusion of Informed Consent Forms is non-negotiable, ensuring that trial participants are thoroughly briefed on the trial's risks and benefits. This multi-layered approach to drug development underscores the industry's commitment to advancing safe and effective treatments, adhering to stringent regulatory standards, and respecting patient autonomy.
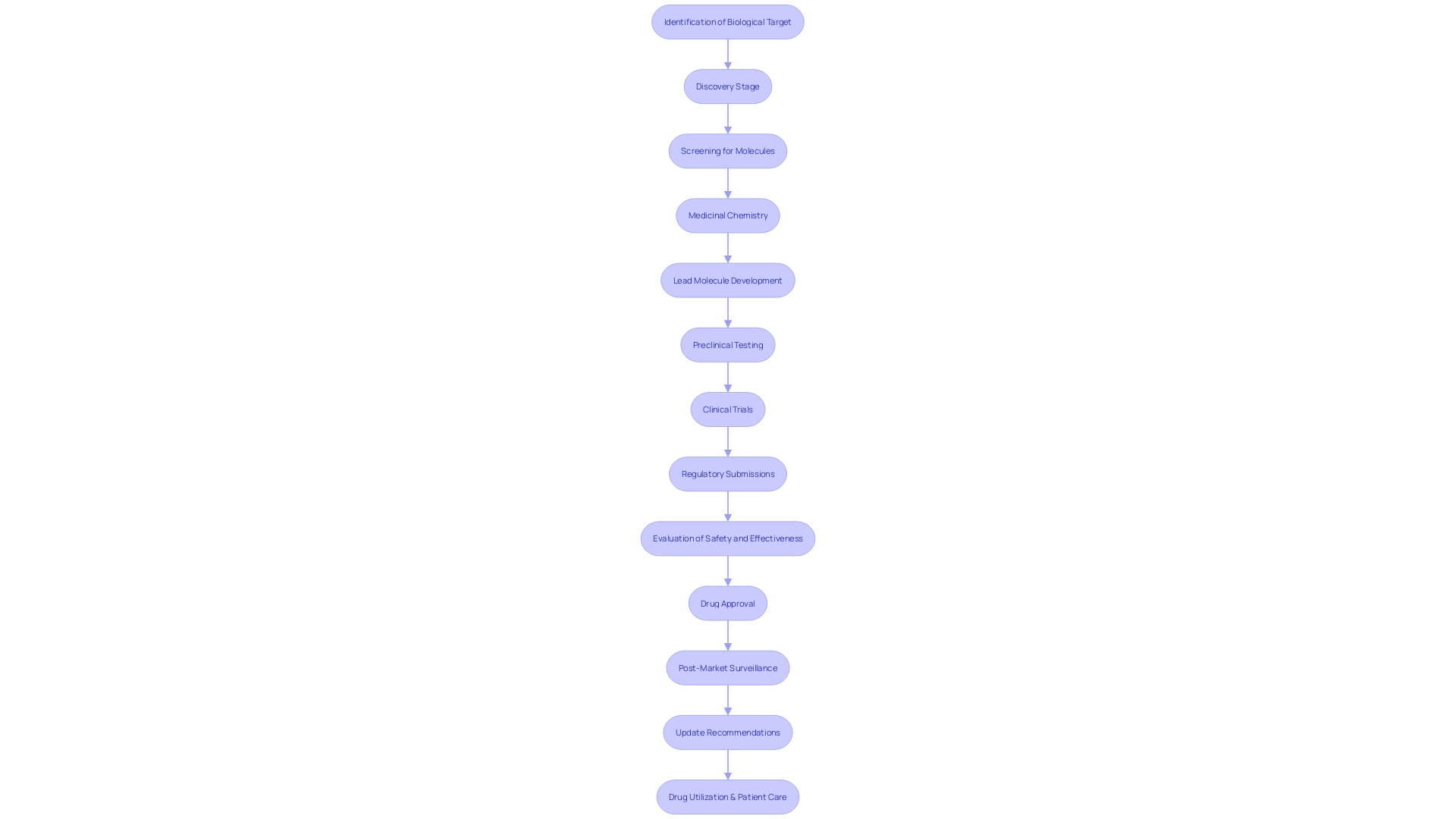
Preclinical Testing and Manufacturing Information
Navigating the complexities of bringing a new drug to the realm of clinical testing is a multifaceted process that hinges not just on discovery, but on rigorous development. The traditional view of drug discovery, seeking an unexpected breakthrough akin to Alexander Fleming's penicillin, overlooks the critical development phase. It took more than a decade before penicillin transitioned from a scientific curiosity to a mass-produced medicine, thanks to the US Army's efforts in scaling up manufacturing.
