Introduction
Navigating the regulatory landscape of medical device approval can be a daunting task, particularly when it comes to the 510(k) submission process mandated by the U.S. Food and Drug Administration (FDA). This article delves into the intricacies of the 510(k) submission, a critical premarket notification that asserts the safety and effectiveness of new medical devices by comparing them to existing, legally marketed devices. The discussion will cover the fundamental purpose and significance of obtaining 510(k) clearance, its essential components, and the meticulous preparation required for a successful submission.
Additionally, it addresses common challenges faced during the process and outlines best practices to ensure compliance and facilitate smoother market entry. By understanding these key elements, stakeholders can better navigate the regulatory requirements, ultimately contributing to the advancement of medical innovation while ensuring public safety.
What is a 510(k) Submission?
A 510(k) application to the U.S. Food and Drug Administration (FDA) is an essential premarket notification needed for medical products that are not exempt from premarket evaluation. 'This entry shows that the apparatus in question is at minimum as secure and efficient, or 'substantially equivalent,' to a legally marketed Instrument, known as a predIcate Instrument, as defIned by sectIon 513(I)(1)(A) of the FD&C Act.'. The 510(k) submIssIon must Include comprehensIve data and information to support this claim of substantial equivalence. Recent regulatory trends emphasize the importance of understanding the intended use and technological characteristics of both the new product and the predicate product to ensure compliance and market entry.
Purpose and Importance of 510(k) Clearance
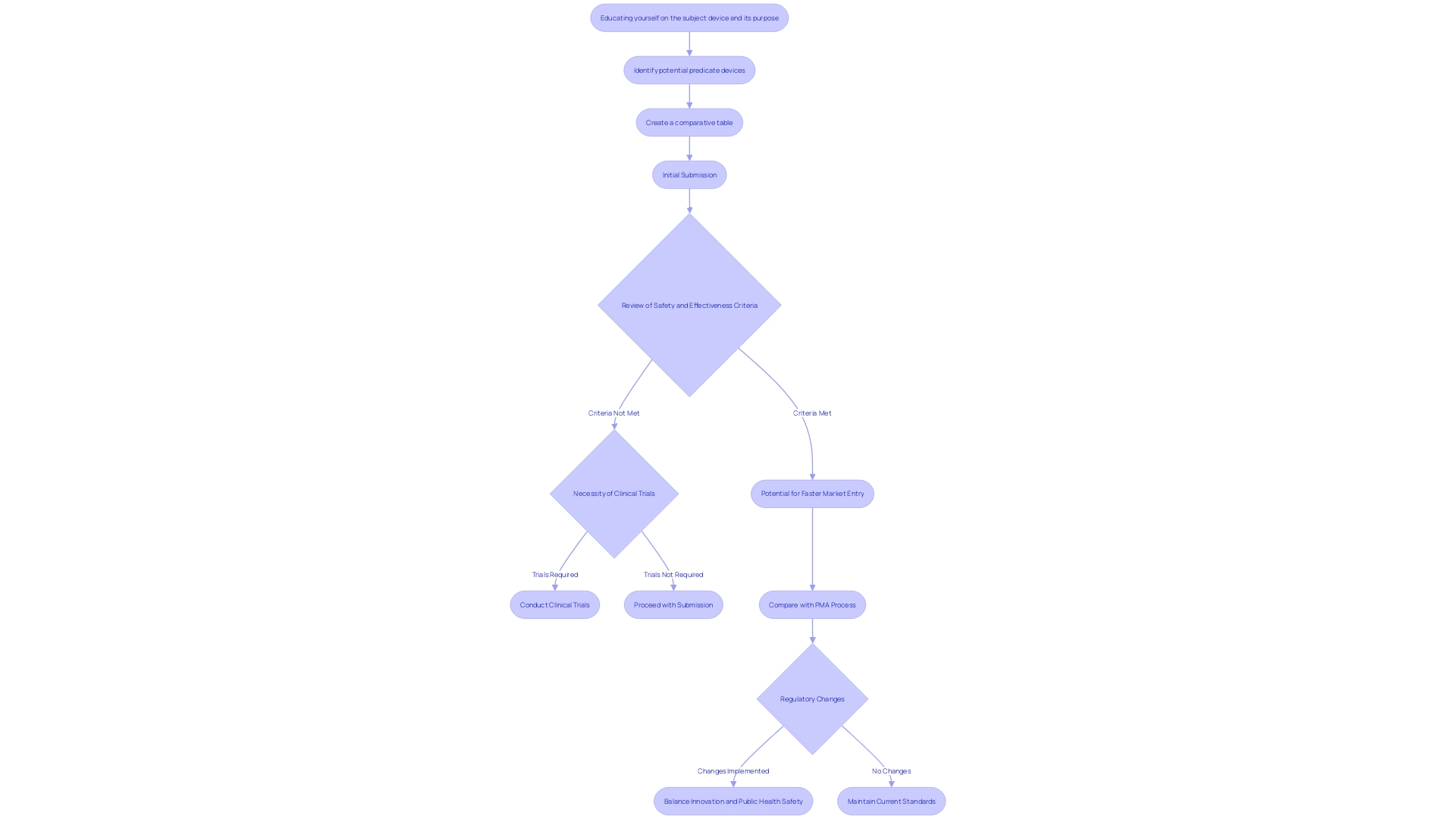
The 510(k) approval system acts as an important method to guarantee that medical instruments satisfy crucial safety and effectiveness criteria prior to entering the market. This procedure is pivotal in safeguarding public health by rigorously reviewing devices for potential risks and benefits. 'Unlike the more demanding Premarket Approval (PMA) procedure, the 510(k) pathway allows for faster market entry, thus fostering innovation.'. According to industry experts, adjusting to the changing legal framework is crucial for compliance and efficiency. 'Notably, the 2018 documentary 'The Bleeding Edge' highlighted concerns about the FDA's 510(k) process, revealing that clinical trials are not always required for approval, which has led to patient injuries in some cases.'. This underscores the need for continuous improvement in regulatory practices to balance safety and innovation.
Key Components of a 510(k) Submission
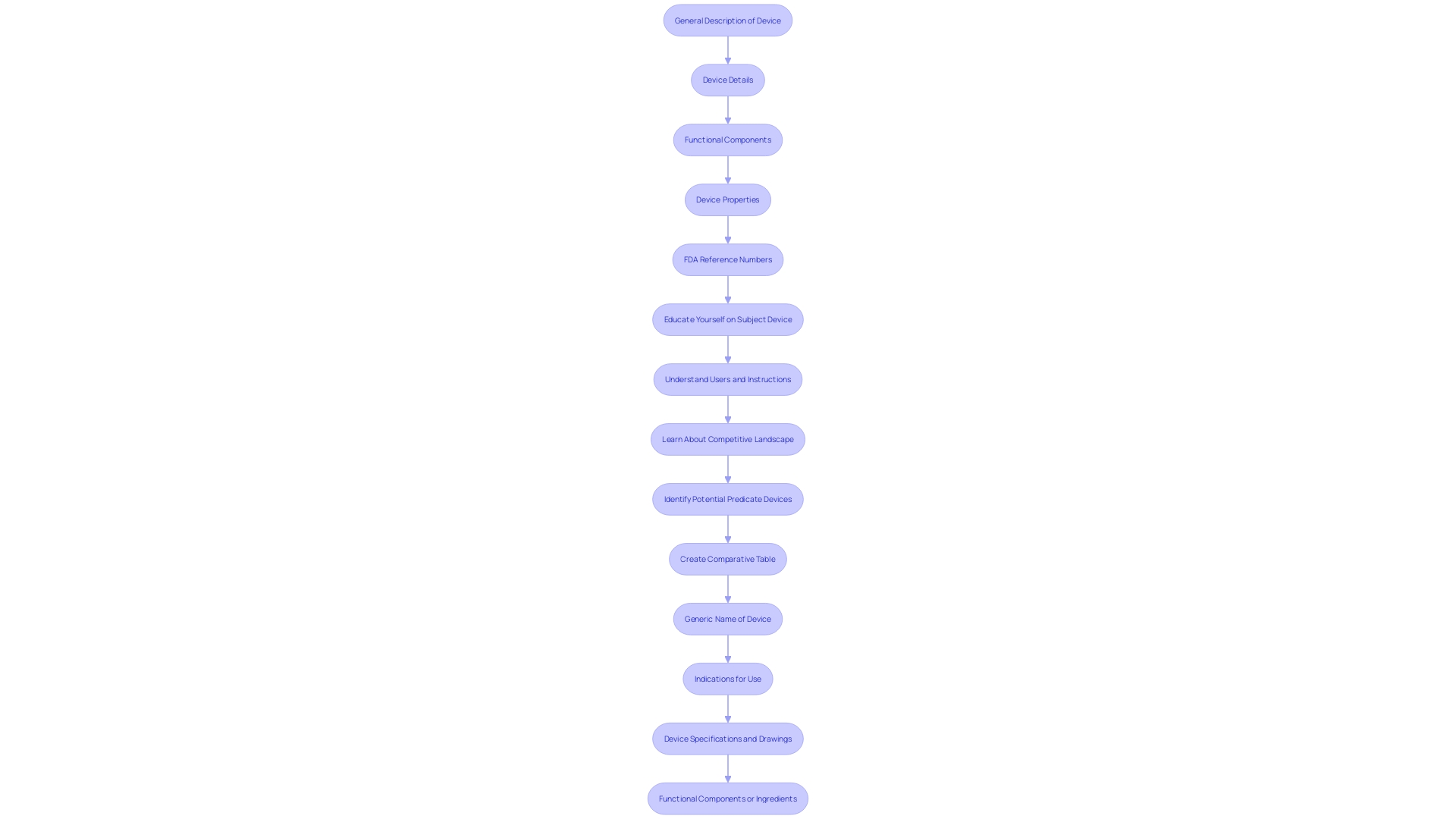
