Introduction
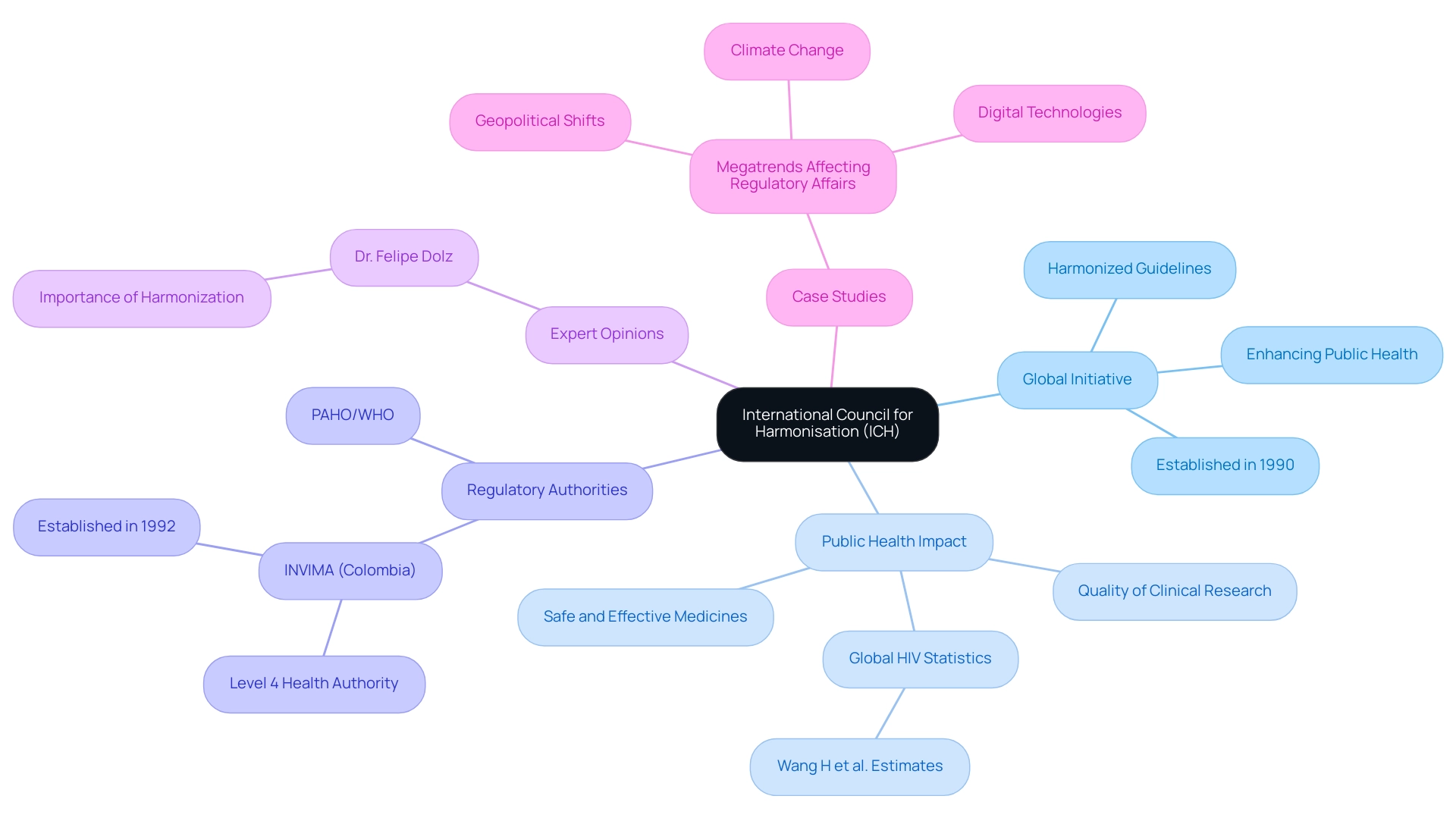
In the ever-evolving landscape of pharmaceutical development, the International Council for Harmonisation (ICH) stands as a cornerstone for regulatory excellence and public health enhancement. Established in 1990, this global initiative unites regulatory authorities and the pharmaceutical industry, aiming to streamline drug registration processes and ensure the delivery of safe, effective, and high-quality medicines.
