Introduction
The International Council for Harmonisation (ICH) has played a pivotal role in standardizing pharmaceutical regulations globally since its inception in the early 1990s. With a mission to simplify drug development and broaden access to safe and effective medicines, ICH has united regulatory bodies and industry representatives from major global markets. What began as a collaboration between the United States, Europe, and Japan has now expanded to include Canada, Switzerland, and other regions.
This expansion reflects ICH's commitment to public health and its aim to bridge the gap between different regulatory systems. Through initiatives like the proposed ICH M14 guideline, which seeks to standardize post-approval studies using real-world data, ICH continues to facilitate a more efficient review process for pharmaceuticals. Furthermore, the collective efforts of ICH and its members have led to significant advancements in healthcare, including the International Statistical Classification of Diseases and Related Health Problems (ICD), which has become an essential tool for healthcare data recording and disease statistics.
As ICH's work evolves, it remains dedicated to promoting accurate and detailed information to the target audience in a formal and professional manner.
History and Evolution of ICH
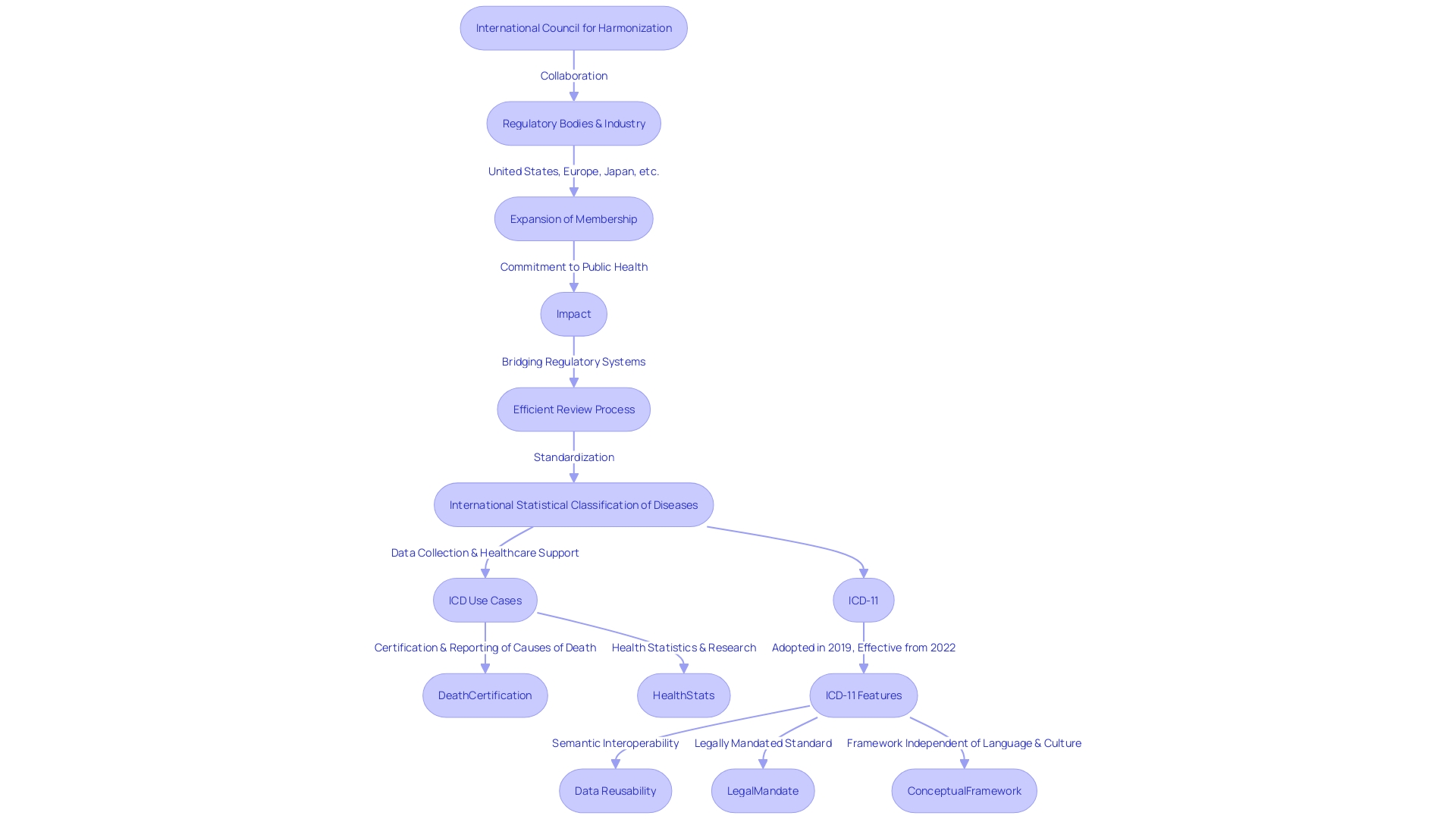
The International Council for Harmonization (ICH)'s inception in the early 1990s marked a pivotal moment in the standardization of pharmaceutical regulations across major global markets. Initially, the collaborative effort involved regulatory bodies and industry representatives from the United States, Europe, and Japan with a shared vision of simplifying the drug development process and broadening the access to medicines that are both efficacious and safe. This was in response to the growing need for a unified approach to addressing the diverse and often complex regulatory requirements that were a hurdle to drug availability worldwide.
