Introduction
The reliability and safety of medical devices are paramount in safeguarding patient health, making stability testing an essential process in the healthcare industry. This systematic methodology not only evaluates how devices perform under various environmental conditions but also plays a crucial role in identifying potential failures before they occur.
As regulatory standards evolve, manufacturers must navigate a complex landscape that includes:
- Accelerated aging
- Real-time testing methodologies
Each offering unique insights into device longevity. With regulatory bodies like INVIMA setting stringent guidelines, the importance of robust stability testing frameworks is underscored by the need for compliance and the protection of public health.
As the industry moves towards innovative solutions, such as the integration of artificial intelligence in testing protocols, understanding these dynamics will be vital for manufacturers aiming to maintain competitive advantage while ensuring the efficacy and safety of their products.
Understanding Medical Device Stability Testing: Definition and Importance
The evaluation of medical equipment reliability includes medical device stability testing as a structured approach for assessing the dependability and lifespan of instruments across various environmental conditions. This essential evaluation guarantees that medical equipment maintains its intended performance and safety throughout its shelf life, ultimately protecting patient health. The significance of medical device stability testing extends beyond mere compliance; it plays a vital role in detecting potential failures before they arise.
As emphasized by compliance consultant Lisa A. Hornback, manufacturers must analyze 'quality data to identify existing and potential nonconforming product, or other quality problems.' This proactive strategy enables manufacturers to perform medical device stability testing to assess how products react to various stressors, including temperature, humidity, and light exposure. By identifying crucial deterioration points, manufacturers can make informed choices about product development and marketing, while also complying with regulatory standards established by INVIMA, Colombia's National Food and Drug Surveillance Institute, which supervises the approval and monitoring of health-related products, including medical device stability testing.
The Directorate for Health Products and other Technologies within INVIMA is responsible for establishing technical standards, monitoring compliance, and conducting post-market surveillance to ensure ongoing safety and effectiveness of health products. 'INVIMA's designation as a Level 4 health authority by PAHO/WHO highlights its capability in guaranteeing the safety, efficacy, and quality of health products.' Importantly, the protocol for maintaining consistency can be modified according to present circumstances, demonstrating the adaptability required to manage the intricacies of healthcare equipment evaluation.
In line with industry standards, producers should perform evaluations on at least three batches to determine suitable expiration dates, guaranteeing reliable data for regulatory adherence. With recent advancements and best practices being examined, especially in early-phase clinical trials for cellular and gene therapy products, the significance of medical device stability testing continues to gain prominence in ensuring the reliability of medical instruments. For example, the FDA's initiatives in organizing recall information emphasize the difficulties encountered in the industry, as comprehending the subtleties of recall categories can enhance the identification of quality issues and improve overall product safety.
Katherine Ruiz, a specialist in Regulatory Affairs for Medical Equipment and In Vitro Diagnostics in Colombia, emphasizes the crucial importance of comprehensive evaluation, including medical device stability testing, and compliance with regulatory standards in preserving equipment integrity and safeguarding public health.
Types of Stability Testing Methods: Accelerated Aging vs. Real-Time Testing
Medical equipment reliability evaluation includes two main approaches: accelerated aging assessment and real-time evaluation. Accelerated aging evaluation subjects apparatus to elevated stress conditions—such as increased temperature, reaching up to 125 degrees Celsius and a supply voltage of 1.8 V for up to 1100 hours—thereby expediting the aging process. This approach facilitates quicker results, allowing manufacturers to predict long-term stability effectively.
Regulatory authorities, including INVIMA, have recognized the validity of Accelerated Aging data as a reliable method for rapid data collection, especially when correlated with real-time samples. On the other hand, real-time evaluation entails keeping equipment under standard conditions and periodically measuring their performance over a prolonged period. While this method provides the most precise depiction of a gadget's longevity, it requires a longer time frame to obtain results.
Both testing methodologies, including medical device stability testing, are essential for ensuring the safety and efficacy of medical instruments. For example, a case study named 'Loss Analysis in Metal Nanoparticle Arrays' demonstrated that losses slightly exceeding 10 dB/μm were achieved for both transverse and longitudinal waves, suggesting that optimized metal nanoparticle structures could still allow the practicality of stand-alone sub-micron systems. As Douglas Stockdale, President of Stockdale Associates Inc., aptly stated, 'If it's not documented, it never happened.'
