Introduction
The Corrective and Preventive Action (CAPA) process is a critical framework in the medical device industry, ensuring that devices meet the highest standards of quality and safety. From initial device launch to ongoing improvement, CAPA plays a foundational role in the pursuit of excellence and patient safety. This comprehensive process involves identifying issues, conducting root cause analysis, developing corrective and preventive actions, and verifying their effectiveness.
CAPA is essential in preventing problem recurrence and fostering ongoing improvement in the diverse and complex field of medical devices. Adherence to stringent regulatory standards is crucial, with regulatory bodies categorizing devices based on risk. The CAPA system is indispensable for regulatory compliance and enhances consumer confidence while streamlining the approval process for medical devices.
By understanding the key components of the CAPA process and navigating the evolving regulatory landscape, manufacturers can drive continuous improvement and ensure the quality and safety of medical devices.
What is a CAPA Process?
The Corrective and Preventive Action (CAPA) process is integral to the life cycle of medical devices, playing a crucial role in ensuring their quality, safety, and effectiveness. This comprehensive process starts with identifying issues that may arise either during the manufacturing process or post-market release. A notable consideration during initial device launch is to prioritize what's critical without overburdening the system, as the true success of a product is often not fully known until it reaches the market. Effective CAPA systems, therefore, must be adaptable and scalable, capable of evolving with the device's lifecycle from initial release to full production ramp-up if market demand increases.
Root cause analysis is at the heart of CAPA, where identifying the underlying causes of problems helps to develop robust corrective and preventive actions. These steps are essential for preventing problem recurrence and fostering ongoing improvement, which is particularly critical in a field as diverse and complex as medical devices. The sector encompasses an array of products, from simple items like bandages to sophisticated systems like MRI machines and pacemakers. With over 10,000 types of medical devices recognized by the World Health Organization, the breadth of technologies includes not just biomedical engineering but extends to materials science, electronics, and information technology.
Ensuring conformity to stringent regulatory standards is a key component of CAPA. Regulatory bodies like the FDA categorize medical devices into classes based on risk, and the rigorous regulatory processes for high-risk devices, such as class three, underscore the importance of a solid CAPA process. These standards, developed by Standards Development Organizations (SDOs) based on consensus principles, ensure transparency and stakeholder involvement. As such, adherence to voluntary consensus standards is not only about regulatory compliance but also about enhancing the quality and efficiency of the healthcare industry.