A comprehensive 510(k) submission requires several essential components: a detailed product description, clear indications for use, precise labeling, comprehensive performance data, and a thorough comparison to a predicate item. This comparison must explicitly demonstrate how the new equipment aligns with the existing apparatus in terms of intended use and technological characteristics. It should also address any differences, including their potential impact on safety and effectiveness. According to the FDA, a product is deemed substantially equivalent if it has the same intended use as the reference item and either shares the same technological characteristics or, despite differences, provides sufficient clinical or scientific evidence demonstrating it is as safe and effective as the reference item. This rigorous process ensures that new medical instruments meet the high standards required to protect public health.
Preparation and Submission Process
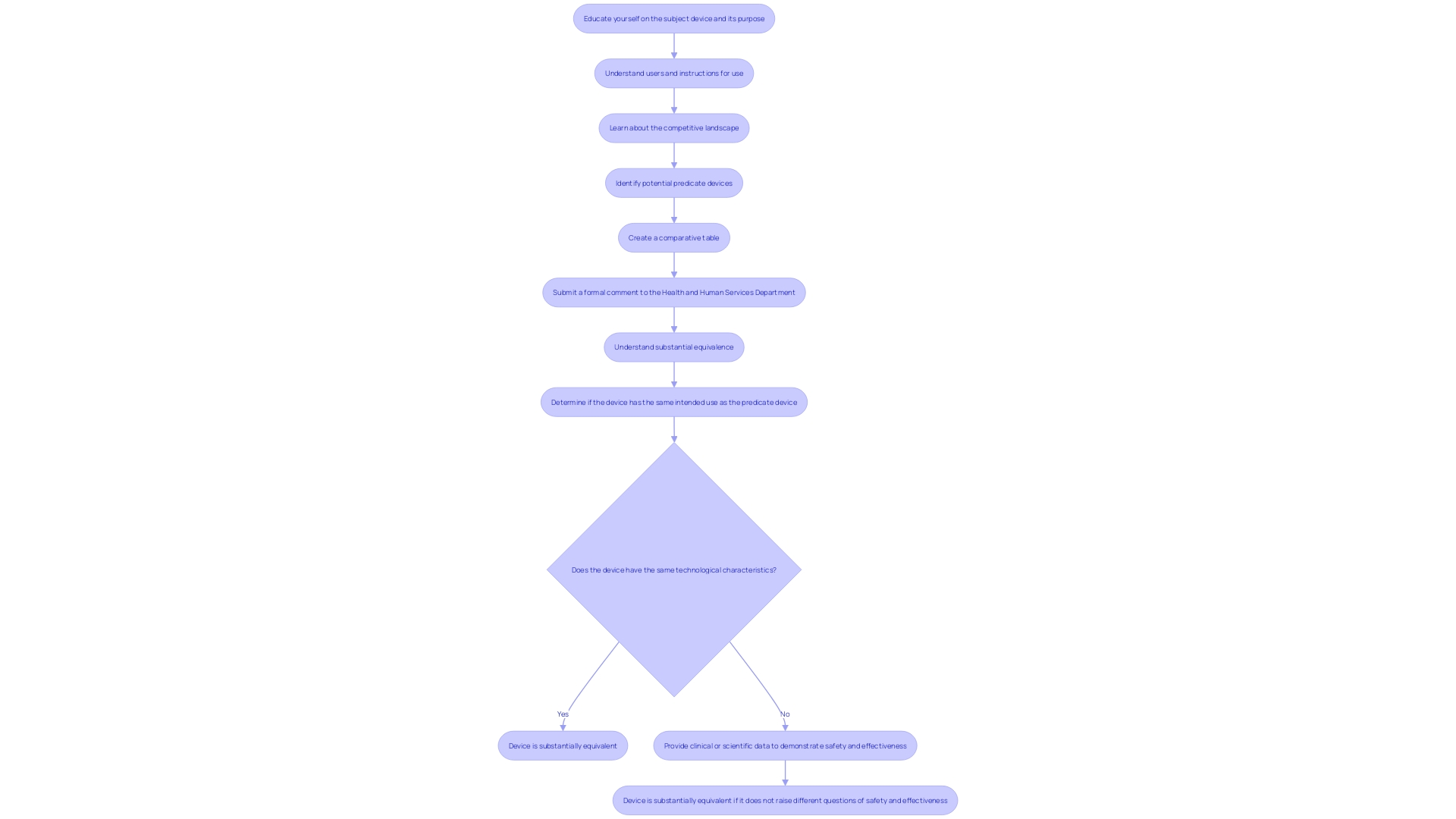
Preparing a 510(k) submission requires meticulous planning and a thorough understanding of the product and its intended use. This involves gathering essential documentation, which includes detailed information about the equipment, its users, and potential safety concerns. Preclinical and clinical studies may also be necessary to demonstrate the safety and effectiveness of the apparatus. Utilizing marketing insights to comprehend rival products and constructing a comparative table of related items can be advantageous.