As ICH's influence grew, its membership extended beyond its original founding regions, embracing regulatory authorities from Canada, Switzerland, and others, thereby enhancing its global footprint and reinforcing its mission. The expansion reflects Ich's commitment to public health and aligns with similar initiatives, such as the International Regulatory Cooperation for Herbal Medicines (IRCH), which was established to safeguard public health through the regulation of herbal medicines.
With regulatory harmonization as a core objective, ICH has been instrumental in bridging the gap between different regulatory systems, facilitating a more efficient review process for pharmaceuticals. This is underscored by recent developments like the proposed ICH M14 guideline, which seeks to standardize post-approval studies using real-world data to optimize pharmacovigilance practices. The growing availability of this data underscores the need for such harmonized guidance to alleviate inefficiency for both sponsors and regulatory bodies.
The collective efforts of ICH and its members have led to significant advancements in the field, including the International Statistical Classification of Diseases and Related Health Problems (ICD), which has become an essential tool for healthcare data recording and disease statistics. The ICD's diagnostic guidance standardizes data collection, thereby enabling large-scale research and supporting various healthcare system functions, from payment systems to service planning and quality management.
The ICH's historical progression and its ongoing initiatives reflect a broader movement within the pharmaceutical industry towards a more cooperative and unified approach to drug regulation, ultimately aimed at improving public health outcomes across the globe.
Objectives and Mission of ICH
The International Council for Harmonization (ICH) is dedicated to promoting public health by ensuring that safe, effective, and high-quality medicines are available globally. The primary goal of ICH is the harmonization of guidelines and standards to streamline the development of new pharmaceuticals, thus expediting their availability while upholding quality, safety, and efficacy. A cornerstone of Ich's mission is the alignment of regulatory requirements that must be met before new medical devices and wellness technologies can enter the market.
Harmonization is also critical in the post-approval phase, as evidenced by the ICH M14 initiative, which is developing guidelines for post-approval non-interventional studies using real-world data. The absence of internationally harmonized guidance in this area has previously led to inefficiencies for both sponsors and regulatory bodies. The anticipated release of a new multidisciplinary guideline in June 2025 aims to address these challenges and facilitate the use of epidemiological studies in pharmacovigilance and safety evaluation.
In line with harmonization efforts, the Global Substance Registration System (GSRS) supports the documentation of substances found in medicines by providing a universal identifier for each substance. This approach aligns with the ISO 11238 standard and supports a consistent definition of substances globally, including those under clinical investigation.
Furthermore, the importance of balancing innovation with patient safety is a prevailing theme in regulatory guidelines for clinical research. As the utilization of artificial intelligence (AI) and machine learning (ML) technologies grows, regulatory bodies such as the FDA, EU, and EMA have proposed guidelines to ensure these innovations are implemented safely. The proposed EU AI Act, for example, takes a risk-based approach and demands transparency from AI providers.
The ICH's commitment to harmonization extends beyond new technologies to include the protection of human subjects in clinical research, aligning with the U.S. Department of Health and Human Service (HHS) Common Rule. This commitment ensures that clinical research remains efficient without compromising participant rights, a principle that has been upheld since the inception of the Harmonized Tripartite Guideline for Good Clinical Practice in 1996. These guidelines have served as a foundation for maintaining the credibility and reliability of data in clinical trials, a standard that continues to be vital amidst technological advancements and evolving regulatory frameworks.
