Introduction
Panama is rapidly emerging as a pivotal hub for medical device research in Latin America, driven by its strategic geographic advantages and robust healthcare infrastructure. The nation is witnessing an influx of international companies eager to tap into its favorable regulatory environment and diverse patient populations for clinical trials.
However, navigating the complexities of this evolving landscape poses challenges, particularly for US Medtech firms contending with regulatory hurdles and resource fragmentation. As collaborations and initiatives flourish, such as those between prominent organizations and local clinical research entities, Panama is solidifying its role in advancing medical technology development.
This article delves into the current state of medical device research in Panama, examining the regulatory landscape, key players, prevailing challenges, and exciting opportunities that lie ahead for this burgeoning sector.
Overview of Medical Device Research in Panama: Current Landscape and Trends
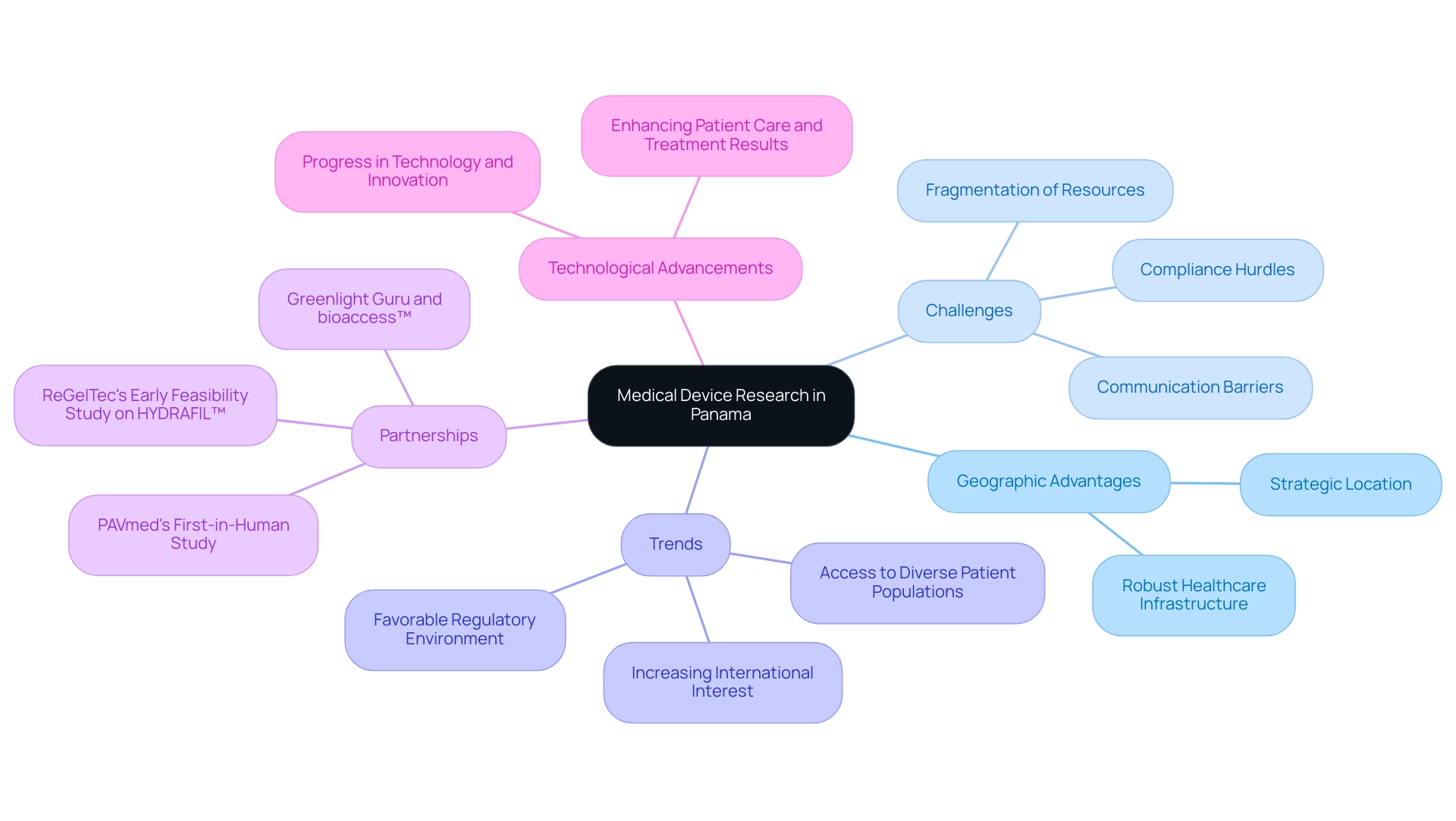
Panama has emerged as a significant player in Medical Device Research Panama, with a rapidly evolving landscape in the medical device development sector within Latin America. The country benefits from a strategic geographic location, robust healthcare infrastructure, and a growing number of clinical research organizations (CROs) that facilitate the conduct of clinical trials. However, US Medtech companies face challenges such as compliance hurdles, communication barriers, and the fragmentation of resources when navigating this landscape.