Furthermore, the recent expansion of testing facilities, such as UL Solutions' laboratory in Michigan, demonstrates the industry's commitment to addressing potential risks including quality, safety, and cybersecurity concerns. This commitment is crucial for maintaining the pace of innovation while ensuring the protection of medical device users.
In summary, the CAPA process is a critical framework that ensures medical devices meet the highest standards of quality and safety. From the focused launch of new devices to the ongoing improvement of manufacturing practices, CAPA plays a foundational role in the medical device industry's pursuit of excellence and patient safety.
Importance of CAPA in Regulatory Compliance
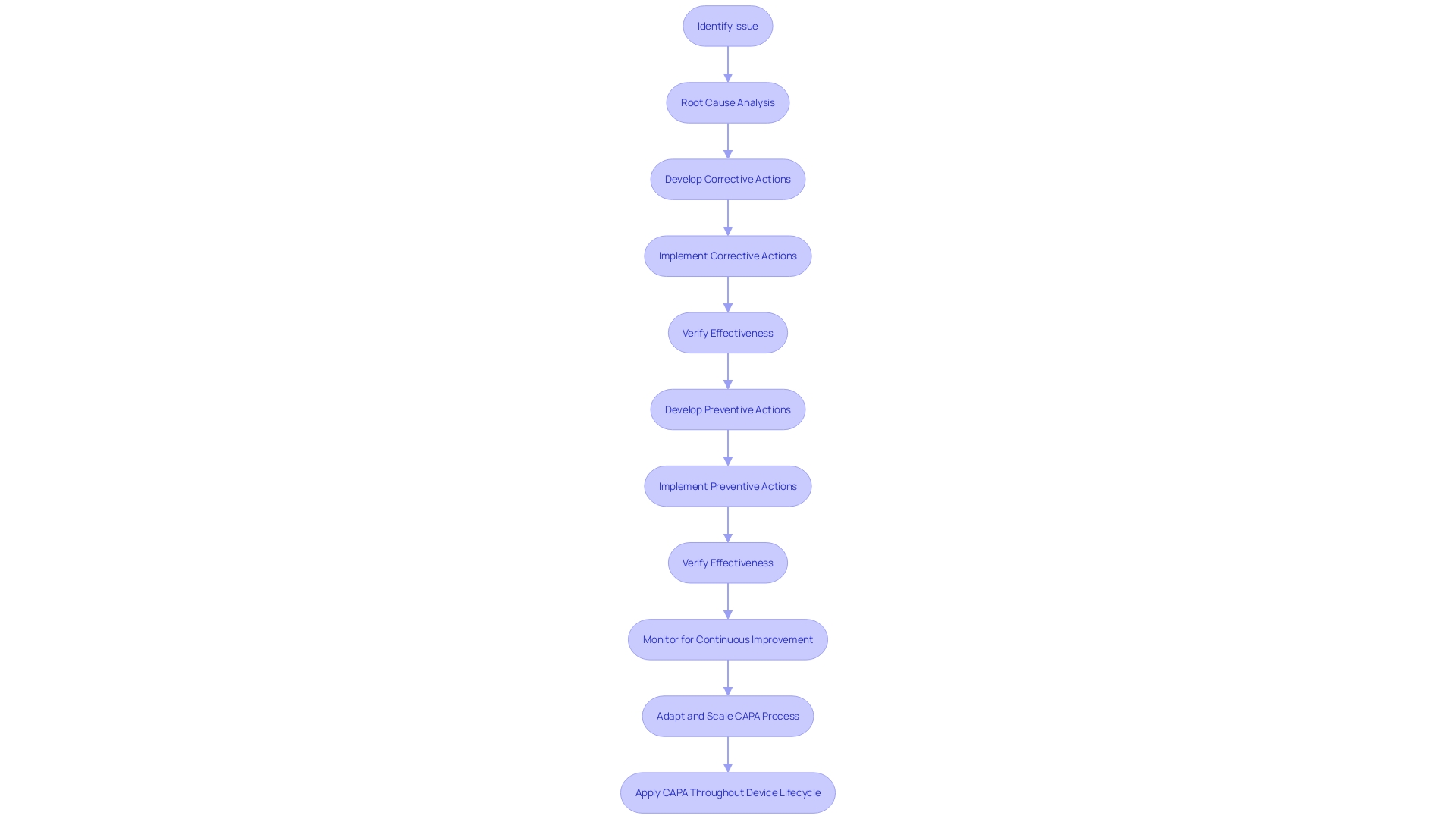
The Corrective and Preventive Action (CAPA) system is an indispensable element in the medical device industry, serving as a pivotal mechanism to meet stringent regulatory demands and ensure the integrity of medical devices. Regulatory agencies, notably the FDA, mandate that device manufacturers maintain a dynamic CAPA process. This is not merely a procedural formality; it is a critical practice that underscores a manufacturer's dedication to product quality and adherence to safety standards. A well-executed CAPA system not only enhances consumer confidence but also can streamline the approval process for medical device products.
Recent industry reports underscore the importance of preparedness in facing the evolving regulatory landscape. Professionals within the field have noted the necessity for a proactive approach in navigating the rise of regulatory requirements, which directly affects the development and market introduction of new medical devices. These challenges are met with a variety of strategies to ensure compliance while maintaining efficiency, particularly in the preparation of regulatory and safety documentation.
The FDA's classification of medical devices into three levels based on risk factors influences the complexity of the regulatory process. Class three devices, for instance, which are critical to life-sustaining or supporting functions, require a more comprehensive review due to their high-risk nature. The industry has responded to these challenges by advocating for more streamlined regulatory pathways and fostering enhanced collaboration between regulatory bodies and manufacturers.
Innovative initiatives, such as the recent expansion of testing facilities in Michigan, demonstrate the industry's commitment to advancing medical device safety and performance. These facilities enable rapid testing and adaptation to manufacturers' needs, highlighting how regulatory compliance is integral to product innovation and market readiness.
Furthermore, educational resources like the Regulatory Pathway Assessment Series provide regulatory professionals with valuable insights into strategic planning. These resources are crucial for understanding the nuances of regulatory frameworks and for making informed decisions about product development and market entry strategies.
In conclusion, as the medical device sector continues to grow, the CAPA system remains a central focus for manufacturers aiming to navigate regulatory complexities efficiently. The industry's preparedness, coupled with strategic collaborations and educational advancements, is pivotal in driving the continuous improvement of medical device quality and safety.
