Introduction
In the intricate landscape of pharmaceutical development, Investigational New Drug Applications (INDs) play a pivotal role in transitioning innovative therapies from the laboratory to clinical trials. These applications, submitted to the U.S. Food and Drug Administration (FDA), are not merely bureaucratic hurdles; they are essential frameworks that ensure rigorous evaluation of a drug's safety and efficacy before it reaches human subjects.
As the complexities of drug development continue to evolve, understanding the IND process becomes imperative for sponsors aiming to navigate regulatory requirements effectively. This article delves into the multifaceted nature of INDs, exploring:
- The steps involved in filing an application
- The criteria that necessitate an IND
- The FDA's critical role in safeguarding patient safety throughout the process
By shedding light on these vital components, stakeholders can better appreciate the significance of meticulous planning and compliance in bringing new therapies to market.
Understanding Investigational New Drug Applications (INDs)
What is an ind fda? Investigational New Substance Applications (INDs) represent a crucial element of the medication development process, submitted to the U.S. Food and Administration (FDA) by sponsors aiming to commence trials with new pharmaceutical compounds. An IND application, which relates to what is an ind fda, is essential for the testing of innovative medications on human subjects, ensuring that their safety and efficacy are rigorously evaluated. This application functions as a comprehensive regulatory framework, detailing essential information about the investigational drug, including what is an ind fda, its chemical composition, preclinical research findings, and the proposed protocols for trials.
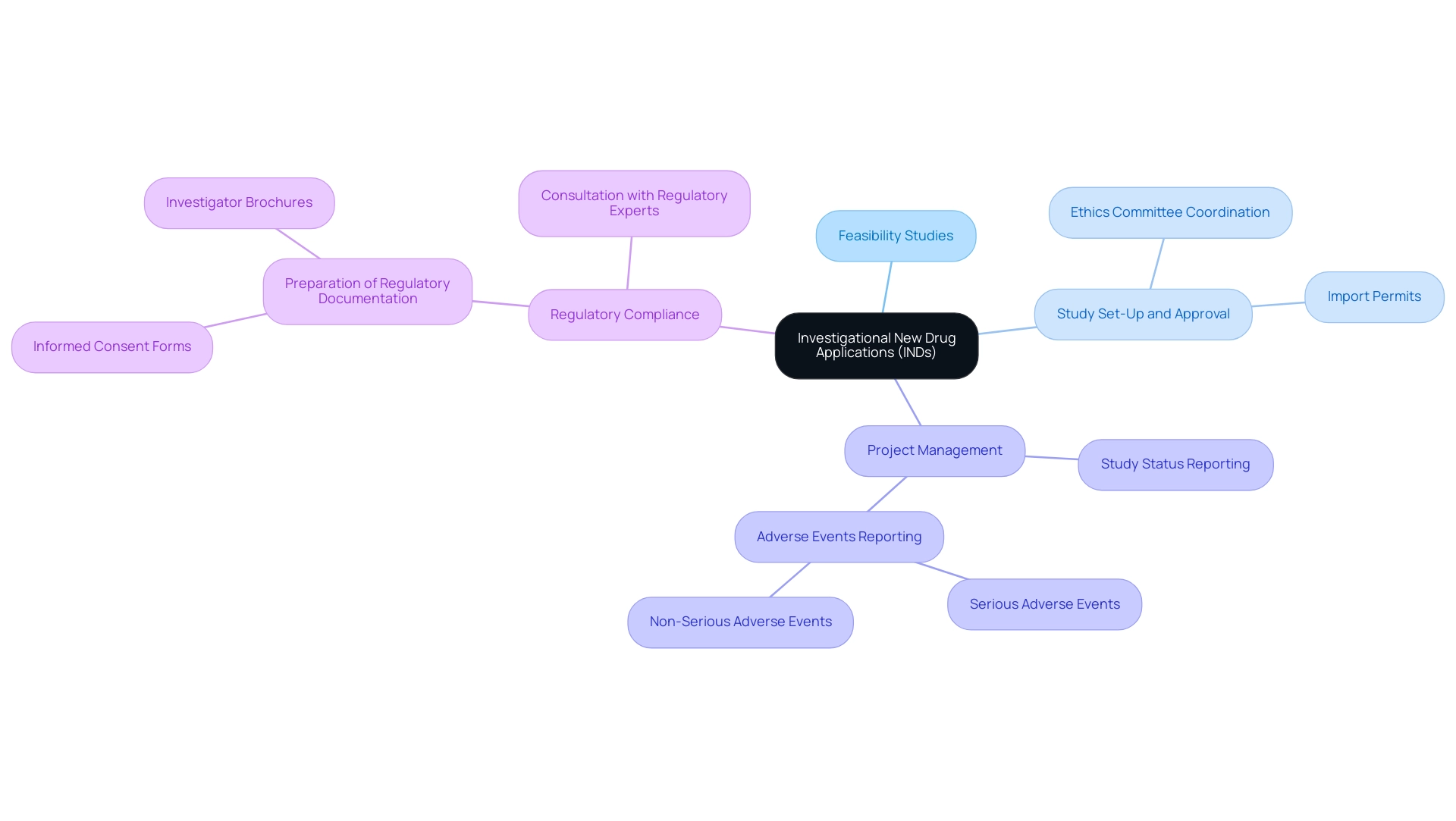
Our service capabilities encompass:
- Feasibility studies
- Selection of research sites and principal investigators (PIs)