This underscores the distinction between discovery and the pivotal 'Development' in R&D—a lesson in the importance of product development and delivering value.
Preclinical research is a crucial step that includes both in vitro (tests conducted outside a living organism) and in vivo (tests within a living organism) studies. An example of the depth of preclinical research can be seen at Neuralink, where studies are classified into bench, pilot, R&D, and good laboratory practices (GLP). Bench studies provide the initial 'proof of concept', while pilot studies refine the approach, ensuring compatibility and feasibility with existing standards.
The transition to human trials necessitates a comprehensive IND application, which should encapsulate not only the preclinical data, but also the manufacturing process, quality control, and product stability. This is particularly vital in gene therapy development, where speed is of the essence due to patient urgency. The development process, while complex, can be streamlined using a platform approach with scalable equipment, which allows for time efficiency from project inception to manufacturing.
As we integrate emerging technologies like AI into healthcare, the potential to revolutionize patient care is significant. However, the balance between promise and practical application is delicate. While AI could solve critical health system challenges, there is also the risk that these innovations may not reach the market, or worse, exacerbate existing inequities or inflate costs.
The biopharmaceutical industry's shift towards re-embracing core development skills, paired with the strategic use of AI and platform approaches, offers a promising avenue to accelerate drug development. The goal is not just to discover but to effectively develop drugs that meet the stringent requirements of safety and efficacy for the betterment of patient health.
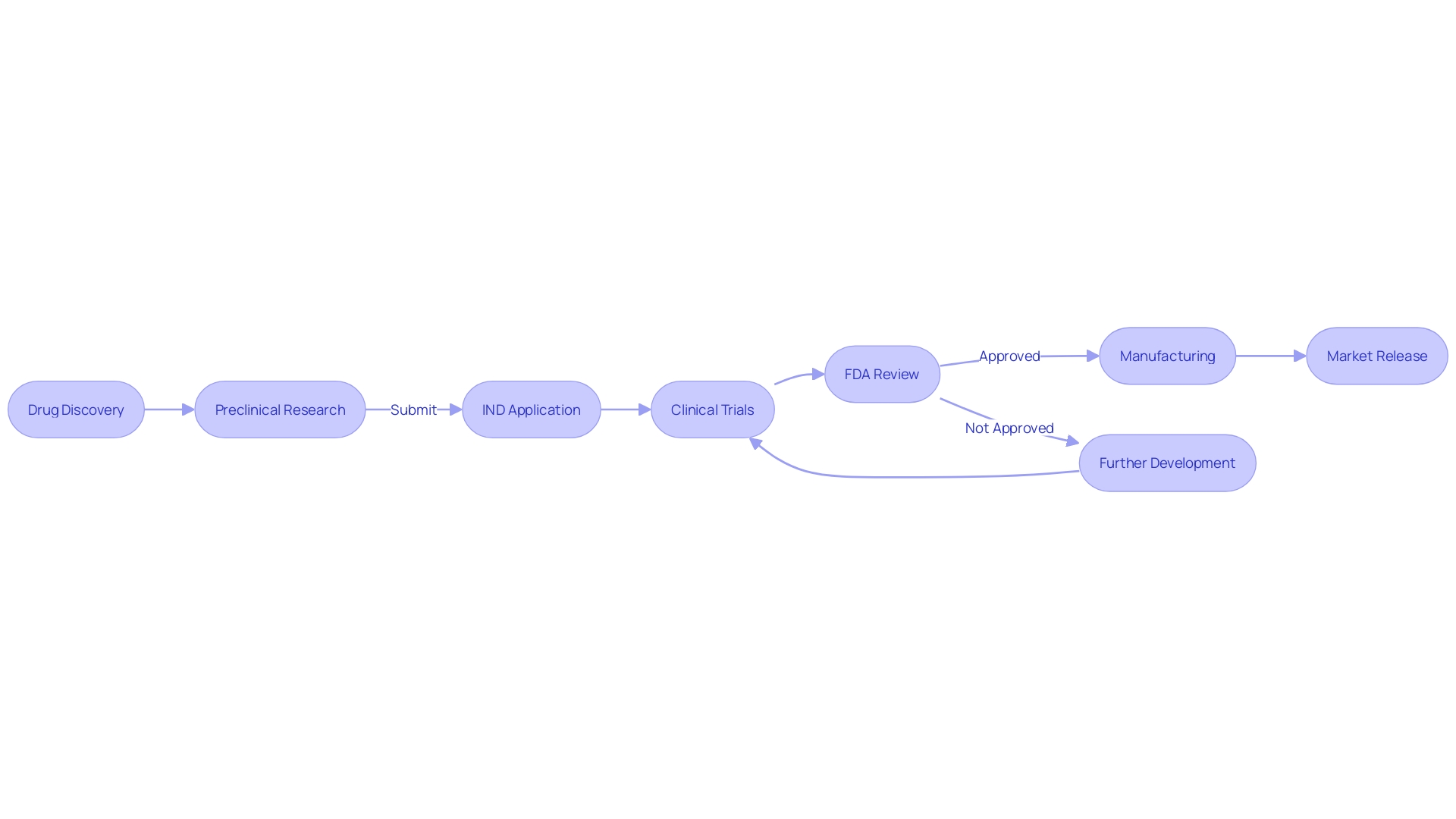
Clinical Trial Protocols and Investigator Information
A pivotal element in the Investigational New Drug (IND) application process is the formulation of detailed clinical trial protocols. These documents are the cornerstone of a proposed clinical study, meticulously delineating the objectives, methodology, and desired outcomes or endpoints. Companies like Intellia Therapeutics, advancing their pipeline with gene editing therapies such as NTLA-2001 for transthyretin (ATTR) amyloidosis, underscore the importance of these protocols in their regulatory filings and press releases.