The importance of ICH guidelines cannot be overstated, particularly in regions like Colombia, where local regulatory bodies such as INVIMA play a crucial role in overseeing compliance and safeguarding public health. As the complexities of global drug development continue to grow, understanding the framework established by the ICH becomes essential for stakeholders navigating the intricate interplay of regulatory requirements and scientific advancements.
This article delves into the pivotal role of ICH guidelines in clinical research, the challenges faced in their implementation, and the future trajectory of global clinical standards, highlighting the necessity for harmonization in an increasingly interconnected world.
Defining ICH: The International Council for Harmonisation
The International Council for Harmonization (ICH) functions as a crucial global initiative bringing together governing bodies and the pharmaceutical sector to tackle the scientific and technical aspects of drug registration. Established in 1990, the ich full form in medical refers to the organization's primary mission to enhance public health by ensuring that safe, effective, and high-quality medicines are developed and registered efficiently. By establishing harmonized guidelines across various regions, the ICH significantly streamlines the global development of pharmaceuticals, driving improvements in the quality of clinical research.
This is particularly relevant in Colombia, where INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) plays a vital role as the national oversight authority. Created in 1992, INVIMA is responsible for supervising health products and ensuring compliance with health standards. Its Directorate for Medical Devices monitors and controls medical devices, suggesting technical standards for their manufacturing, marketing, surveillance, and quality assurance.
This emphasizes its classification as a Level 4 health authority by PAHO/WHO, which underscores its competence in safeguarding public health and its ability to implement effective governance practices. As oversight professionals navigate an increasingly complex landscape influenced by global megatrends such as climate change and geopolitical shifts, understanding the ich full form in medical becomes crucial for stakeholders involved in drug development. This framework not only ensures compliance with international standards but also supports the evolving demands of global drug registration, particularly in light of recent initiatives such as the EMA's pilot project 'OPEN,' which emerged during the COVID-19 pandemic to facilitate international participation in scientific evaluations.
Dr. Felipe Dolz, Head of Global Science and Policy at Sanofi, emphasizes the importance of ICH, stating, 'The harmonization of standards is essential for improving the efficiency and effectiveness of drug registration processes.' Additionally, the statistics from Wang H et al. highlighting global HIV incidence, prevalence, and mortality from 1980 to 2015 emphasizes the essential role of harmonization in addressing urgent public health concerns.