Structure and Governance of ICH
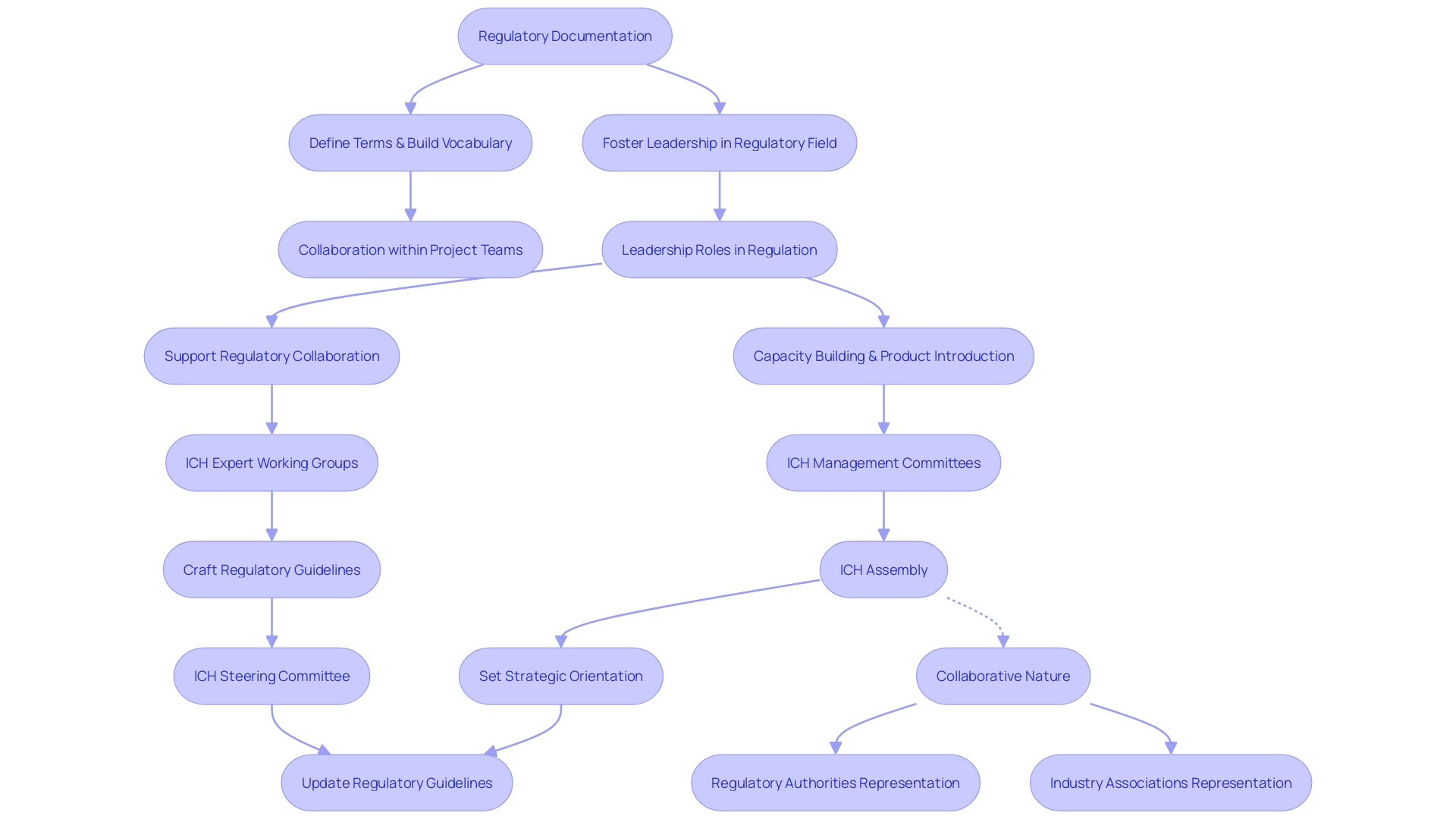
The International Council for Harmonization (ICH) is an organization dedicated to achieving greater harmonization worldwide to ensure that safe, effective, and high-quality medicines are developed and registered in the most resource-efficient manner. At its core, ICH is sustained by a structured and robust governance system, comprising expert working groups and management committees that are responsible for the crafting and updating of regulatory guidelines for drug development.
At the pinnacle of this structure is the Assembly, which consists of regulatory authorities and industry representatives from the member countries. This pivotal body sets the strategic orientation and gives the final nod to the guidelines that will steer pharmaceutical research and development.