The IND not only carries this crucial information but also presents data on the principal investigator, including their credentials and experience, which is vital in demonstrating the capability to conduct the trials effectively and safely.
In the dynamic landscape of clinical research, the stakes are high, and the risks are many. Intellia's forward-looking statements reflect the inherent uncertainties that accompany clinical trials and product development. The success of such endeavors hinges on numerous factors, from maintaining intellectual property rights to navigating the clinical trial authorization process, and from ensuring patient enrollment to meeting development timelines.
McKinsey's analysis reveals that the average clinical trial duration has increased, and an alarming 80 percent of trials do not conclude as scheduled. This amplifies the pressure on biopharma companies to expedite the development process in a bid to be first to market, especially when considering the increased competition among companies targeting similar mechanisms of action.
Furthermore, the design of clinical trials is not only a regulatory requirement but also a strategic decision that could impact a product's market potential. The labeled 'Indication' of a product, driven by clinical trial design, determines its claimed effectiveness, shaping the entire product development plan. This approach is reflected in industry trends, where the first product to market often enjoys significant success.
The recent US Inflation Reduction Act further intensifies this landscape by influencing the choice of indications companies pursue, thereby accentuating the importance of market timeliness.
The implications of clinical trial design extend beyond regulatory compliance, influencing the trajectory of medical advancements and patient outcomes. With numerous diseases lacking effective treatments, such as rare diseases and certain cancers with low survival rates, the need for well-structured clinical trials that can lead to successful and timely product development is more critical than ever.
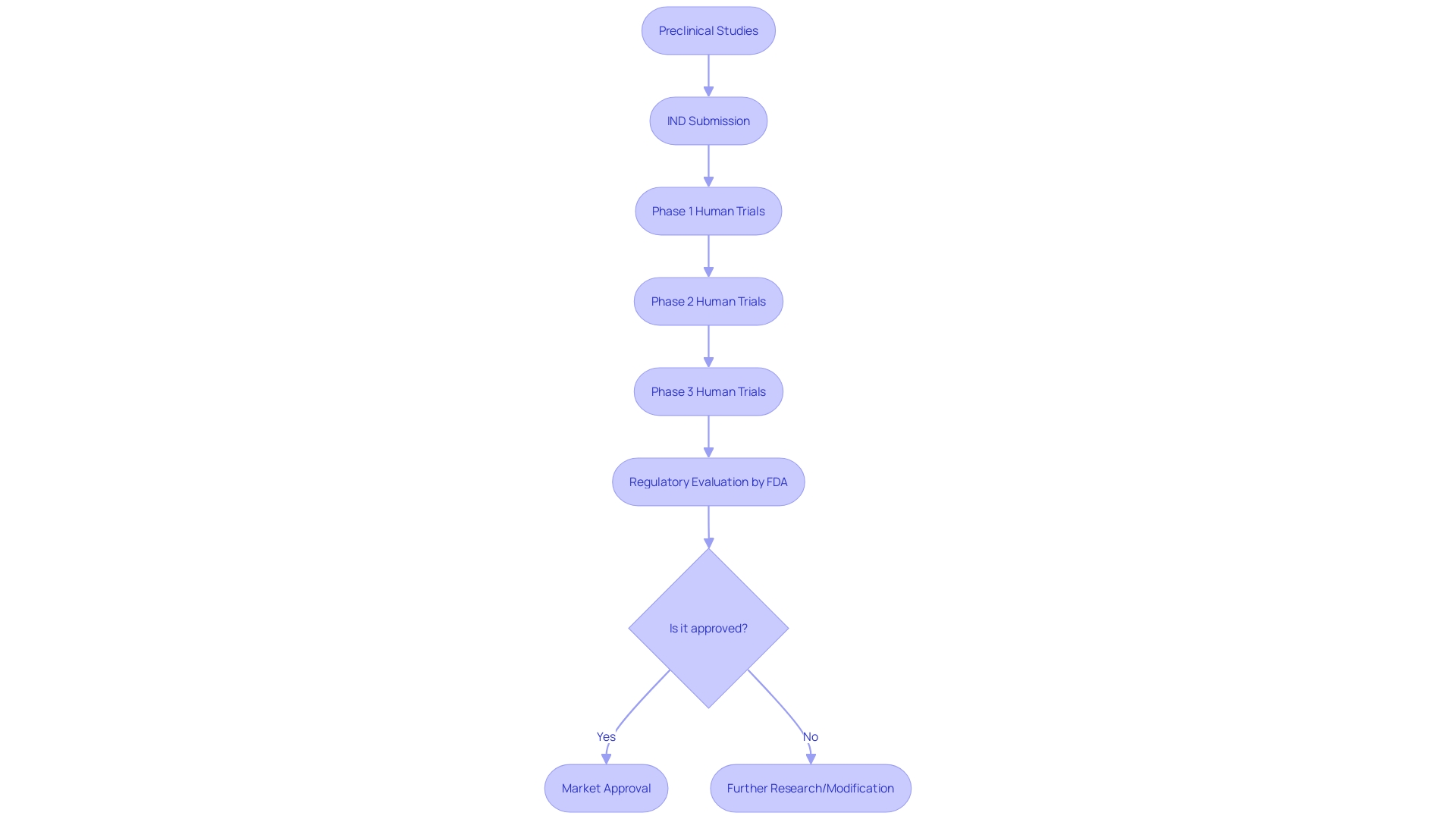
Emergency Use and Treatment INDs