- Review and feedback on study documents to ensure compliance with country requirements
- Study set-up, start-up, and approval processes—including ethics committee and health ministry coordination
- Import permits
- Nationalization of investigational devices
- Effective study project management
- Thorough reporting on study status, inventory, and serious and non-serious adverse events
According to Amsberry, 'Preparing a regulatory-compliant IND application is extremely labor intensive and time-consuming. Successful preparation and submission of INDs, or what is an ind fda, require substantial strategic planning and precise timing.'
This emphasizes the labor-intensive nature of the IND process and underscores the necessity of consulting with regulatory experts like Katherine Ruiz, who specializes in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia. Her expertise ensures that all regulatory documentation, including Informed Consent Forms and Investigator Brochures, is properly prepared and included in the IND application. With the recent reorganization of the Office of New Drugs, including changes to the Office of Translational Sciences and the Office of Pharmaceutical Quality, staying informed about these developments has become crucial for researchers.
As the environment of medical research progresses, understanding what is an ind fda becomes increasingly important for ensuring compliance and promoting successful studies.
The Process of Filing an IND: Steps and Requirements
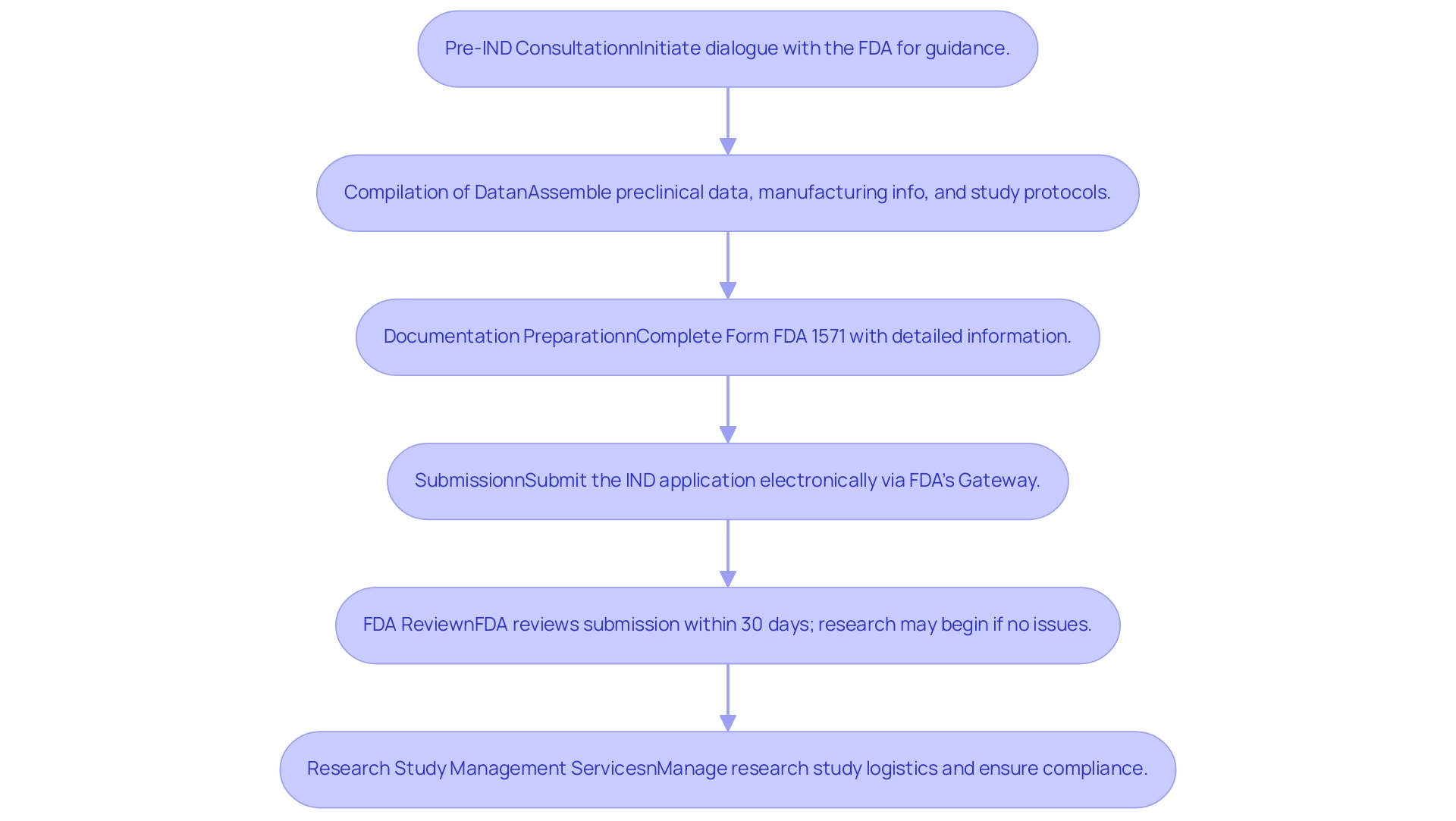
Filing an Investigational New Drug (IND) application involves several critical steps, and understanding what is an ind fda is essential for ensuring rigorous attention to detail.
-
Pre-IND Consultation:
Initiating dialogue with the FDA early in the process is essential. This consultation allows sponsors to discuss their development plans and receive invaluable guidance tailored to their specific projects.
-
Compilation of Data:
It is imperative to assemble a comprehensive array of documents, which includes preclinical data, detailed manufacturing information, and well-defined study protocols. This data forms the backbone of the IND submission, which illustrates what is an ind fda and provides the FDA with the necessary information to assess the proposed investigations.
-
Documentation Preparation:
Completing Form FDA 1571 is a crucial step. This form requires the IND sponsor's detailed information and outlines the proposed studies. Accuracy and thoroughness in this documentation are vital to prevent delays in the review process.
-
Submission:
The IND application must be submitted electronically via the FDA’s Electronic Submissions Gateway. This platform streamlines the submission process but requires familiarity with electronic formatting and compliance standards.
-
FDA Review:
Following submission, the FDA undertakes a review within 30 days. If the evaluation raises no issues, the sponsor may begin research studies. However, as highlighted by John Jenkins, M.D., director of CDER's Office of New Drugs, > But it's no guarantee <, emphasizing the potential for unforeseen challenges during the process of what is an ind fda, which can complicate timelines and outcomes.
-
Thorough Research Study Management Services:
At this stage, utilizing thorough research study management services becomes essential. Our capabilities encompass feasibility and selection of research locations and lead researchers, review and feedback on study documents to ensure adherence to country requirements, setup and approval by ethics committees and health ministries, and import permit processes for investigational devices.
We also provide detailed reporting on study status, inventory, and both serious and non-serious adverse events. Efficient project management is crucial to guarantee a seamless research study advancement, tackling obstacles that may emerge during the process of what is an ind fda filing.