Supporting this overarching entity is the ICH Steering Committee, which is tasked with the crucial role of ensuring the smooth coordination and management of ICH activities. This committee is a composite of representatives from regulatory agencies and industry associations, reflecting the collaborative spirit that underpins the ICH.
This collaborative approach echoes the structure seen in other successful governance models, such as the Internet Architecture Board (IAB), which brings together experts to guide the technical evolution of the Internet without imposing strict controls. Similarly, Ich's governance structure aims to foster an environment where diverse stakeholders can work together towards common goals in the pharmaceutical landscape.
ICH's work, while technical and complex, has profound implications not only for the healthcare industry but for global public health at large. It is instrumental in ensuring that the pharmaceutical industry can adapt to the challenges of the modern world, including those highlighted in the recent technological revolution. The technical and regulatory standards set by ICH facilitate the development of medicines that can withstand the scrutiny of various health systems and are consistent with the global push for innovation and sustainability.
Moreover, as we've seen with the adoption of the International Statistical Classification of Diseases and Related Health Problems (ICD-11), such standards are not only about compliance but also about enabling research, informing healthcare policies, and ultimately improving patient outcomes on a global scale. The Ich's regulatory guidelines, akin to the ICD's diagnostic guidance, aim to create a standardized approach to drug development that can be applied universally, fostering international cooperation and trust in pharmaceuticals.
Process of Harmonization
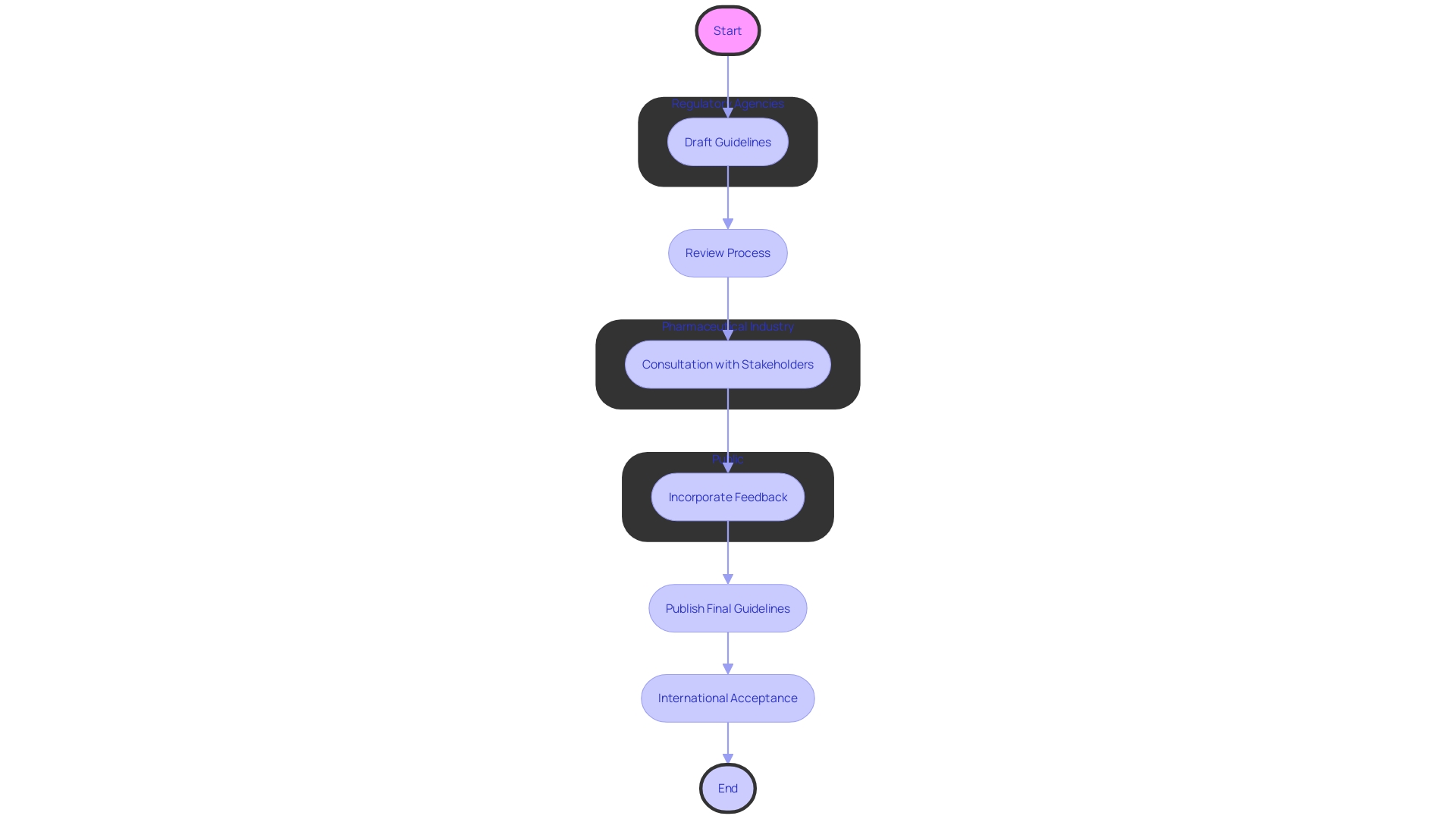
The International Council for Harmonization (ICH) embraces a concerted effort to establish guidelines that resonate globally. Teams of experts from regulatory agencies and the pharmaceutical industry collaborate in working groups to create draft guidelines, which are then meticulously reviewed through a comprehensive consultation process. This process involves input from various stakeholders, including regulatory bodies, industry players, and the public, ensuring the final guidelines are robust and internationally accepted.