In the landscape of clinical treatment, the U.S. Food and Drug Administration (FDA) has provisions such as the Emergency Use Investigational New Drug (IND) and Treatment IND to address critical situations where conventional treatments are unavailable. The Emergency Use IND is specifically designed to enable the use of an investigational drug for patients in life-threatening scenarios where there is no time to secure a standard IND. This regulatory pathway provides immediate access to potentially lifesaving treatments under conditions of dire urgency.
On a parallel note, the Treatment IND is another regulatory mechanism that facilitates access to investigational drugs for patients with severe or terminal illnesses who have no other viable treatment options. This is particularly relevant when patients have exhausted all available therapies and are facing significant health risks without alternative interventions.
These INDs are part of a broader framework that ensures patient safety while responding to public health emergencies. For instance, in events that prompt an emergency response, the FDA can authorize the use of a product without it being considered unapproved, adulterated, or misbranded, thus allowing for the swift introduction of crucial medical interventions during crises. This framework is essential for institutions like the Francis Crick Institute and the University of Cambridge, which are at the forefront of biomedical discovery and disease treatment innovation.
Understanding these regulatory pathways is crucial for clinical research directors, as they navigate the complexities of bringing new drugs and biological products to market. The FDA's Center for Drug Evaluation and Research (CDER) offers guidance on study design and data requirements for drug applications, ensuring that innovative treatments can be assessed with rigor and reach patients in need. As new drugs are developed, they offer fresh hope and advancements in healthcare, with some drugs being novel introductions to clinical practice.
These emergency provisions underscore the FDA's commitment to facilitating access to potentially life-saving treatments while maintaining strict regulatory oversight to ensure public safety during health emergencies. The flexibility and responsiveness of these regulations reflect a balance between rapid access to experimental therapies and adherence to scientific evaluation, as part of the FDA's mission to advance public health.