The typical duration to introduce a medication to the market in 2022 is projected to be no less than 10-15 years, a period that highlights the importance of the IND filing process. Each of these steps is not only procedural but also foundational to ensuring compliance with FDA regulations, particularly in understanding what is an ind fda.
Additionally, the case study titled 'Document Development System' illustrates the importance of having a clear document review platform and defined roles for all participants involved in the IND submission process. Acumen's submission managers help organize and manage the logistics around document development, ensuring that authors, reviewers, and approvers are aligned.
With an average of 89.8 months spent in research studies for FDA-approved medications between 2014 and 2018, attention to detail at the IND filing phase can significantly influence the overall timeline for introducing a treatment to the market.
When Is an IND Required? Criteria and Exemptions
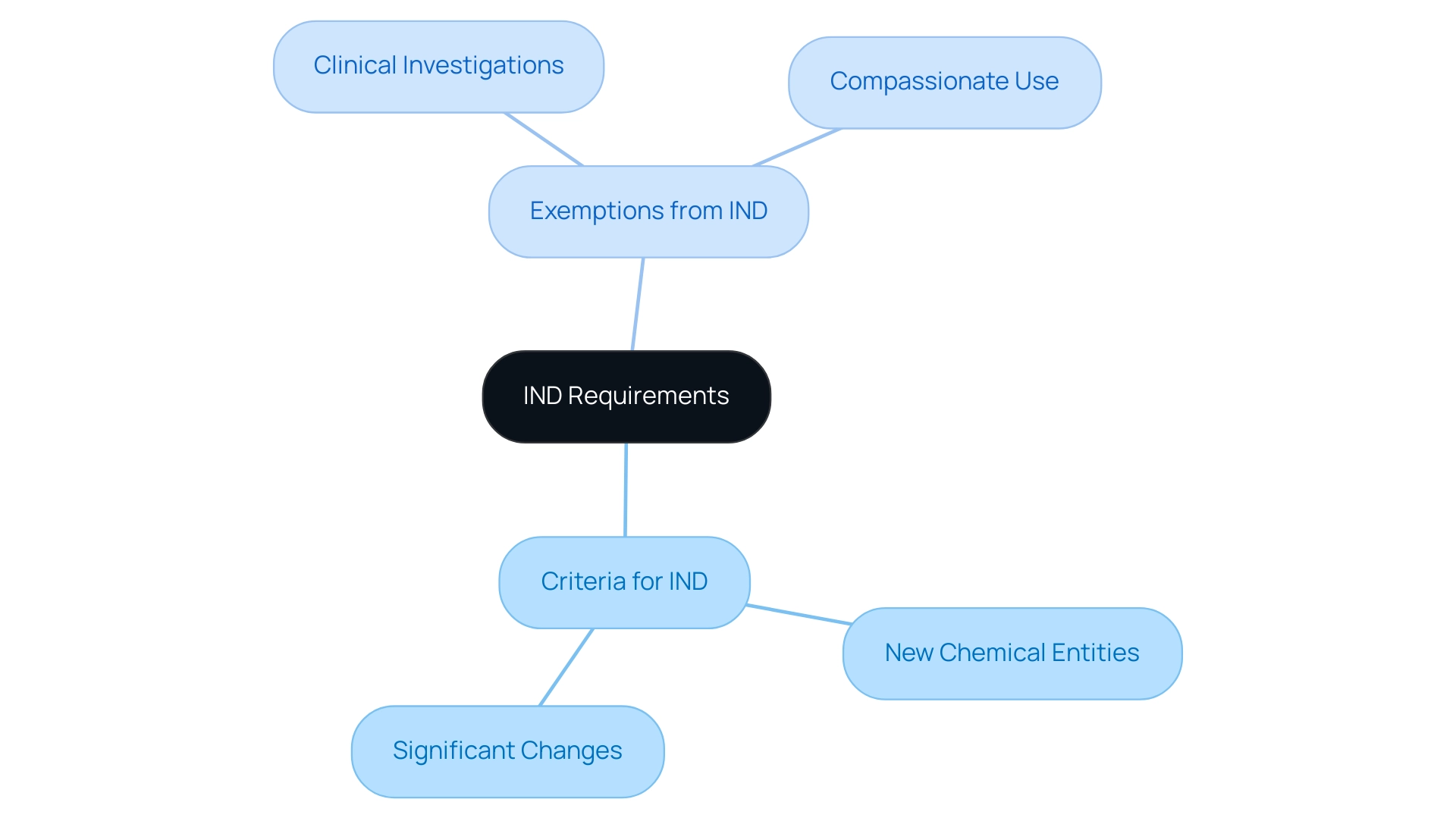
When a sponsor intends to start research studies with a new substance that has not yet obtained marketing authorization, they must submit an Investigational New Drug (IND) application, which leads to the inquiry of what is an ind fda. The primary criteria warranting an IND include:
- New Chemical Entities: This encompasses any drug that has not been previously approved by regulatory agencies.