The harmonization process has been instrumental in addressing significant challenges in healthcare, such as the need for consistent data collection methods across different regions. For instance, the identification and definition of core data elements (CDEs) for assessing medication safety during pregnancy exemplify the collaborative efforts to standardize data, which is crucial for expediting consensus on medication safety.
A testament to this collaborative spirit is the Innovative Medicines Initiative (IMI) and its successor, the Innovative Health Initiative (IHI), which have catalyzed partnerships in health research to enhance patient care and accelerate medical science. These initiatives underscore the vital role of sharing health data and standardizing data collection for precompetitive collaboration.
Recent developments in guidelines, such as the inclusion of a data governance section in the European good clinical practice (GCP) guidance, reflect the evolving landscape of clinical trials. This update, expected to be implemented by August/September 2024, highlights the significance of patient safety, the evaluation of trial objectives, and the utilization of technology to improve trial design and processes.
The collaborative nature of the ICH's harmonization process is not only about setting standards but also about fostering an environment that upholds the rights and safety of trial participants while ensuring the reliability of trial results. This underscores the collective responsibility of all stakeholders in maintaining an enabling environment for research and innovation while adhering to international human rights standards.
Key Areas of Harmonization
The International Council for Harmonization (ICH) plays a crucial role in the pharmaceutical industry by providing a standardized approach across different regions, focusing on improving the quality, safety, efficacy, and regulatory aspects of pharmaceutical products. The harmonization efforts are structured into distinct series, each targeting a core area of drug development and management. The ICH Q series concentrates on the quality of pharmaceutical products, establishing guidelines for ensuring the production of high-quality medications. The ICH S series addresses the safety protocols during the drug development process, mitigating risks and protecting patient health. Efficacy is the focus of the ICH E series, which provides a framework for the clinical evaluation of medicines, ensuring they deliver their intended benefits. Lastly, the ICH M series delves into the multidisciplinary aspects of pharmaceutical regulation, covering a broad spectrum of regulatory issues and ensuring that all facets of drug approval and post-approval processes are aligned.
These guidelines not only strive to enhance health outcomes and consistency of care but also aim to reduce uncertainty for clinicians by serving as a reference point for delivering high-quality medical care. However, despite the best intentions, guidelines can sometimes fall short in practice, not fully achieving their aims for better patient care due to various challenges. For instance, the complexity and scope of some guidelines can be daunting, requiring significant time and investment to develop and implement new technologies and systems that meet the expectations set forth. This is exemplified by the challenges faced in the implementation of new EMA guidelines within a six-month timeframe, which has proven to be a complex task for the industry.
Moreover, the inclusion of a data governance section in the European GCP guidance is a testament to the evolving nature of clinical guideline development, underscoring the importance of designing clinical trials that prioritize the safety and wellbeing of participants while ensuring the reliability of results. This recent update to the ICH E6 GCP (revision three) guidelines, expected to be implemented in August/September 2024, highlights the need for a meticulous evaluation of the scientific objectives and associated risks of clinical trials, focusing on critical activities.