Furthermore, the case study titled 'Megatrends Affecting Regulatory Affairs' illustrates how global megatrends are reshaping oversight practices, necessitating that professionals in this area adapt to these changes to enhance the overall efficiency of their operations. Katherine Ruiz, a specialist in Affairs for Medical Devices and In Vitro Diagnostics in Colombia, exemplifies the vital role of local expertise in navigating these complex compliance landscapes.
The Role of ICH Guidelines in Clinical Research and Good Clinical Practice
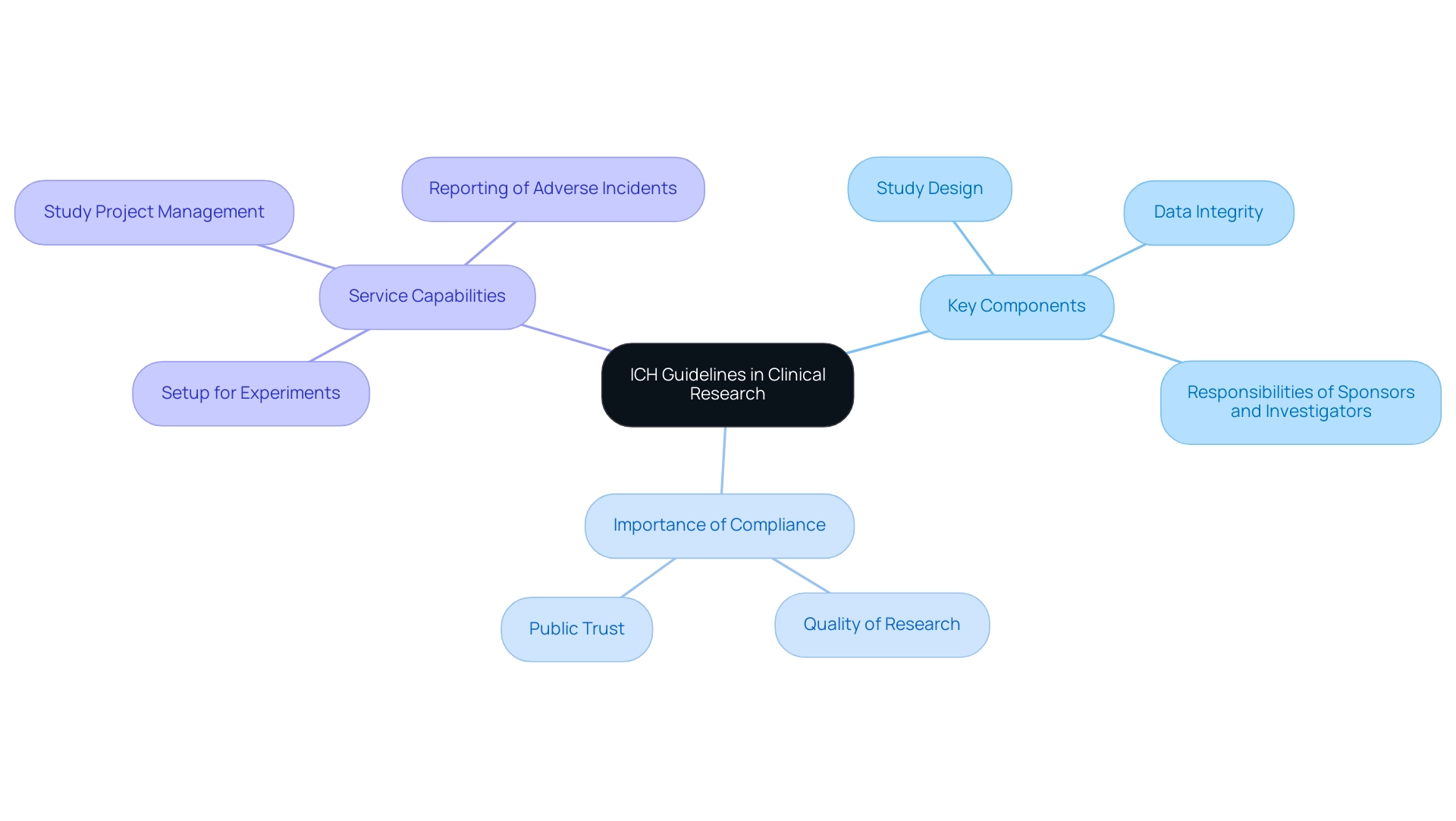
The ICH full form in medical refers to guidelines that act as a crucial framework for Good Practice in Research (GCP), setting an internationally acknowledged standard that regulates the ethical and scientific quality of studies. These guidelines are designed to protect the rights, safety, and well-being of participants while ensuring that the data generated are both credible and appropriate for submissions, such as those required by INVIMA, Colombia's National Food and Drug Surveillance Institute, recognized as a Level 4 health authority by PAHO/WHO. For instance, the research trial on immune activation of Bio-Germanium involved 130 human subjects, underscoring the importance of participant involvement in research.
Key components of the ICH guidelines encompass:
- Study design
- Data integrity
- The delineation of responsibilities for sponsors and investigators
Following these guidelines is not just a compliance requirement; it improves the quality of medical research, which is essential for advancing healthcare knowledge, including the ich full form in medical, and enhancing patient outcomes. Katherine Ruiz, a specialist in Affairs for Medical Devices and In Vitro Diagnostics in Colombia, asserts that clear guidance from approval organizations is key to driving change in the pharmaceutical industry.
By embedding the principles of the ICH full form in medical guidelines into their protocols, researchers foster a culture of quality and compliance, which is essential for gaining public trust and ensuring the efficacy of newly developed therapies. This dedication to compliance is further confirmed by the recognition from oversight organizations regarding the significance of conformity in research studies, as illustrated in the case study titled 'Adherence Interventions in Research Settings,' which highlights strategies to improve adherence and lessen variability in results. Furthermore, it is crucial to note that all written comments regarding compliance must include the document's docket number: FDA-1997-D-0508, emphasizing the ongoing dialogue between researchers and oversight bodies.