- Significant Changes: This refers to modifications made to an existing drug, particularly those that could impact its safety profile or therapeutic efficacy.
However, under specific conditions, exemptions from IND requirements are applicable, such as:
- Clinical Investigations: These involve drugs that are already approved for other indications, allowing for expanded use without the necessity of an IND.
- Compassionate Use: This scenario arises when immediate access to a drug is crucial for patients with serious or life-threatening conditions, potentially bypassing standard IND protocols.
Grasping these criteria is vital for ensuring compliance with regulatory standards and facilitating effective research planning. Our extensive research management services offer essential assistance in feasibility studies, site selection, compliance evaluations, study setup, import permits, project oversight, and reporting on significant and non-significant adverse events.
As regulatory specialists such as Katherine Ruiz and Ana Criado highlight, grasping these components is essential for maneuvering through the intricacies of medical studies in Colombia. For immediate assistance or inquiries related to what is an ind fda applications and our services, sponsors can contact the FDA’s Office of Emergency Operations at 1-866-300-4374, ensuring they stay informed about evolving guidelines and exemptions.
The FDA's Role in Reviewing IND Applications
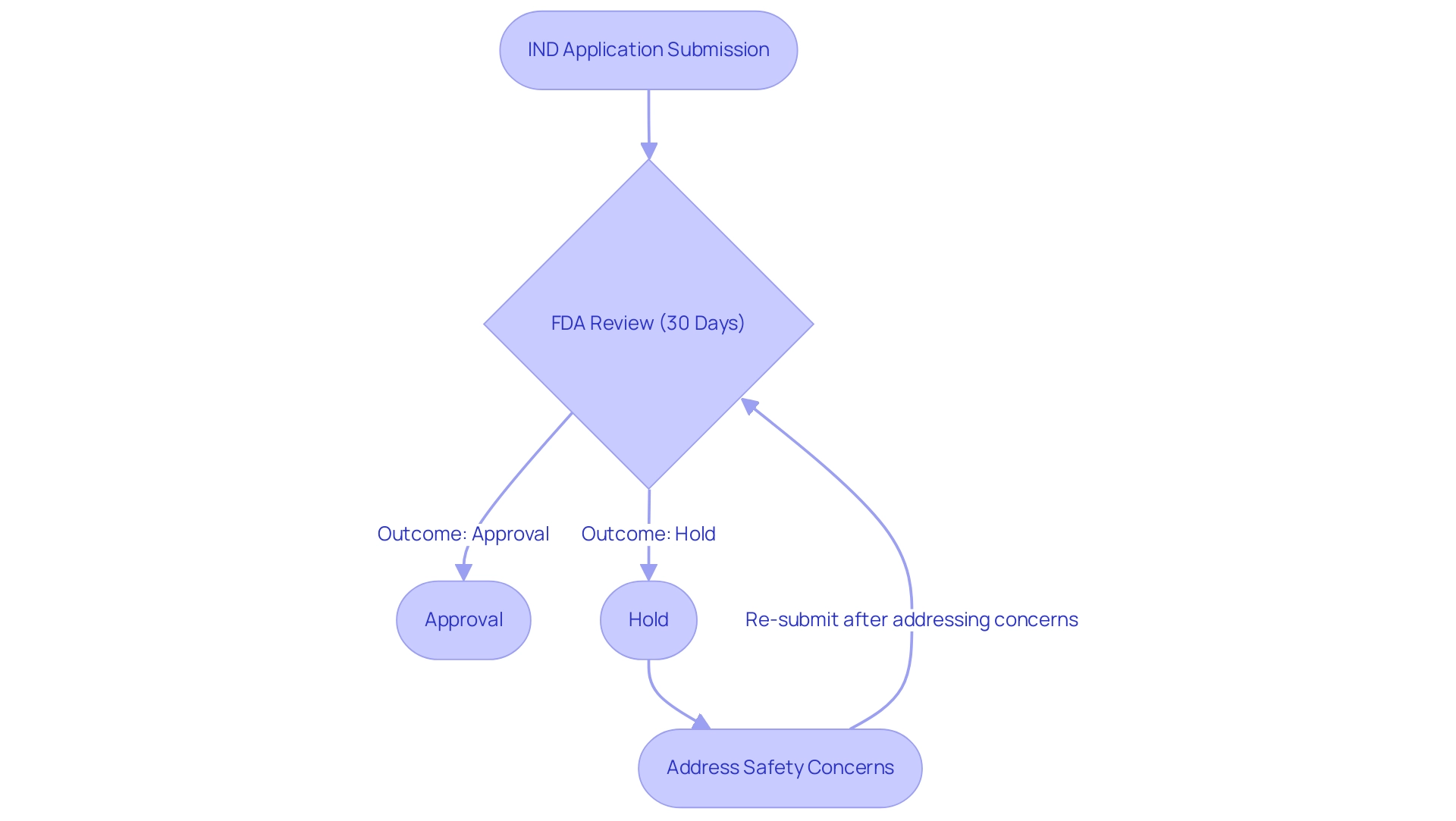
The FDA's oversight is essential in understanding what is an IND FDA process, meticulously reviewing applications to safeguard patient safety and uphold scientific rigor. This regulatory body evaluates whether proposed trials are structured to protect participants and minimize potential risks, ensuring that the methodologies employed are grounded in sound scientific principles. Within a 30-day period, the FDA evaluates the IND submission and maintains the authority to impose a hold if serious concerns about participant safety or data integrity arise.