The ICH's efforts are supported by global health organizations like the International Federation of Pharmaceutical Manufacturers and Associations (IFPMA) and the International Medical Device Regulators Forum (IMDRF), which foster international regulatory cooperation to expedite patient access to new medicines and medical devices. As the pharmaceutical landscape continues to evolve, the harmonization of guidelines remains pivotal in facilitating the global availability of safe and effective medical products.
Guidelines and Technical Requirements
The International Council for Harmonization (ICH) guidelines serve as a pivotal set of standards for the pharmaceutical industry, encompassing a wide range of processes from the development stage to post-approval surveillance of medical products. Specifically, the guidelines address critical areas such as quality control, clinical trial methodologies, safety evaluations, and the stability of pharmaceuticals to ensure the consistent production of high-quality medicines.
Taking into account recent developments, a significant step forward has been made in the context of pregnant women's health—a demographic often sidelined in clinical trials. The emergence of a comprehensive framework of Core Data Elements (CDEs), developed through a scoping review and expert consultation, aims to standardize data collection across different countries. This framework, which includes 98 datasets across 14 tables, is vital for assessing medication safety during pregnancy. Notably, 63 datasets have been deemed crucial for evaluating the risk of adverse outcomes on pregnant women, fetuses, and infants, while 71 are essential for analyzing long-term effects on children.
Moreover, with the anticipated release of the ICH M14 guideline in June 2025, there is an active effort to harmonize post-approval non-interventional studies that utilize real-world data. This initiative addresses a gap in international guidance that has led to inefficiencies for both sponsors and regulators. As the availability of real-world data increases globally, the need for standardized methodologies becomes more apparent.
In light of these advances, it is imperative for stakeholders to heed the call for public comments, ensuring confidentiality and responsibility, as outlined in the submission instructions. Comments are integral to the continuous improvement of the ICH guidelines, which in turn, enhances global healthcare outcomes.
The significance of these guidelines and frameworks becomes even more pronounced when considering the challenges faced by the industry in adhering to complex and multilayered regulations within tight timelines. The journey toward compliance involves numerous steps, including planning, piloting, testing, validating, and implementing changes to meet future expectations.
Furthermore, recent research conducted in partnership with Consilium Scientific underscores the urgency of producing robust clinical data for both premarket and post-market phases. The study highlights the prevalence of weak evidence in clinical trial results, underscoring the necessity for better-designed studies that accurately reflect population demographics and treatment pathways.
In essence, the ICH guidelines are not static; they evolve to incorporate new scientific insights and technological advancements. The integration of this dynamic knowledge base into the guidelines ensures that the global pharmaceutical industry remains at the forefront of safety, efficacy, and quality in medicine production and distribution.
Benefits of ICH Harmonization
The International Council for Harmonization (ICH) plays a pivotal role in enhancing public health by establishing globally recognized standards for pharmaceutical product quality, safety, and efficacy. This harmonization is essential for regulatory authorities, streamlining the evaluation of new medicines and bolstering international collaboration. Such standardization is not just a matter of compliance but is a gateway to fostering innovation and addressing pressing health challenges, such as rare diseases and the use of AI in healthcare, as outlined by the Innovative Health Initiative. For the pharmaceutical industry, ICH guidelines minimize redundant clinical trials and expedite market entry, which is particularly crucial for medical devices and emerging wellness technologies that require rigorous assessment before reaching patients. This process is exemplified by the significance of Early Feasibility Studies (EFS) in the EU, which are vital for assessing the initial clinical safety and performance of new medical devices. Ultimately, for healthcare professionals and patients, consistent ICH standards mean enhanced confidence in medical products and a more robust approach to patient safety, reflecting in the broad themes discussed at healthcare summits and the proactive engagement of regulatory science experts. As the regulatory landscape evolves, with the emergence of large consortia aiming to tackle healthcare challenges, the harmonization fostered by ICH will continue to be a cornerstone of global health innovation.
Global Impact and Membership
The International Council for Harmonization (ICH) plays a pivotal role in shaping global health care by establishing standards that ensure the quality, safety, and efficacy of medicines and fostering cooperation among regulatory authorities. The consortium's influence extends worldwide, with a diverse membership that includes regulatory bodies from Europe, the United States, Japan, Canada, and Switzerland. Industry associations contribute to the formulation and implementation of ICH guidelines, which is crucial for maintaining a consistent and harmonized approach to healthcare regulation across different countries. This global collaboration is echoed in the sentiments of Ivan Florez, Editor of Clinical and Public Health Guidelines, who emphasizes the importance of advancing guideline science on an international scale to support guideline developers, researchers, and implementers.