Key Components of a CAPA Process
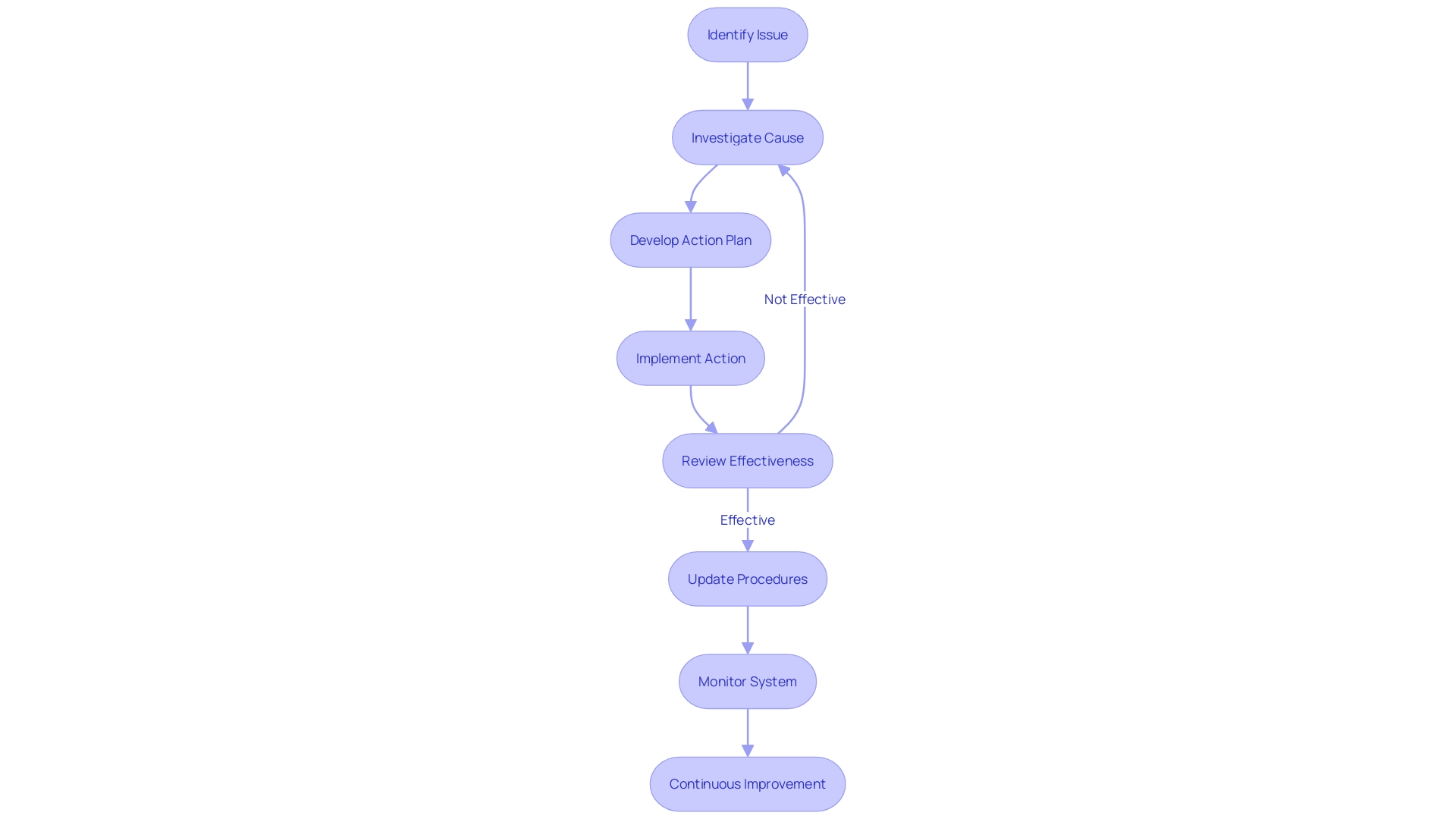
A robust Corrective and Preventive Action (CAPA) system in the medical device arena is critical to ensure the delivery of safe and effective devices. At its core, the CAPA process involves identifying and documenting issues, conducting root cause analysis, developing and implementing corrective and preventive actions, and verifying the effectiveness of these measures.