Recent statistics indicate a significant rise in treatment holds, emphasizing the FDA's steadfast dedication to patient safety, a sentiment reflected by Jonathan P. Jarow, MD, who underscores the FDA's role in ensuring the safety of individuals receiving investigational therapies. This diligent review process ultimately strengthens confidence in medical studies and the medications being developed for public use.
Furthermore, our extensive research management services, directed by specialists such as Katherine Ruiz in Regulatory Affairs for medical devices and in vitro diagnostics in Colombia, guarantee thorough feasibility studies, efficient site selection, compliance assessments, and streamlined study preparation, including the import permit and nationalization of investigational devices.
Our services also encompass thorough review and feedback on study documents to comply with country requirements. A case study on regulatory documentation verification demonstrates what is an IND FDA and illustrates the necessity of thorough preparation of all regulatory documentation, including Informed Consent Forms and safety reporting procedures, which are crucial for the successful submission of IND applications. This meticulous verification process not only supports regulatory compliance but also enhances patient safety, illustrating what is an IND FDA and the importance of the FDA's role in the IND process.
Furthermore, we place significant emphasis on reporting study status and monitoring serious and non-serious adverse events to ensure compliance and maintain the integrity of clinical trials. For further insights, refer to the article published in Ther Innov Regul Sci, volume 51, issue 2, pages 246–249.