Furthermore, our service capabilities encompass:
- Setup for experiments
- Study project management
- Thorough reporting of both serious and non-serious adverse incidents
This ensures that all aspects of research management are aligned with ICH guidelines and regulatory expectations.
Key ICH Guidelines and Their Impact on Drug Development
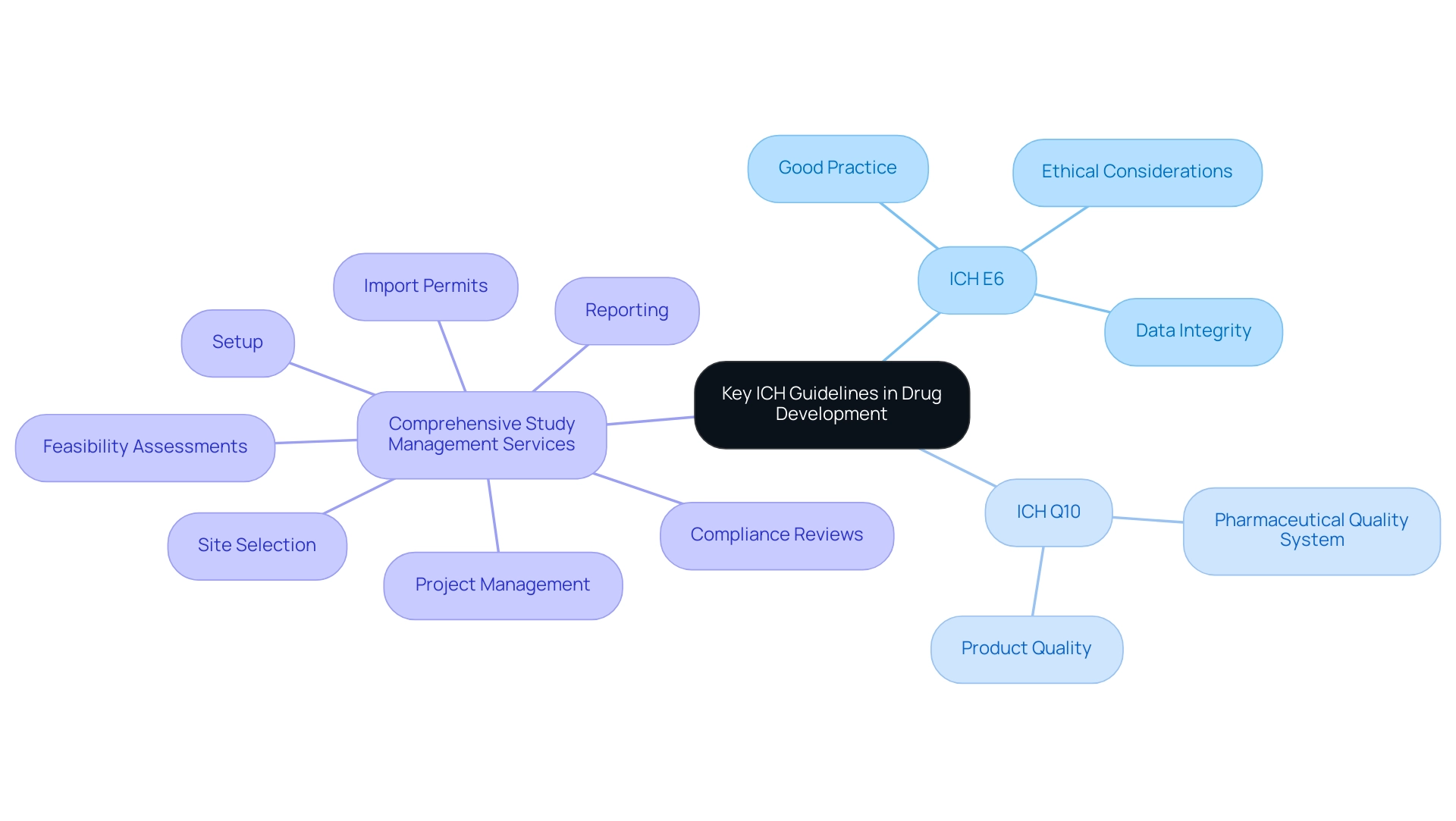
Key ICH guidelines, particularly ICH E6 and ICH Q10, are essential in shaping the environment of research and drug development, highlighting the ich full form in medical. The ich full form in medical refers to ICH E6, which establishes Good Practice in research, prioritizing ethical considerations in studies to ensure participant safety and the integrity of gathered data. This guideline is particularly vital in improving research ethics, as it offers a detailed framework that investigators must follow, which aligns with the ich full form in medical.