In a rapidly evolving digital era, the standardization of practices and interoperability of technologies becomes increasingly significant. Digital standards are established to ensure that various technologies can seamlessly work together, promoting cooperation and enhancing the functionality of devices and software. These standards are not only vital for technological advancements but also play a significant geopolitical role, influencing a wide spectrum of areas from trade and security to human rights.
Recent updates on the work of the ICH reflect the organization's commitment to leveraging real-world data to inform post-approval studies of medical interventions through initiatives like ICH M14. The goal is to harmonize guidelines internationally, addressing inefficiencies and regulatory challenges. Such efforts underscore the continuing evolution of Ich's impact and the importance of maintaining a global perspective on science and healthcare standards, as emphasized by the mission of the International Science Council to advance science as a global public good.
The ICH's efforts resonate with the broader scientific community's recognition of science as a universal language, defined by principles that are systematically organized and tested against reality. As science becomes increasingly globalized, it is imperative to have globally accepted standards for cooperation, a view shared by thought leaders who advocate for the distinction between science and science systems to promote international scientific cooperation.
Real-World Evidence and Future Directions
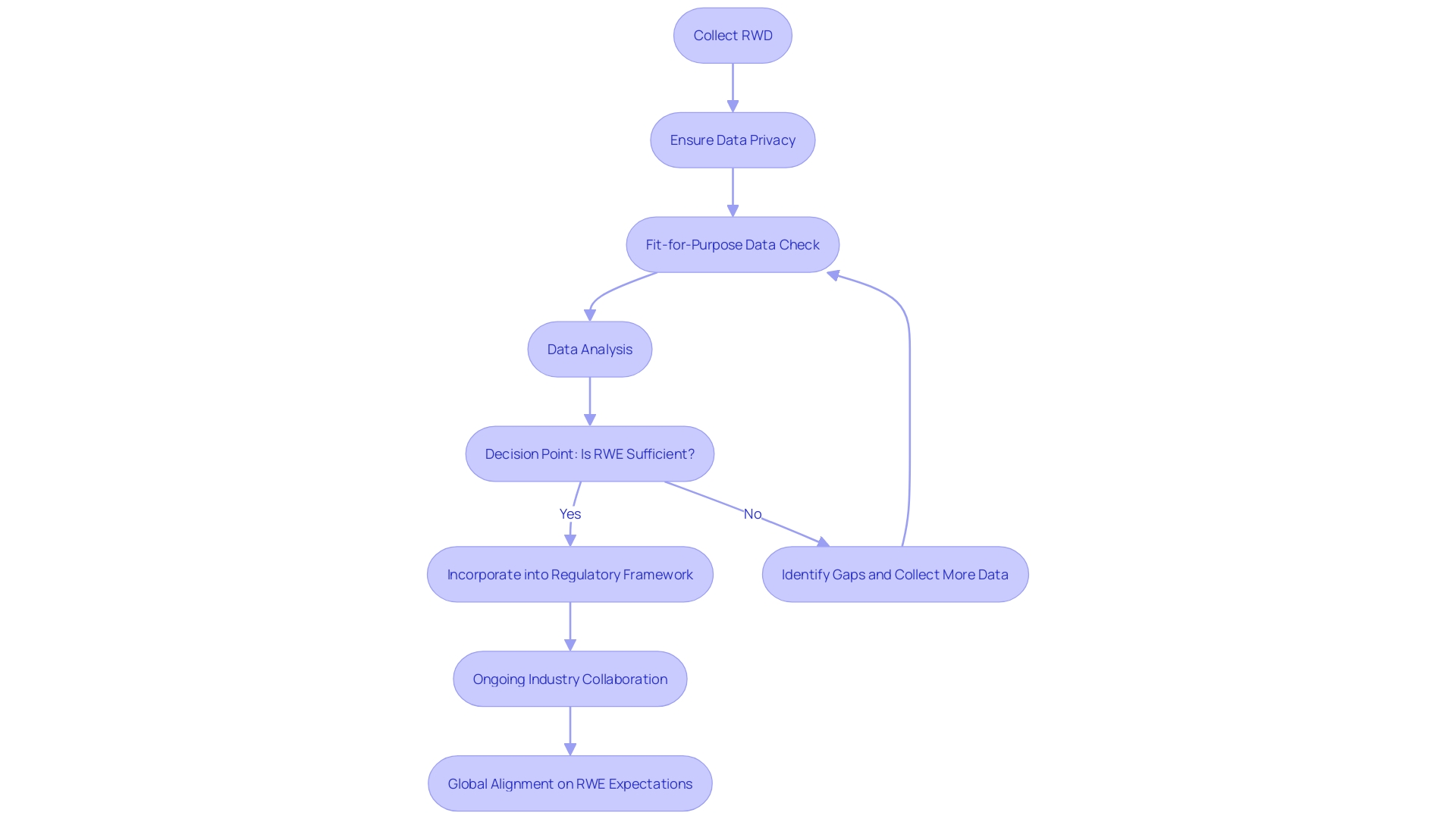
In response to the dynamic nature of the healthcare and pharmaceutical industries, the International Council for Harmonization (ICH) is actively incorporating real-world evidence (RWE) into its regulatory framework. Recognizing the vital role of RWE, which originates from various sources such as electronic health records, insurance claims, and digital health technologies, ICH is committed to integrating this data into its guidelines. The objective is to establish a robust evidence base that informs the development and evaluation of therapeutic products. Cardinal Health's case study exemplifies the strategic approach to regulatory submissions, which underscores the necessity for a comprehensive understanding of RWE's potential to enhance the regulatory process. Challenges such as ensuring fit-for-purpose real-world data (RWD) and maintaining patient privacy while granting data access to regulatory bodies are being addressed through ongoing collaborations across the industry.
Regulators worldwide, including the FDA and EMA, are grappling with the diversity in research objectives, study designs, and analytical methods, which complicates standardization efforts. The aspiration for a harmonized approach is echoed by Regeneron Pharmaceuticals, advocating for clarity in definitions and study parameters. The European Health Data Space (EHDS) initiative symbolizes one of the European Commission's main priorities, aiming to establish a reliable health data ecosystem that ensures quality, relevance, and interoperability. This initiative will tackle the challenges posed by data privacy, access, fragmentation, and analytical familiarity. Moreover, the FDA's draft guidance highlights the importance of high-quality, systematically collected RWD for regulatory decision-making.