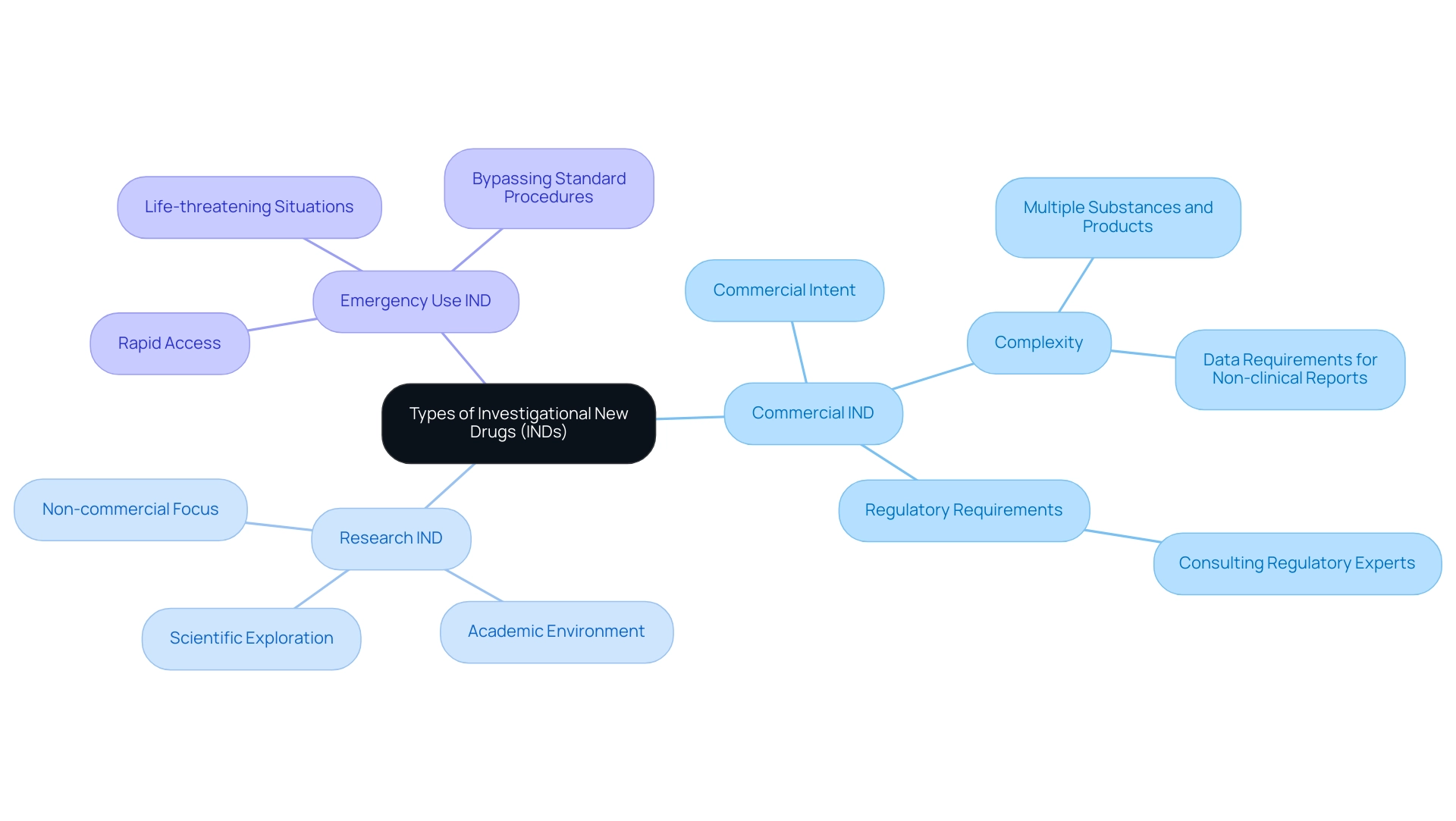
Types of INDs: Exploring the Different Pathways
There are three primary types of Investigational New Drugs (INDs), each designed to fulfill distinct regulatory requirements and objectives:
-
Commercial IND: This type is submitted by sponsors who intend to market the medication post-approval. Given its commercial intent, a Commercial IND encompasses extensive data requirements and is typically the most intricate of the three types.
Kent Amsberry, the Director of CMC at Cardinal Health Regulatory Sciences, observes,
Some items that may contribute to the complexity of an initial IND submission include multiple substances and products and data requirements for non-clinical reports.
This complexity necessitates precise planning and execution of the submission process to meet FDA standards. Consulting with regulatory experts familiar with FDA regulations can expedite IND preparation and submission, helping to navigate the intricate requirements effectively. -
Research IND: Primarily utilized for clinical investigations not aimed at commercial outcomes, Research INDs are often filed within academic environments. These applications focus on exploring the scientific aspects of medication efficacy and safety, allowing researchers to delve into innovative therapies without the pressures of immediate marketability. In 2024, statistics indicate that a significant percentage of IND filings are for research purposes, reflecting the ongoing commitment to scientific advancement.