For instance, a data analyst from Switzerland highlighted the practical challenges of archiving Trial Master Files (TMFs), noting that the lack of accessible archives can complicate participant safety and data retrieval in cases of adverse events. Conversely, the ICH full form in medical refers to ICH Q10, which focuses on the pharmaceutical quality system, offering a structured approach to maintaining product quality throughout the drug lifecycle. The implementation of these guidelines, particularly the ich full form in medical, has markedly streamlined drug development processes, effectively reducing redundancies and enhancing the quality of research outputs.
Comprehensive study management services, including:
- feasibility assessments
- site selection
- compliance reviews
- setup
- import permits
- project management
- reporting
are essential for adherence to these guidelines. Specifically, the review and feedback on study documents ensure compliance with country requirements, while effective setup and monitoring facilitate smoother operations. A recent case study highlighted the clarity and usefulness of the ICH E6 GCP guidance, which participants found beneficial for training and as a globally accepted standard for research, illustrating the ich full form in medical.
Moreover, Jeppe Ragnar Andersen and Inger Byrjalsen have complete access to all trial information and are accountable for maintaining data integrity and accuracy, highlighting the significance of responsibility in research studies. The efficient management of these services not only advances medical research but also positively impacts local economies through job creation and healthcare improvement. As a result, the timelines for bringing new therapies to market have significantly shortened, providing patients with timely access to innovative treatments.
Recent feedback indicates a growing appreciation for the guidance on human subject protections and risk-based monitoring, highlighting the evolving standards in research that are informed by the pivotal ICH full form in medical guidelines.
Challenges in Implementing ICH Guidelines
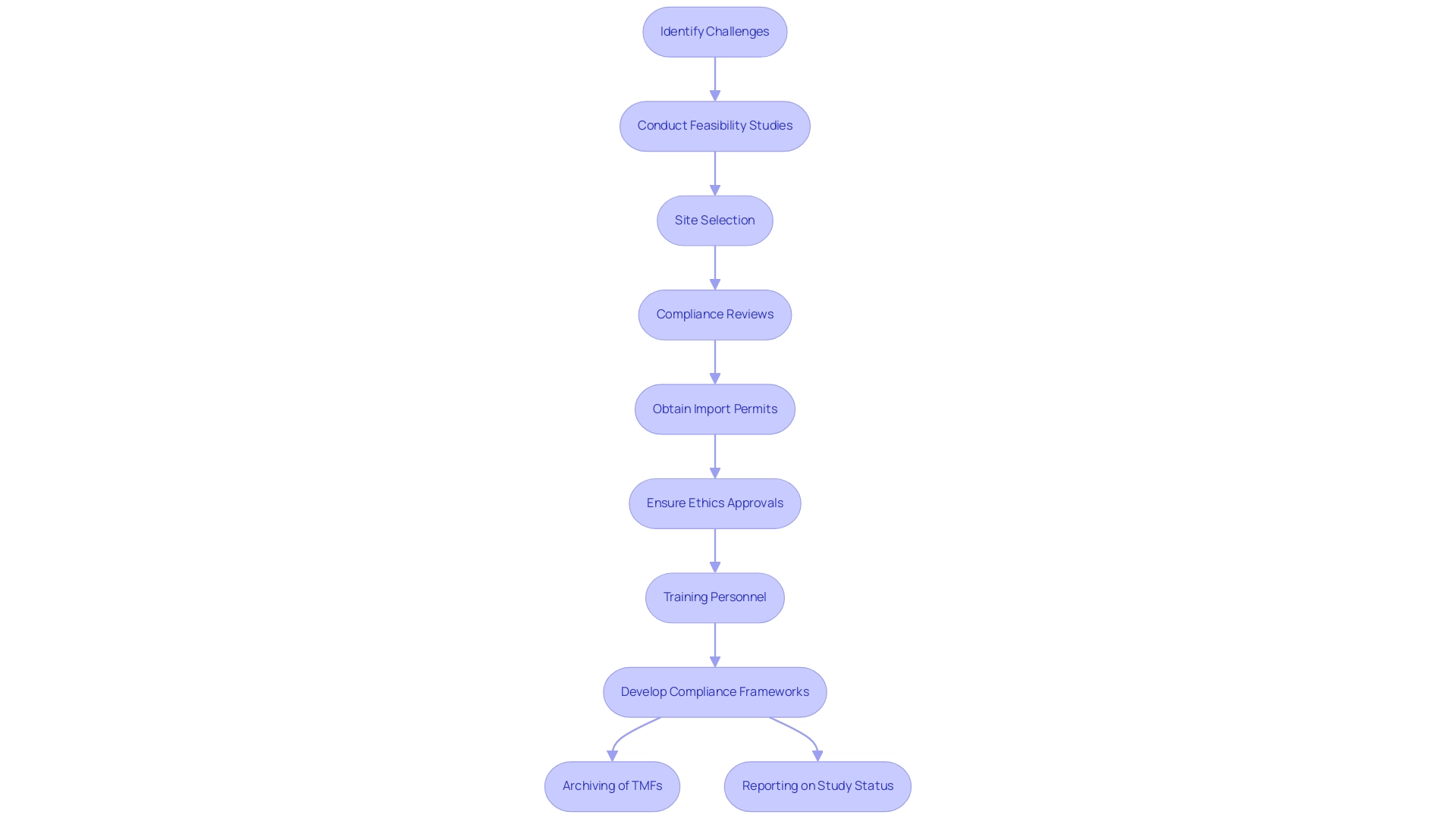