Recent trends indicate an increasing number of international companies looking to Panama for conducting clinical trials, highlighting the importance of Medical Device Research Panama due to its favorable regulatory environment and access to diverse patient populations. Significantly, partnerships such as that between Greenlight Guru and bioaccess™ are enhancing the quality of clinical trials, while successful initiatives like PAVmed's first-in-human study and ReGelTec's Early Feasibility Study on HYDRAFIL™ showcase the importance of Medical Device Research Panama in technology development. Furthermore, progress in technology and innovation in healthcare tools are propelling medical device research in Panama, focused on enhancing patient care and treatment results, establishing the country as an appealing center for health technology development.
Navigating the Regulatory Landscape: Medical Device Registration in Panama
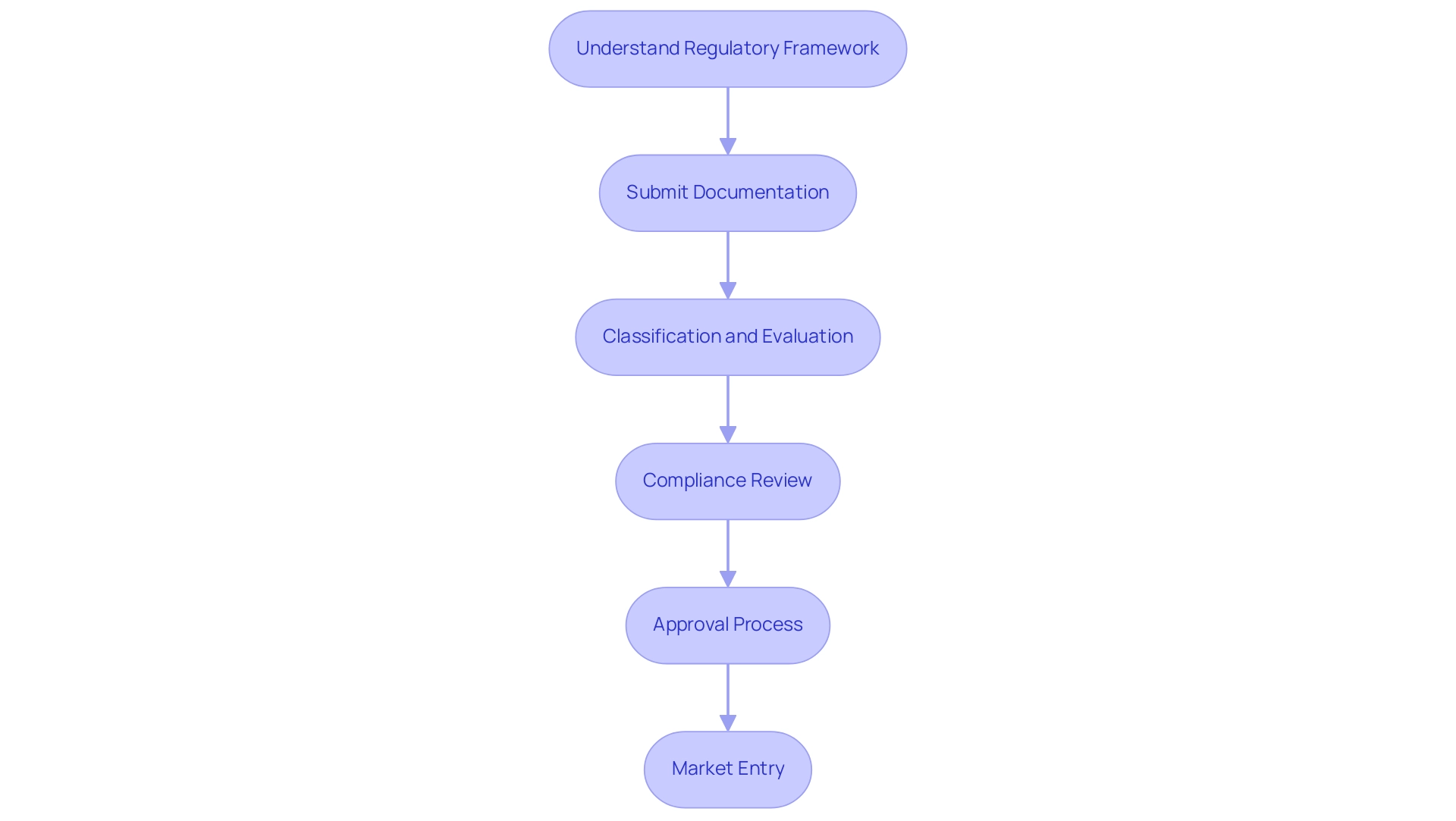
The governing structure for healthcare equipment registration in Panama, as part of the Medical Device Research Panama, is mainly supervised by the Ministry of Health (MINSA) and the National Directorate of Medicines and Health Technologies. Companies seeking to introduce healthcare devices must navigate a structured process that involves adhering to specific guidelines tailored to the classification, evaluation, and registration of devices, which vary according to their associated risk levels, especially in the realm of Medical Device Research Panama. The registration process requires the submission of extensive documentation, including clinical data, technical specifications, and proof of compliance with international standards.
As Julio G. Martinez-Clark, CEO of bioaccess® LATAM CRO EXPERTS, states, 'Grasping the compliance nuances in Panama is essential for companies aiming to succeed in Medical Device Research Panama.' Recent changes in Panama's governance framework have streamlined the process, making Medical Device Research Panama more efficient for companies to register their products. Companies like [Example Company] have successfully navigated these regulations, showcasing the importance of staying informed about the evolving landscape.
With bioaccess®'s comprehensive clinical trial management services for Medical Device Research Panama, including:
- Feasibility studies
- Site selection
- Compliance reviews
- Trial setup
- Import permits
- Project management
- Reporting
stakeholders can effectively navigate these oversight environments. Katherine Ruiz, a specialist in compliance matters for healthcare tools and in vitro diagnostics in Colombia, highlights the importance of grasping local laws to enhance global health through international cooperation and innovation in Medtech. As Panama continues to improve its oversight framework, it is essential for stakeholders involved in Medical Device Research Panama to stay alert regarding any alterations or updates that could influence their investigations and product development strategies.
Effectively navigating this regulatory environment is essential for Medical Device Research Panama to ensure that health products meet established safety and efficacy standards, thereby facilitating a successful market entry.