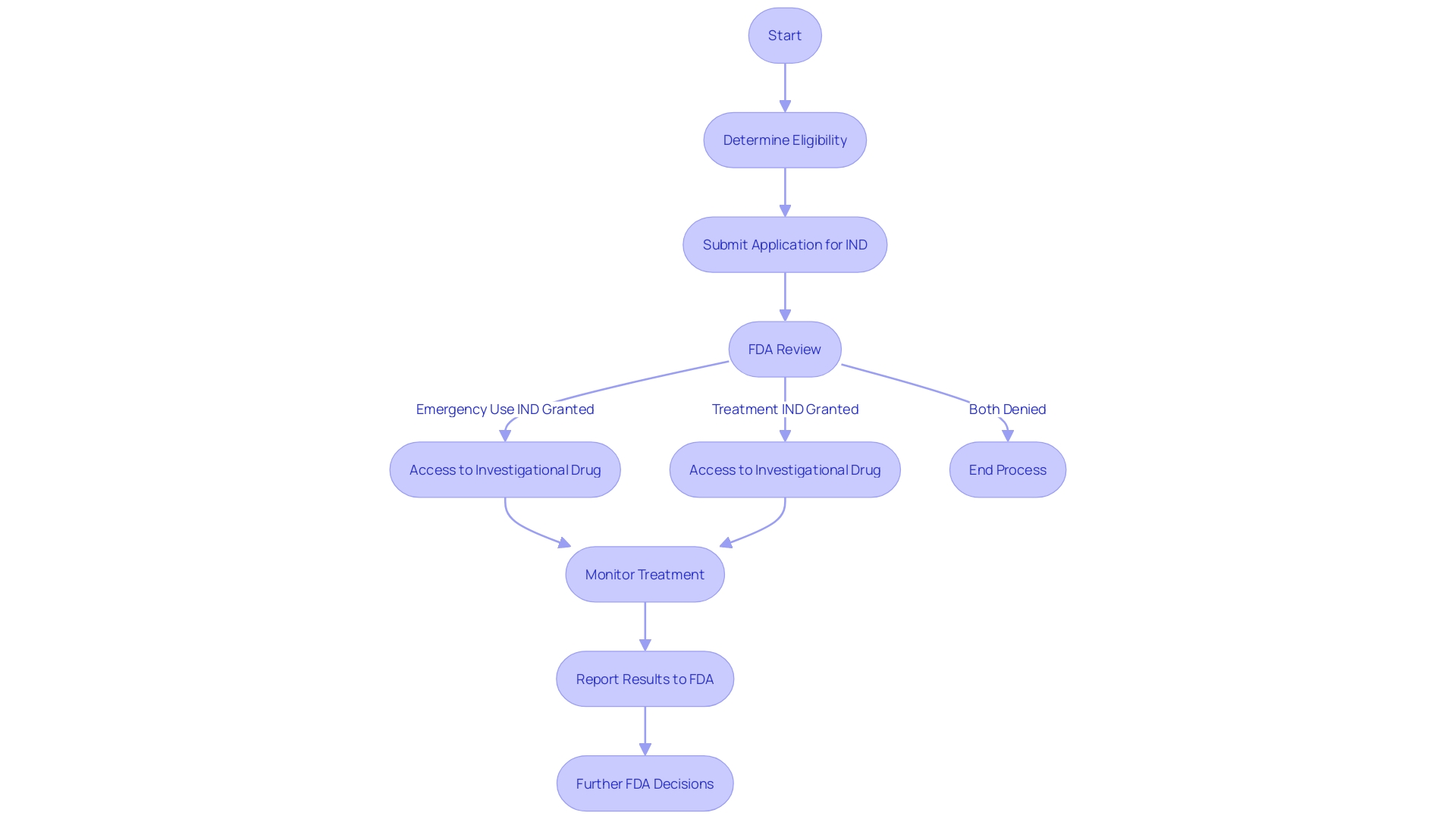
Expanded Access and Compassionate Use
Expanded Access, often termed Compassionate Use, is a pathway for patients to receive investigational drugs outside the confines of clinical trials. This critical program is designed for patients with serious or immediately life-threatening conditions who lack therapeutic alternatives and are ineligible for participation in any ongoing clinical studies. The governance of this program is subject to a robust framework of ethical, legal, and social considerations, ensuring that the use of investigational drugs is both appropriate and justifiable.
The drug in question must meet certain criteria, such as not being approved for any other use, and the potential benefit to the patient must justify the potential risks. The FDA oversees this process rigorously, providing guidance to healthcare professionals who must navigate a complex array of market incentives, intellectual property concerns, and the drug's properties – which may include toxicity and mechanism of action.
Furthermore, orphan drugs, which are intended for rare diseases or conditions, are subject to specific FDA regulations. A designated orphan drug receives exclusive approval for seven years post-FDA approval, barring subsequent sponsors from gaining approval for the same drug and indication unless the new drug is clinically superior.
Clinical trials remain a cornerstone of medical advancement, providing generalizable knowledge for future patients' care. As such, the FDA continually works to advance clinical research, streamline regulations, and ensure participant protection, all while fostering medical innovation. In fact, the FDA's Center for Drug Evaluation and Research (CDER) annually approves a diverse array of new molecular entities and therapeutic biological products, after thorough consideration of study design elements and the scientific knowledge pertaining to the product.
In conclusion, Expanded Access presents a vital option for patients in need, backed by a stringent regulatory framework that balances the urgency of patient needs with the imperative for safety and efficacy. It encapsulates the FDA's commitment to protecting public health while enabling access to potentially life-altering treatments.