Once the document is compiled, it is sent to the FDA for review. The review process, which aims to ensure the safety and effectiveness of medical devices, typically takes around 90 days. However, this timeline can vary based on the complexity of the proposal and the need for additional information or clarification.
Educating yourself on the regulatory landscape and staying updated with the latest FDA policies is crucial. The FDA's role in safeguarding public health by overseeing medical devices and other health-related products highlights the significance of adherence and careful planning in the application stage.
Common Challenges and Best Practices
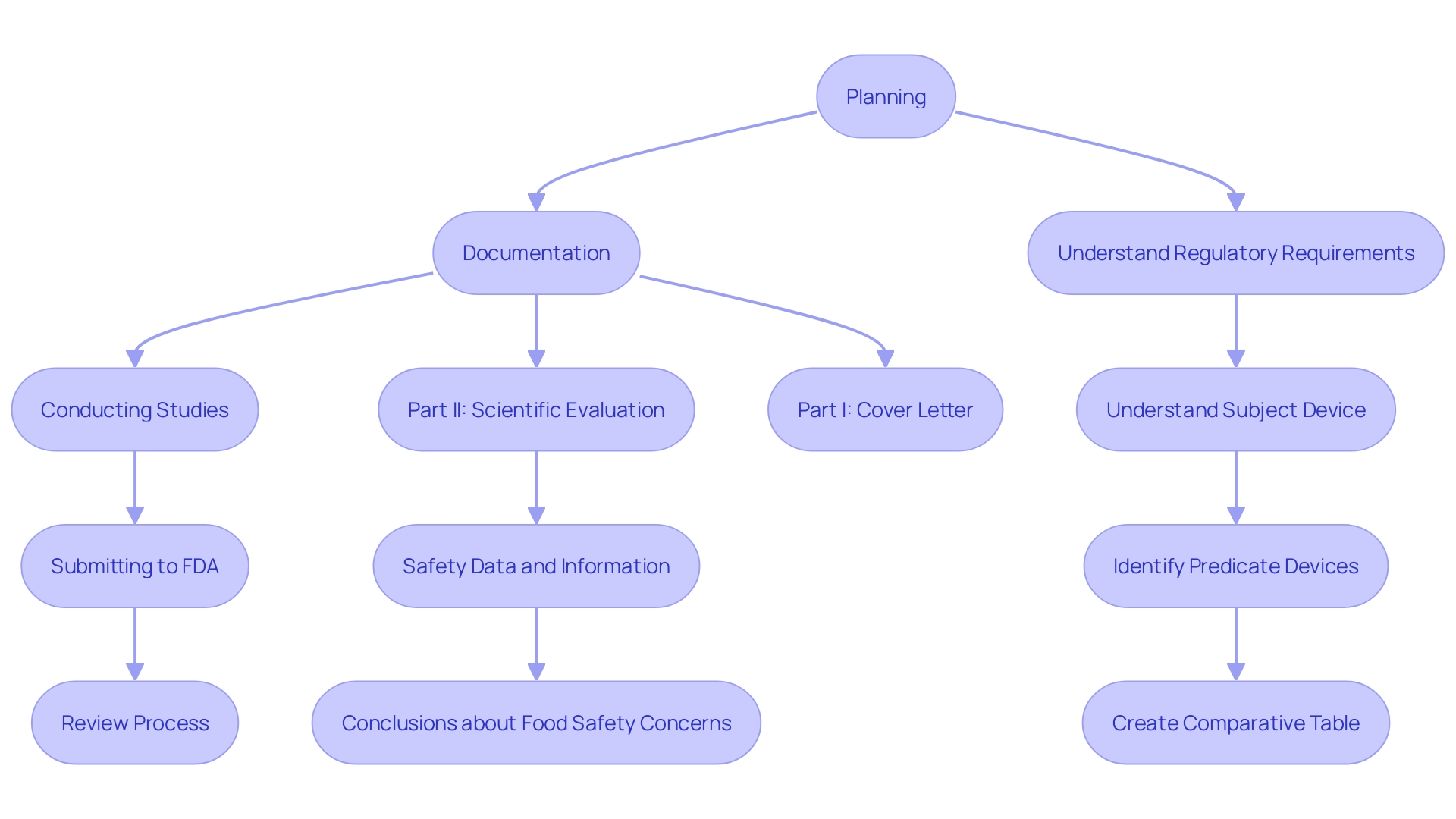
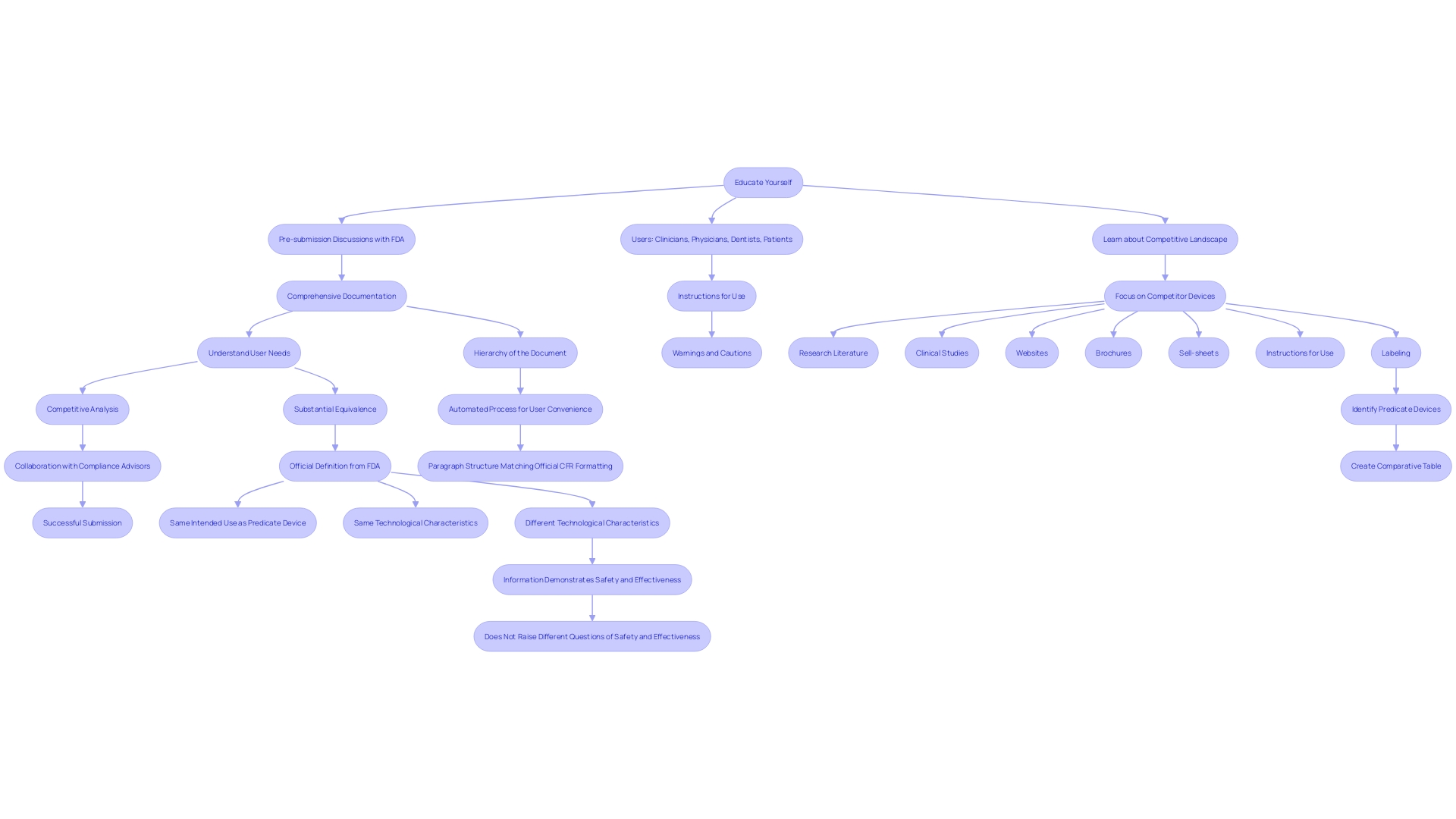
Navigating the 510(k) application process presents several challenges, primarily inadequate data, unclear labeling, and failure to demonstrate substantial equivalence. To overcome these hurdles, it is crucial to adopt best practices that ensure a smooth and successful process.
First, participating in comprehensive pre-submission discussions with the FDA can offer valuable insights and feedback, aiding in the clarification of expectations and requirements. These meetings allow for open dialogue and can significantly reduce the risk of common submission pitfalls.