The implementation of the ICH full form in medical guidelines is crucial for enhancing the quality and efficiency of medical research, yet it often presents significant challenges for organizations, particularly in regions where local regulations diverge. Adapting existing processes to align with ICH standards requires meticulous attention to feasibility studies, site selection, and compliance reviews. Organizations must navigate the complexities of obtaining import permits and ensuring that trial setups comply with ethics committee and health ministry approvals.
As noted in a recent survey, while some stakeholders found the shift toward risk-based monitoring introduced in the (R2) revision to be clear and helpful, the overall adaptation remains daunting. Omega Statistics, known for its dedication to quality standards in research with an A+ rating from the Better Business Bureau, exemplifies the significance of strong compliance frameworks. Training personnel to thoroughly understand and adhere to these guidelines demands substantial resources.
Recent recommendations from Japanese stakeholders underscore the necessity for good clinical practice renovations, particularly through structured training programs to ensure compliance with ICH guidelines. For example, challenges in the archiving of master files (TMFs) highlight the problematic nature of documentation storage, especially when such documentation is situated far from the investigator's site, which can hinder accessibility and complicate responses to later adverse events. Furthermore, reporting on study status, inventory, and both serious and non-serious adverse events is essential for maintaining transparency and compliance throughout the research process.
To navigate these complexities, organizations should invest in comprehensive training initiatives and develop rigorous compliance frameworks that integrate the ICH full form in medical standards into their standard operating procedures. By nurturing a culture of continuous enhancement and quality assurance, organizations can effectively navigate the complexities of research while upholding compliance with ICH guidelines. Grasping the regulatory roles of INVIMA, Colombia's National Food and Drug Surveillance Institute, and utilizing the knowledge of experts such as Katherine Ruiz in regulatory matters can further improve adherence and operational success in research studies.