FDA Review Process and Clinical Holds
The FDA's scrutiny of Investigational New Drug (IND) applications is a multi-tiered process designed to evaluate the safety and potential patient benefits of new medications. The journey begins with an initial review of the data presented, where the agency meticulously examines the drug's proposed use, expected benefits, and potential risks. When the FDA encounters concerns about patient safety or the sufficiency of the information provided, this can trigger a clinical hold.
Such an action temporarily halts clinical trials, necessitating a resolution to the identified concerns before proceeding. This was exemplified by the agency's recent review of the Impella Connect System—a combination of software and hardware designed to support critical cardiac functions—which necessitated premarket authorization due to its role in monitoring life-threatening conditions and issuing timely alerts to healthcare providers. Similarly, the FDA's classification of 'OcluMed Eye Drops' as a drug highlights the stringent oversight applied to products intended to diagnose, treat, or impact bodily functions, underscoring the importance of regulatory compliance.
Companies are urged to address any violations promptly to avoid legal repercussions, as was emphasized in a recent communication to a firm over unapproved product claims.
Moreover, the FDA's role extends beyond drug safety to encompass the overall public health, regulating a broad spectrum of products and industries. The agency's commitment to transparency is evidenced by its maintenance of lists, such as the one for AI/ML-enabled medical devices, which serve as valuable resources for professionals and the public alike. These regulatory endeavors and the implications for companies navigating the approval process are well-documented, including the potential for significant delays in drug development and the financial and reputational risks associated with non-compliance.
The entire approval timeline, spanning from research to final approval, typically extends between 12 to 15 years, reflecting the depth of evaluation each drug undergoes. As new pharmaceuticals emerge, such as those targeting Alzheimer's disease, the FDA's rigorous assessment of safety, efficacy, and labeling plays a critical role in bringing these innovations to market.
The FDA's regulatory landscape is dynamic, with amendments to regulations influencing not only the cost but also the pace of drug development. This environment necessitates thorough understanding and adherence to FDA guidance, as well as proactive engagement in the regulatory process, including the submission of comments and requests for reconsideration, as detailed in the agency's draft guidance for industry. It is through this diligent oversight and collaboration that the FDA continues to uphold its mission to protect and promote public health.