The initial step in this process often involves a meticulous assessment phase, as evidenced by practices within the NHS where the Digital Service Team evaluates new technology requests for security, appropriateness, and compliance. Such preemptive assessments are akin to the issue identification stage of CAPA, ensuring that any potential problems are acknowledged and documented early on.
Following this, a thorough root cause analysis is imperative. This mirrors the approach taken by the Digital Service Team, which employs questionnaires to understand the adopter's needs and justifications for new technology, paralleling the in-depth investigation required in a CAPA process.
The development and execution of corrective and preventive actions are akin to the 'digital-assurance process,' ensuring new technologies meet high standards before adoption. This proactive approach is essential in CAPA, as it aims to not only correct existing issues but also prevent their recurrence.
Lastly, CAPA's effectiveness is verified through rigorous testing, much like the UL Solutions' Rochester Hills laboratory that evaluates medical devices against various methods and specifications. Such verification is crucial to confirm that CAPA measures have successfully addressed the issues and that the product is safe for market release.
These CAPA components are integral to continuous improvement and align with industry observations that highlight the need for precise expectations and streamlined production processes, as medical devices range from simple to highly complex systems, impacting diverse human factors. With over 10,000 types of medical devices recognized by the World Health Organization, the CAPA process is not just a regulatory requirement but a fundamental practice to ensure the quality and safety of medical devices in a multifaceted and rapidly advancing field.
Step-by-Step CAPA Process
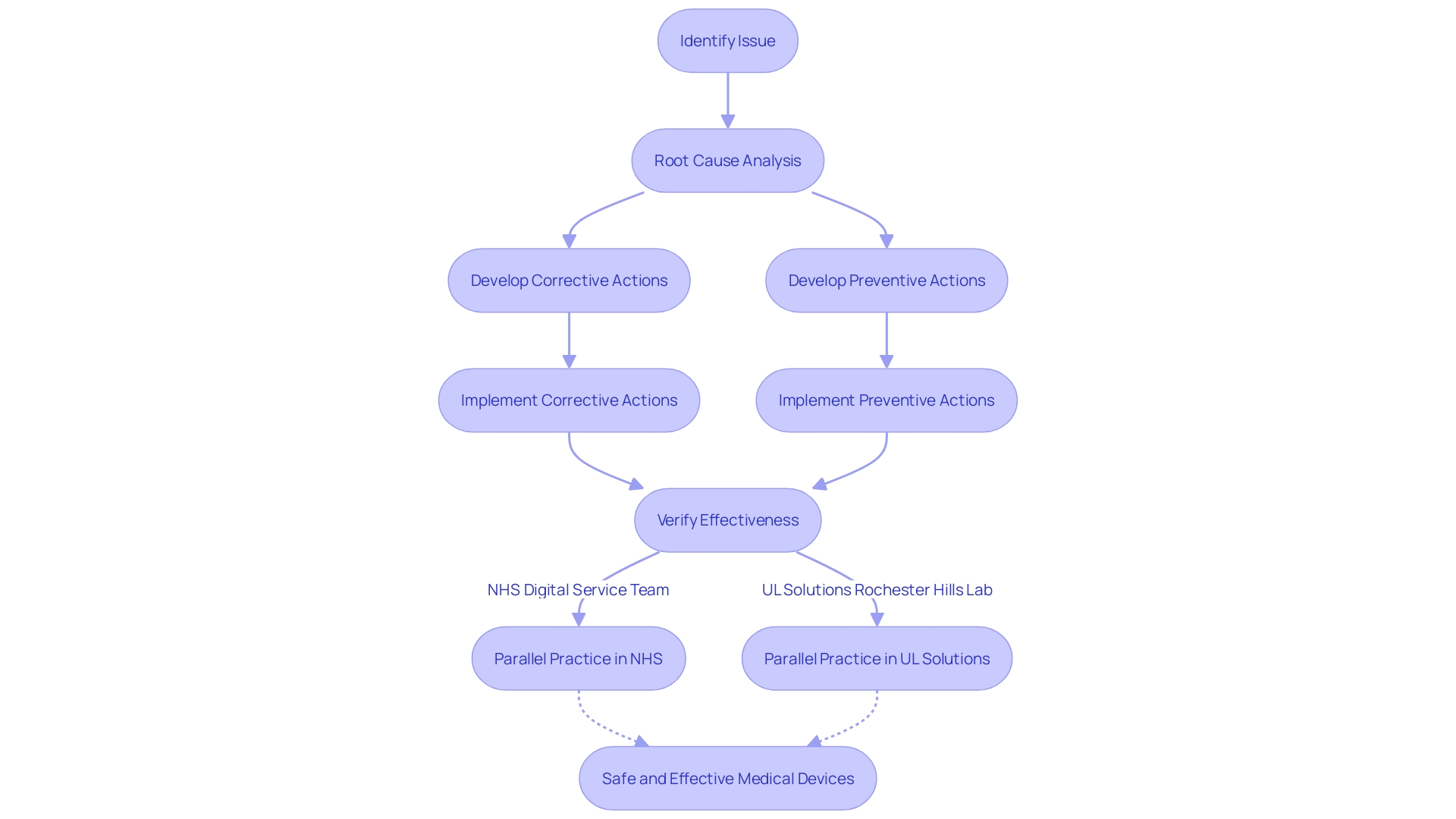
The CAPA process, integral to medical device systems and processes, is a robust, multi-step approach designed to address issues and foster continuous quality improvement. It starts with Identifying and Documenting Issues, a critical initial step where concerns or non-conformances are captured in detail. This sets the stage for the Root Cause Analysis (RCA), where the underlying reasons for the issues are meticulously investigated.