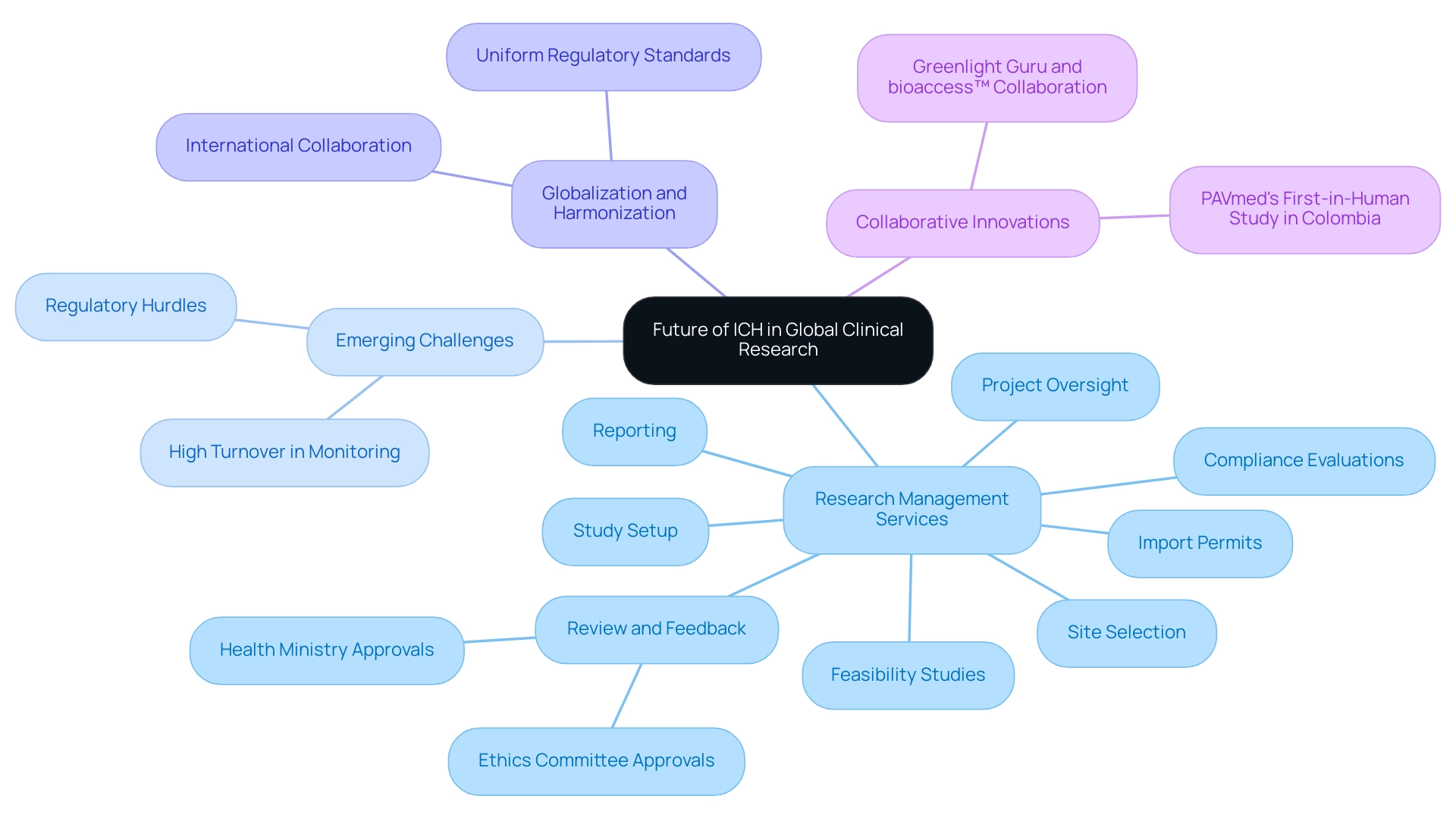
The Future of ICH and Its Role in Global Clinical Research
The landscape of medical research is undergoing significant transformation, with the ICH full form in medical playing a crucial role in establishing global standards. Our extensive research management services include:
- Feasibility studies
- Site selection
- Compliance evaluations
- Study setup
- Import permits
- Project oversight
- Reporting
- Review and feedback on study documents to meet country requirements, including ethics committee and health ministry approvals.
As digital health technologies and personalized medicine gain traction, the ICH full form in medical is anticipated to revise its guidelines to address the emerging challenges and opportunities in drug development.