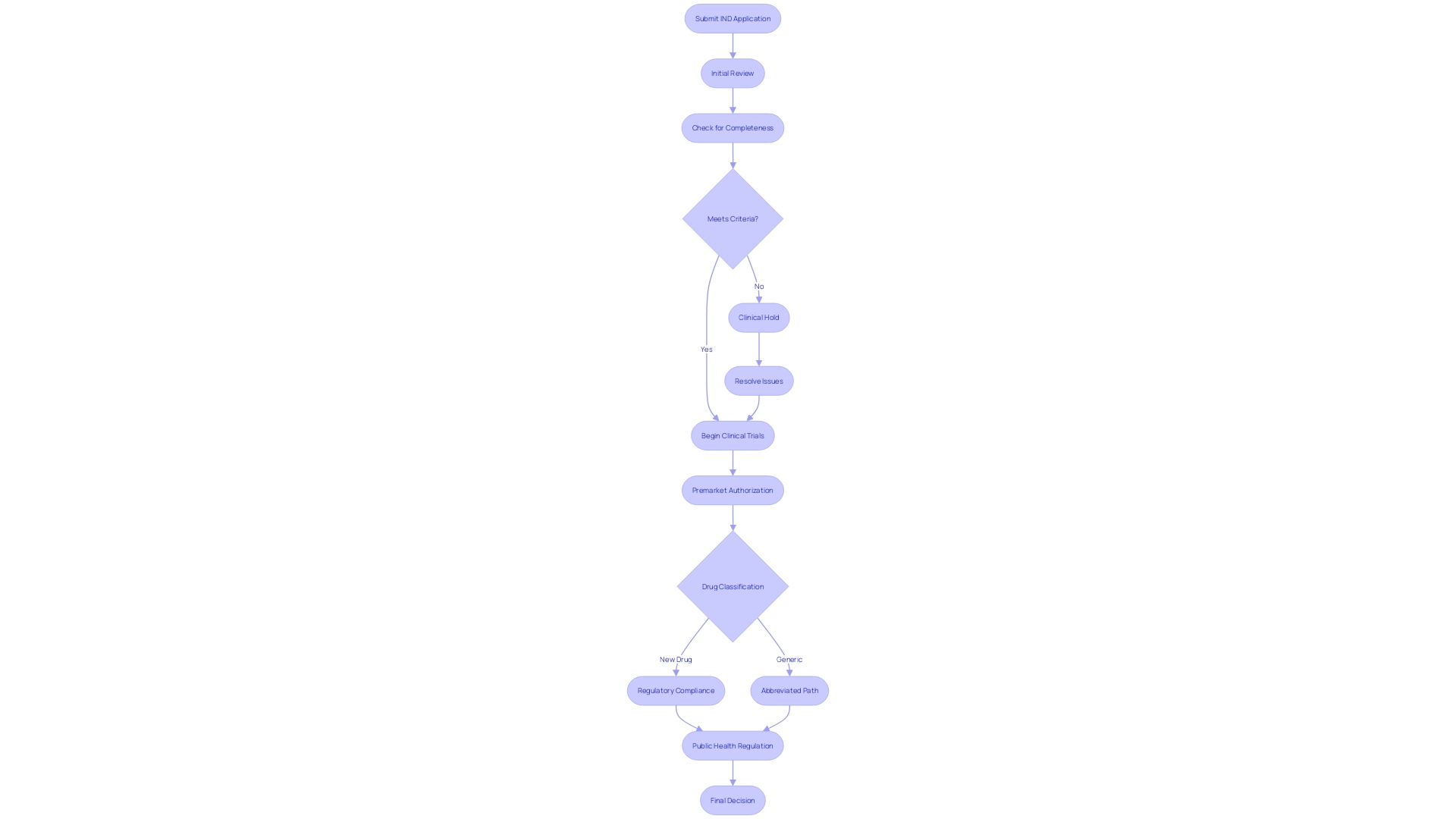
Labeling and Informed Consent Requirements
Investigational drugs are governed by stringent FDA regulations to ensure patient safety and informed decision-making in clinical trials. Labels must unequivocally indicate the drug's investigational status and include critical safety details. The core of these clinical studies is the informed consent process, which is meticulously designed to be clear and concise.
This process involves presenting key information upfront, such as the study's purpose, duration, procedures, and the potential risks and benefits, aiding participants in making an educated choice about their involvement.
The FDA emphasizes the importance of understanding what constitutes an investigational product, including the rights of participants and the special considerations needed for vulnerable populations such as minors, pregnant women, fetuses, neonates, prisoners, and mentally impaired individuals. Ensuring that participants are well-informed about the study they are consenting to is not just ethical but also a regulatory requirement, with recent FDA guidance encouraging investigators to use this key information as a roadmap for consent discussions.
The FDA's commitment to safeguarding public health extends to the clear and neutral presentation of drug information in direct-to-consumer advertising. This includes ensuring that the major side effects are communicated in consumer-friendly language and in a way that is at least as understandable as the rest of the advertisement. The simultaneous use of audio and text in television ads, for example, is mandated to allow for easy comprehension and readability.
Furthermore, understanding the terminology is vital. A 'drug product' refers to a finished dosage form containing an active drug ingredient and may include inactive ingredients. The term encompasses both pharmaceuticals and biological products as defined by the Public Health Service Act.
The roles of licensed practitioners, manufacturers, packers, and distributors are also clearly defined, ensuring accountability and adherence to regulations.
Recent surveys underscore the public's expectation that clinical trials should be an integral part of healthcare, with a significant majority believing that healthcare professionals should discuss trial participation as a standard care practice. Despite this, only a fraction report having had such discussions with their healthcare providers, highlighting an area for improvement. As the industry navigates an abundance of data, pharmaceutical companies are encouraged to leverage this information to foster inclusive clinical trials and adhere to the latest FDA guidelines for diversity in participant recruitment and site selection.