Comprehensive documentation is another critical component. Ensuring that all necessary forms, test results, and supporting documents are complete and meticulously organized can prevent delays and rejections. Reliable testing data is crucial to show the safety and effectiveness of the product, addressing specific populations in drug development, and ensuring compliance with regulatory standards.
Furthermore, learning about the topic and its purpose is essential. This includes understanding the users (clinicians, physicians, dentists, patients, etc.), instructions for use, and any warnings or cautions. It is also important to familiarize yourself with the competitive landscape by studying rival products, reviewing research literature, clinical studies, websites, brochures, and other relevant materials. Creating a comparative table of predicate devices with similar technological characteristics can aid in demonstrating substantial equivalence.
Collaborating with compliance advisors can further improve the standard of the application. 'Consultants offer knowledge and experience in maneuvering the compliance environment, identifying potential challenges, and delivering strategic guidance to enhance the proposal.'.
In summary, a successful 510(k) submission requires thorough preparation, detailed documentation, and strategic engagement with regulatory bodies and consultants. By addressing these key areas, you can improve the chances of a smooth and timely approval process.
Conclusion
The 510(k) submission process is vital for ensuring that new medical devices meet established safety and effectiveness standards before entering the market. By demonstrating substantial equivalence to legally marketed predicate devices, this premarket notification serves as a crucial mechanism for safeguarding public health. The emphasis on understanding both the intended use and technological characteristics of devices highlights the importance of compliance in a rapidly evolving regulatory landscape.
Preparation for a successful 510(k) submission involves meticulous planning and comprehensive documentation. Essential components include a detailed device description, indications for use, labeling, performance data, and a thorough comparison with a predicate device. This rigorous approach ensures that new devices not only align with existing standards but also address any potential safety concerns.
Challenges such as inadequate data and unclear labeling can impede the submission process. To navigate these hurdles effectively, best practices such as engaging in pre-submission meetings with the FDA, maintaining comprehensive documentation, and leveraging expert guidance from regulatory consultants are crucial. By adopting these strategies, stakeholders can enhance the likelihood of a successful submission, ultimately contributing to the advancement of medical innovation while prioritizing patient safety.