A recent report from Applied Trials highlights that turnover for monitoring remains high, reflecting ongoing challenges within the industry, particularly for US Medtech companies facing regulatory hurdles and limited resources. For example, a workshop highlighted the importance of data in medical studies, as indicated in the 2021 Tufts report, which recorded a rise in intricate study designs generating greater amounts of data from various sources. This trend aligns with Timothy C. Hardman's assertion that, by 2050, 'if you are working in research studies, you will be a data scientist,' underscoring the importance of a data-centric approach in the field.
Moreover, the globalization of medical studies emphasizes the necessity for harmonization, particularly regarding the ICH full form in medical, to guarantee that regulatory standards are uniform across different jurisdictions. The impact of Medtech research studies on local economies, such as job creation and healthcare improvement, cannot be overstated, paving the way for international collaboration. By bridging gaps in clinical research and innovation, organizations can enhance their research capabilities and significantly contribute to advancements in medical science, as exemplified by the collaboration between Greenlight Guru and bioaccess™ to accelerate Medtech innovations and clinical trials in Latin America, particularly highlighted by PAVmed's first-in-human study in Colombia.
Conclusion
The International Council for Harmonisation (ICH) plays an indispensable role in the regulatory landscape of pharmaceutical development, particularly in fostering compliance and enhancing public health outcomes. As outlined in the article, ICH guidelines serve as a comprehensive framework for Good Clinical Practice (GCP), ensuring that clinical trials are conducted ethically and that the data generated are credible and reliable. This is especially relevant for stakeholders in Colombia, where local regulatory bodies such as INVIMA uphold these standards to safeguard public health.
Despite the clear benefits of ICH guidelines in streamlining drug development and improving the quality of clinical research, challenges remain in their implementation. Organizations must navigate complex local regulations while adapting their processes to align with ICH standards. Investing in robust training programs and compliance frameworks is essential for overcoming these hurdles and fostering a culture of quality assurance within clinical research.
Looking ahead, the future of ICH is poised for further evolution as the landscape of clinical research transforms with advancements in digital health technologies and personalized medicine. The need for harmonization in regulatory requirements across jurisdictions will become increasingly crucial as global collaboration in clinical trials expands. By embracing these changes and prioritizing adherence to ICH guidelines, stakeholders can significantly enhance their research capabilities, ultimately contributing to the development of safe and effective therapies that improve patient outcomes. The commitment to regulatory excellence will not only benefit individual organizations but also support the overarching goal of advancing public health on a global scale.
Frequently Asked Questions
What is the International Council for Harmonization (ICH)?
The ICH is a global initiative established in 1990 that brings together governing bodies and the pharmaceutical sector to address the scientific and technical aspects of drug registration, with the goal of enhancing public health through the development of safe, effective, and high-quality medicines.
What is the primary mission of the ICH?
The primary mission of the ICH is to enhance public health by ensuring that medicines are developed and registered efficiently through the establishment of harmonized guidelines across various regions.
How does the ICH impact pharmaceutical development?
By establishing harmonized guidelines, the ICH significantly streamlines the global development of pharmaceuticals and drives improvements in the quality of clinical research.
What role does INVIMA play in Colombia?
INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos) is Colombia's national oversight authority responsible for supervising health products and ensuring compliance with health standards.
What is INVIMA's classification by PAHO/WHO?
INVIMA is classified as a Level 4 health authority by PAHO/WHO, highlighting its competence in safeguarding public health and implementing effective governance practices.
Why is understanding the ICH important for stakeholders in drug development?
Understanding the ICH is crucial for stakeholders as it ensures compliance with international standards and supports the evolving demands of global drug registration.
What recent initiative emerged during the COVID-19 pandemic related to ICH?
The EMA's pilot project 'OPEN' was initiated during the COVID-19 pandemic to facilitate international participation in scientific evaluations.
What are the key components of the ICH guidelines?
The key components of the ICH guidelines include study design, data integrity, and the delineation of responsibilities for sponsors and investigators.
How do ICH guidelines improve medical research?
Following ICH guidelines improves the quality of medical research, which is essential for advancing healthcare knowledge and enhancing patient outcomes.
What are some service capabilities related to ICH guidelines in research?
Service capabilities include setup for experiments, study project management, and thorough reporting of both serious and non-serious adverse incidents, ensuring alignment with ICH guidelines and regulatory expectations.