-
Emergency Use IND: Designed to facilitate rapid access to investigational drugs during life-threatening situations, the Emergency Use IND allows healthcare providers to bypass standard IND procedures in order to expedite treatment when no viable alternatives exist. This pathway underscores the critical need for timely intervention in acute medical scenarios. Recent case studies highlight successful applications of Emergency Use INDs in treating patients during health crises, further emphasizing their importance. Understanding the distinctive purposes of each IND type is crucial for researchers, ensuring that their regulatory submissions align with their specific objectives and comply with the requisite standards.
Additionally, staying informed about the latest types of INDs and their regulatory pathways is essential for maintaining compliance and achieving successful outcomes.
Conclusion
The Investigational New Drug (IND) application process is a fundamental aspect of pharmaceutical development that ensures the safety and efficacy of new therapies before they reach human subjects. As detailed in the article, understanding the steps involved in filing an IND, including:
- Pre-IND consultations
- Data compilation
- The submission process
is crucial for sponsors navigating the regulatory landscape. Each step requires meticulous attention to detail, as the average timeframe for bringing a drug to market can span a decade or more.
Moreover, recognizing when an IND is required is essential, particularly in the context of new chemical entities and significant changes to existing drugs. While exemptions exist for certain clinical investigations and compassionate use cases, being well-versed in these criteria is vital for ensuring compliance and facilitating effective research planning. The role of the FDA in reviewing IND applications cannot be overstated; their rigorous evaluation process safeguards patient safety and upholds scientific integrity throughout clinical trials.
Finally, the distinction between the various types of INDs—commercial, research, and emergency use—highlights the diverse regulatory pathways available to sponsors. Each type serves a specific purpose and understanding these nuances is key to aligning regulatory submissions with project objectives. In summary, a comprehensive grasp of the IND process is indispensable for stakeholders aiming to successfully navigate the complexities of drug development and bring innovative therapies to market, thereby reinforcing the critical importance of thorough planning and compliance in this vital field.
Frequently Asked Questions
What is an Investigational New Drug (IND) application?
An IND application is a submission to the U.S. Food and Drug Administration (FDA) by sponsors who wish to begin trials with new pharmaceutical compounds. It is essential for testing innovative medications on human subjects, ensuring their safety and efficacy are evaluated.
What information is included in an IND application?
An IND application includes detailed information about the investigational drug, such as its chemical composition, preclinical research findings, and proposed trial protocols.
What are the key steps involved in filing an IND application?
The key steps include: 1. Pre-IND Consultation with the FDA 2. Compilation of comprehensive data 3. Documentation preparation, including completing Form FDA 1571 4. Electronic submission of the IND application 5. FDA review of the application.
Why is pre-IND consultation important?
Pre-IND consultation allows sponsors to initiate dialogue with the FDA early in the process, discussing development plans and receiving guidance tailored to their specific projects.
How is the IND application submitted?
The IND application must be submitted electronically via the FDA’s Electronic Submissions Gateway, which requires familiarity with electronic formatting and compliance standards.
What happens after the IND application is submitted?
After submission, the FDA conducts a review within 30 days. If there are no issues, the sponsor may begin research studies, but there are no guarantees, as unforeseen challenges may arise.
What services are offered to assist with the IND process?
Services include feasibility studies, selection of research sites and principal investigators, compliance review of study documents, study setup and approval processes, import permits, project management, and thorough reporting on study status and adverse events.
How long does it typically take to bring a medication to market?
The typical duration to introduce a medication to the market is projected to be no less than 10-15 years, highlighting the importance of the IND filing process.
What role does documentation play in the IND process?
Proper documentation is crucial for compliance with FDA regulations and includes preparing Informed Consent Forms and Investigator Brochures, which must be included in the IND application.
Why is attention to detail important during the IND filing phase?
Attention to detail at the IND filing phase can significantly influence the overall timeline for introducing a treatment to the market, as procedural accuracy is foundational for compliance and successful studies.