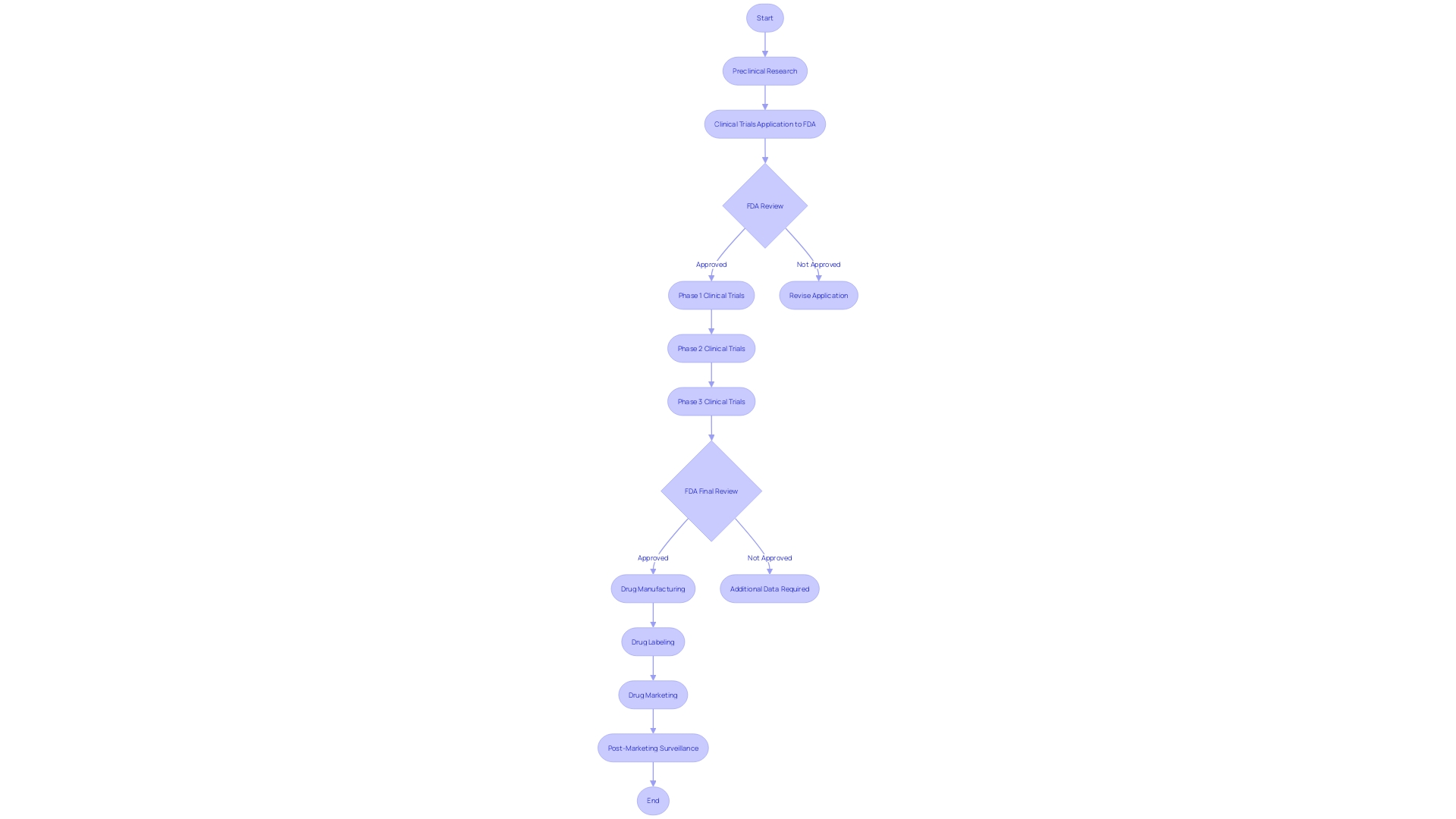
Conclusion
The Investigational New Drug (IND) application is a critical step in the drug development process, serving as a formal request to the U.S. Food and Drug Administration (FDA) for permission to conduct human clinical trials. Understanding the IND application process is crucial to navigate the complexities of drug development and ensure the advancement of safe and effective treatments.
Key components of an IND application include the Investigator's Brochure, Chemistry, Manufacturing, and Control (CMC) Information, Preclinical Data, Clinical Trial Protocols, and Informed Consent Forms. These components demonstrate the drug's potential for safe human use, adherence to regulatory standards, and respect for patient autonomy.
Preclinical testing and manufacturing information play a crucial role, ensuring the transition from discovery to clinical testing. The integration of emerging technologies, such as artificial intelligence (AI), offers promising avenues to streamline drug development while maintaining safety and efficacy.
Emergency Use and Treatment INDs provide pathways for patients facing life-threatening conditions or lacking alternative treatments. These mechanisms enable access to investigational drugs in critical situations and public health emergencies.
Expanded Access, also known as Compassionate Use, allows patients to access investigational drugs outside of clinical trials. This program follows a robust framework of ethical, legal, and social considerations, ensuring appropriate and justifiable use of investigational drugs.
The FDA's review process evaluates the safety and potential patient benefits of new medications, with clinical holds temporarily halting trials when concerns arise. Compliance with FDA regulations and guidance is crucial to navigate the approval process efficiently.
Labeling and informed consent requirements are essential to ensure patient safety and informed decision-making in clinical trials. The FDA's emphasis on clear and neutral drug information extends to direct-to-consumer advertising, promoting consumer understanding.
In conclusion, the IND application process is a crucial step in bringing new treatments to the public. It requires meticulous attention to detail, adherence to regulatory standards, and a commitment to patient safety. Understanding the complexities of drug development and navigating the FDA's guidelines are essential to ensure the advancement of safe and effective treatments.