Key Players in the Medical Device Research Sector
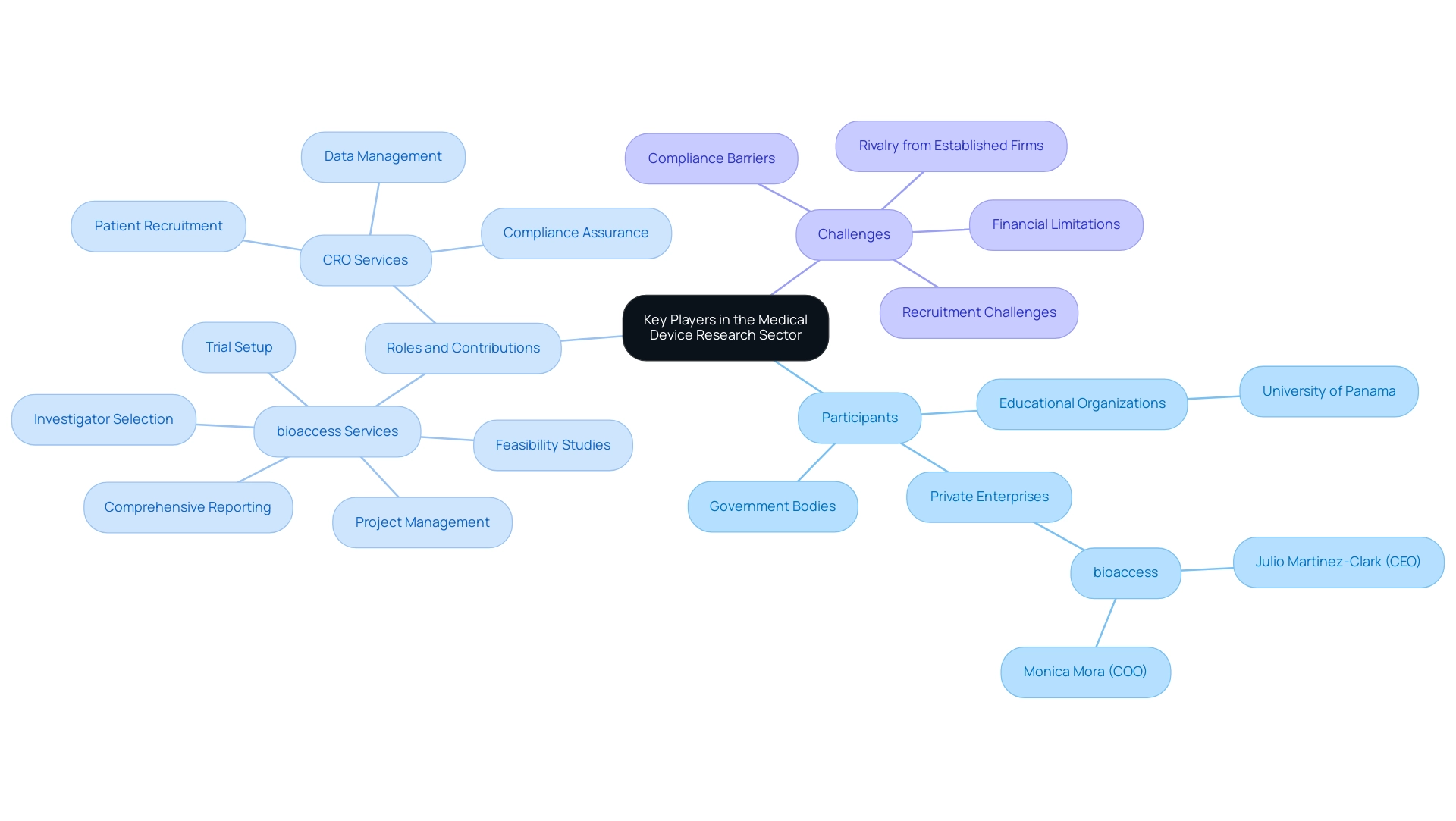
The Medical Device Research Panama sector is supported by a varied range of essential participants, including government bodies, educational organizations, and private enterprises in the healthcare equipment study. Julio Martinez-Clark, CEO of bioaccess, advocates for Medtech clinical studies in Latin America, emphasizing the operationalization of clinical trials and the promotion of regional opportunities for growth. Monica Mora, the Chief Operating Officer, specializes in the complexities of operations, logistics, and compliance strategies for medical device companies in the region.