With causes identified, the next phase encompasses Developing and Implementing Corrective and Preventive Actions. This involves creating strategic plans to not only rectify the current problem but also prevent future occurrences. The final step is Verifying the Effectiveness of CAPA, ensuring the actions taken have successfully resolved the issues and are preventing recurrence.
Coupled with these steps, medical device development relies on adhering to stringent regulatory standards to ensure patient safety and product efficacy. The qualification process is governed by Good Manufacturing Practice (GMP) guidelines, with compliance to standards such as ISO 13485 and US FDA regulations. However, the complexity and diversity of medical devices, ranging from simple instruments like spectacles to advanced MRI machines, demand a comprehensive approach to CAPA.
According to the World Health Organization, over 10,000 types of medical devices are available, and the qualification of these devices can face challenges such as regulatory constraints and an overemphasis on compliance at the expense of value. Effective CAPA processes, therefore, require a balanced approach that addresses the multifaceted nature of medical devices and the regulatory environment.
In light of this, medical device manufacturers are encouraged to streamline development and manufacturing. For instance, reducing the number of parts needed in a device can significantly cut down on supply chain complexities, quality issues, and overall time and cost. As Mick Fry from Minnetronix Medical suggests, simplifying device design early on can greatly enhance production efficiency without compromising functionality.
The FDA categorizes medical devices into three classes, with class three devices such as pacemakers requiring the most rigorous processes due to their high-risk nature. Despite representing only 10% of medical devices regulated by the FDA, these high-risk devices have longer approval times due to their critical role in sustaining life.
Implementing a CAPA system within this regulatory framework, while challenging, is vital for ensuring the consistent quality and safety of medical devices. It's a necessary process that, when executed effectively, can lead to significant improvements in patient care and the successful launch of innovative medical products.
Conclusion
The CAPA process is critical in the medical device industry, ensuring devices meet high standards of quality and safety. It involves identifying issues, conducting root cause analysis, developing corrective and preventive actions, and verifying their effectiveness. Adherence to stringent regulatory standards is crucial for CAPA, as devices are categorized based on risk.
The CAPA system enhances consumer confidence, streamlines the approval process, and ensures regulatory compliance. Manufacturers need to understand the evolving regulatory landscape to drive continuous improvement. CAPA is pivotal for meeting regulatory demands, enhancing consumer confidence, and driving continuous improvement.
Manufacturers must navigate regulatory requirements and plan for compliance while maintaining efficiency. The key components of a robust CAPA process involve identifying and documenting issues, conducting root cause analysis, developing and implementing corrective and preventive actions, and verifying their effectiveness. These components are essential for continuous improvement and align with industry observations.
The step-by-step CAPA process starts with issue identification and documentation, followed by root cause analysis. It then involves developing and implementing corrective and preventive actions to rectify current problems and prevent future occurrences. The effectiveness of CAPA is verified through rigorous testing.
In conclusion, CAPA is integral to the medical device industry, ensuring the delivery of safe and effective devices. It is vital for regulatory compliance, enhancing consumer confidence, and driving continuous improvement. Manufacturers must navigate the evolving regulatory landscape and adhere to the key components of the CAPA process to ensure quality and safety in this rapidly advancing field.