The use of RWE in the EU is subject to multiple barriers, emphasizing the need for close scrutiny of data privacy, relevance, and quality. As the industry navigates these challenges, the value of RWE in regulatory settings is increasingly recognized, with potential uses ranging from identifying trial participants to serving as comparator arms in externally controlled trials. The alignment of global expectations for RWE and fitness for purpose remains a key goal, with the acknowledgment that a diverse range of stakeholders must work in concert to achieve the best outcomes for regulatory processes and ultimately, patient care.
Conclusion
In conclusion, the International Council for Harmonisation (ICH) has been instrumental in standardizing pharmaceutical regulations worldwide since the early 1990s. With a focus on simplifying drug development and expanding access to safe and effective medicines, ICH has brought together regulatory bodies and industry representatives from major global markets.
Through initiatives like the proposed ICH M14 guideline, which aims to standardize post-approval studies using real-world data, ICH continues to streamline the review process for pharmaceuticals. The collective efforts of ICH and its members have resulted in significant advancements in healthcare, including the International Statistical Classification of Diseases and Related Health Problems (ICD), a crucial tool for healthcare data recording and disease statistics.
ICH's work aligns with a broader movement towards a cooperative and unified approach to drug regulation, ultimately improving public health outcomes on a global scale. The structure and governance of ICH foster a collaborative environment, enabling diverse stakeholders to work together towards common goals in the pharmaceutical landscape.
ICH's guidelines serve as pivotal standards for the industry, addressing areas such as quality control, clinical trial methodologies, safety evaluations, and pharmaceutical stability. By ensuring the consistent production of high-quality medicines, these guidelines enhance healthcare outcomes and provide confidence in medical products.
The harmonization efforts of ICH offer numerous benefits, including streamlined evaluations for regulatory authorities, reduced redundancy in clinical trials for the industry, and enhanced patient safety and confidence. ICH's global impact is reflected in its diverse membership and collaborations with global health organizations, facilitating patient access to new medicines and medical devices.
ICH is actively incorporating real-world evidence (RWE) into its regulatory framework, recognizing its importance in establishing a robust evidence base for therapeutic product development and evaluation. Ongoing collaborations address challenges related to RWE, such as ensuring data fitness and patient privacy.
In summary, ICH's contributions to global pharmaceutical regulation have been significant. Through its initiatives, guidelines, and collaborative approach, ICH simplifies drug development, expands access to safe medicines, and improves public health outcomes worldwide.