Notable institutions like the University of Panama collaborate with international organizations to conduct cutting-edge Medical Device Research Panama. Clinical Research Organizations (CROs) play a crucial role in facilitating clinical trials by providing services such as:
- Patient recruitment
- Data management
- Ensuring compliance with regulations
bioaccess provides essential services including:
- Feasibility studies
- Investigator selection
- Trial setup
- Project management
- Comprehensive reporting to support the operationalization of clinical trials
Nonetheless, healthcare equipment startups encounter considerable obstacles, such as:
- Compliance barriers
- Rivalry from established firms
- Recruitment challenges
- Financial limitations
The expertise of Julio and Monica is crucial in navigating these challenges, as their experience and strategic insights can help startups streamline processes and enhance their chances of success. Collaborations with global healthcare equipment firms have resulted in heightened funding for local innovation initiatives, promoting creativity and enhancing the overall quality of healthcare product development in the area.
Challenges and Opportunities in Medical Device Research
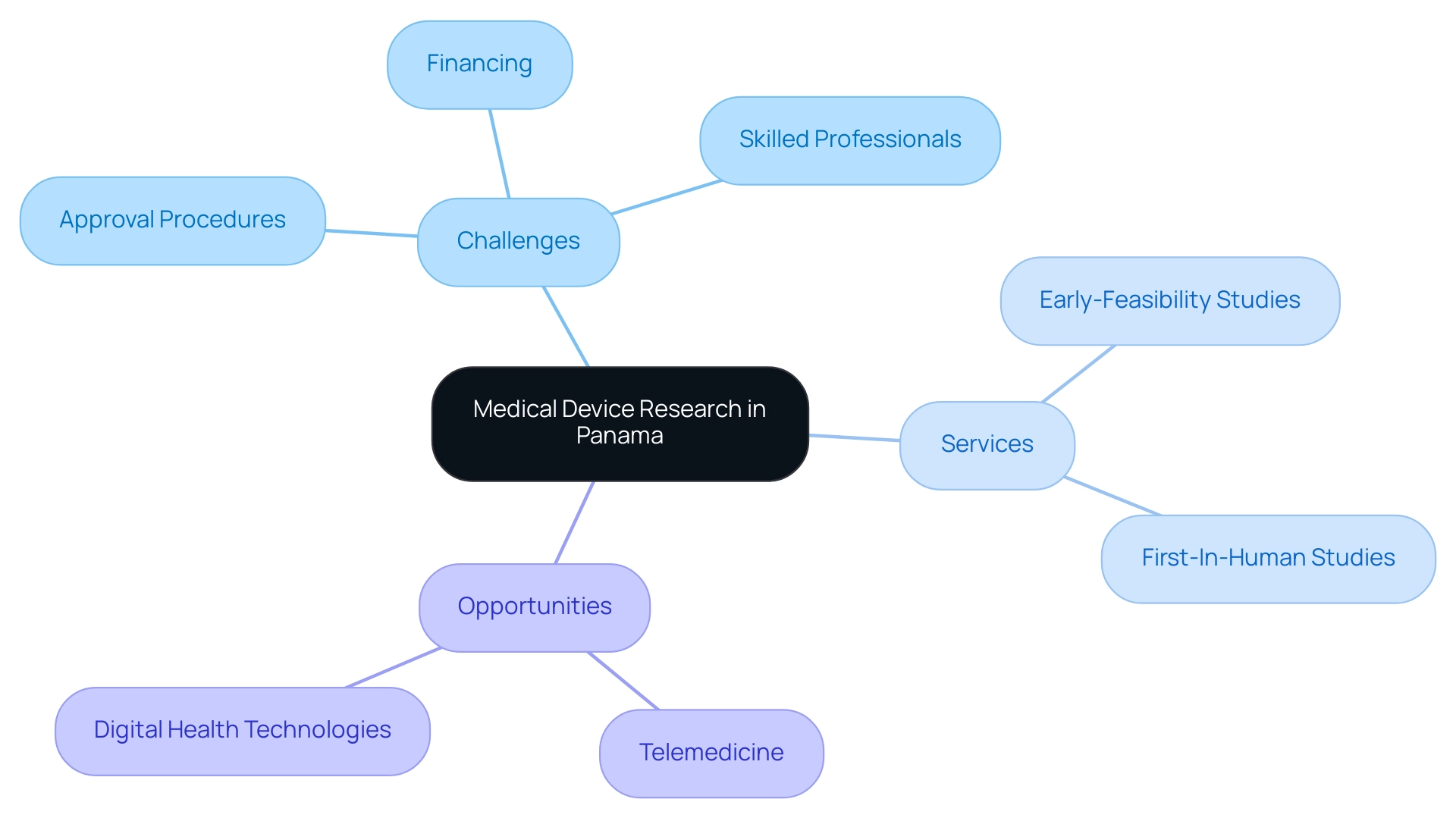
Despite the encouraging environment for Medical Device Research Panama, several obstacles remain, such as:
- Navigating intricate approval procedures
- Securing financing for innovative initiatives
- Addressing the need for skilled professionals in the sector
Regulatory oversight, particularly from INVIMA in Colombia, provides a framework that can guide similar efforts in Medical Device Research Panama, ensuring compliance and facilitating smoother trial setups. bioaccess® offers a range of clinical trial management services, including:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
These services are crucial for navigating these regulatory environments.
Opportunities abound, especially in telemedicine and digital health technologies, which have gained traction due to the COVID-19 pandemic. By utilizing progress in technology and promoting cooperation among stakeholders, including specialized clinical trial management services like bioaccess®, Panama can enhance Medical Device Research Panama and strengthen its role as a leader in healthcare innovation. This strategic approach will ultimately contribute to improved patient outcomes, economic growth, job creation, and international recognition in the healthcare sector.
Future Directions for Medical Device Research in Panama
Looking forward, healthcare equipment development in Panama is set for substantial expansion, fueled by technological progress and a rising need for inventive health solutions. With bioaccess® offering extensive clinical trial management services—including:
- Early-Feasibility Studies
- First-In-Human Studies
- Feasibility studies
- Site selection
- Compliance reviews
Panama is well-prepared to support agendas centered on personalized medicine, minimally invasive surgical techniques, and the incorporation of artificial intelligence in healthcare tools. Enhancing collaborations among academia, industry, and government, along with global cooperation enabled by skilled teams, will be vital in creating an environment favorable to development and innovation.
As bioaccess®'s services contribute to job creation and economic growth, Panama continues to build on its existing strengths, positioning itself as a leading destination for medical device research and development in Latin America, ultimately contributing to improved healthcare outcomes.
Conclusion
Panama is rapidly establishing itself as a key player in the medical device research sector in Latin America, thanks to its strategic advantages and improving regulatory framework. The influx of international companies seeking to conduct clinical trials in this region is a testament to the favorable environment that Panama offers, characterized by diverse patient populations and a robust healthcare infrastructure. However, challenges remain, particularly for US Medtech firms that must navigate regulatory complexities and resource fragmentation.
The regulatory landscape is evolving, with recent changes aimed at streamlining the registration process for medical devices. This development is crucial for companies aiming to introduce innovative technologies to the market. Collaboration among key players—including government agencies, academic institutions, and private organizations—enhances the quality and efficiency of clinical trials, driving advancements in medical technology and patient care.
Despite the promising landscape, stakeholders must remain vigilant in addressing existing challenges, such as:
- Securing funding
- Navigating regulatory hurdles
- Attracting skilled professionals
Opportunities in telemedicine and digital health technologies are emerging, presenting pathways for growth and innovation. By leveraging these opportunities and fostering collaboration among all stakeholders, Panama is well-positioned to enhance its role in medical device research and development.
In conclusion, the future of medical device research in Panama looks promising, with significant potential for growth and innovation. By capitalizing on its strengths and refining its regulatory framework, Panama can continue to attract international investment and partnerships, ultimately leading to improved healthcare outcomes and economic benefits for the region. The strategic initiatives currently underway will contribute to solidifying Panama's status as a leading hub for medical technology development in Latin America.