This emphasizes the significance of comprehensive documentation in assessing equipment reliability and performance. Additionally, with more than 20 years of experience in Medtech, bioaccess® is distinctly situated to offer expedited clinical study services in Latin America, overseeing:
- Early-Feasibility Studies (EFS)
- First-In-Human Studies (FIH)
- Pilot Studies
- Pivotal Studies
- Post-Market Clinical Follow-Up Studies (PMCF)
with a tailored approach. Westpak experts are also available to assist with any questions or guidance needed, emphasizing the support available for clinical research directors aiming to enhance product reliability and compliance.
Regulatory Guidelines for Medical Device Stability Testing
Regulatory guidelines for medical device stability testing are meticulously crafted by organizations such as the FDA and ISO, outlining essential requirements for the execution of reliability studies. These guidelines dictate a variety of factors, including the specific tests that must be undertaken, the duration of these tests, and the necessary documentation to be maintained. For instance, medical device stability testing often requires assessments of physical, chemical, and microbiological properties to ensure safety and efficacy throughout the product's shelf life.
Following these regulatory frameworks is crucial for manufacturers pursuing market approval, as it directly relates to medical device stability testing and the safety and efficacy of the products in question. Notably, the FDA has highlighted that the adsorption of warm-reactive autoantibodies—a common procedure in immunohematology testing—is generally considered a 1976-Type LDT under enforcement discretion and relies heavily on specialized manual techniques performed by trained laboratory personnel. With the FDA's recent receipt of 14 requests for extensions following the publication of the Notice of Proposed Rulemaking (NPRM), it is evident that ongoing regulatory changes can significantly influence testing protocols and product development strategies.
Experts emphasize that keeping up with these updates is not just recommended, but crucial for ensuring compliance and maintaining competitive edge in the healthcare equipment sector. Furthermore, discussions surrounding legislative recommendations for in vitro diagnostics indicate that the FDA is focused on enhancing safety and effectiveness under its existing statutory authorities. The FDA recognizes the need for potential new legislation to further regulate this sector, highlighting the importance of collaboration with Congress to advance balanced oversight in the field.
With the insights of professionals like Ana Criado, Director of Regulatory Affairs and an expert in biomedical engineering and health economics, along with Katherine Ruiz, another authority in Regulatory Affairs for healthcare products in Colombia, stakeholders can navigate these complex regulations effectively and ensure that their offerings meet all necessary standards. Katherine Ruiz's expertise further enhances the understanding of the regulatory environment, offering additional viewpoints on compliance and assessment standards that are vital for clinical research directors.
The Benefits of Stability Testing for Medical Devices
Medical device stability testing serves as a cornerstone for manufacturers, delivering a spectrum of benefits that encompass enhanced product safety, improved regulatory compliance, and heightened consumer confidence. As regulations grow more rigorous, especially for combination products that need to satisfy at least two sets of regulatory criteria, the significance of medical device stability testing and durability assessment cannot be overstated. By detecting potential issues early in the development phase, manufacturers can proactively implement necessary adjustments, significantly mitigating the risk of costly product recalls.
This proactive approach not only safeguards the manufacturer’s market reputation but also ensures better patient outcomes. Moreover, the information gathered from medical device stability testing supports marketing assertions and simplifies the regulatory approval procedure, ultimately strengthening compliance. According to industry insights, the digitalization of sample management can also mitigate associated risks, enhancing the reliability of research on stability.
As emphasized by Kim Huynh-Ba, incorporating reliability evaluations within wider quality frameworks, such as risk management and change control, guarantees a thorough method for upholding consistency statistics. A significant case study, 'Mitigating the Risks – Digitalization of Sample Management for Quality Assurance,' demonstrates that while quality assurance studies are crucial for ensuring the safety and efficacy of pharmaceutical products, managing associated risks through digitalization can improve the reliability of these studies. In 2024, medical device stability testing is anticipated to gain more focus, as its importance in preventing product recalls and ensuring the safety of healthcare instruments becomes more prominent.
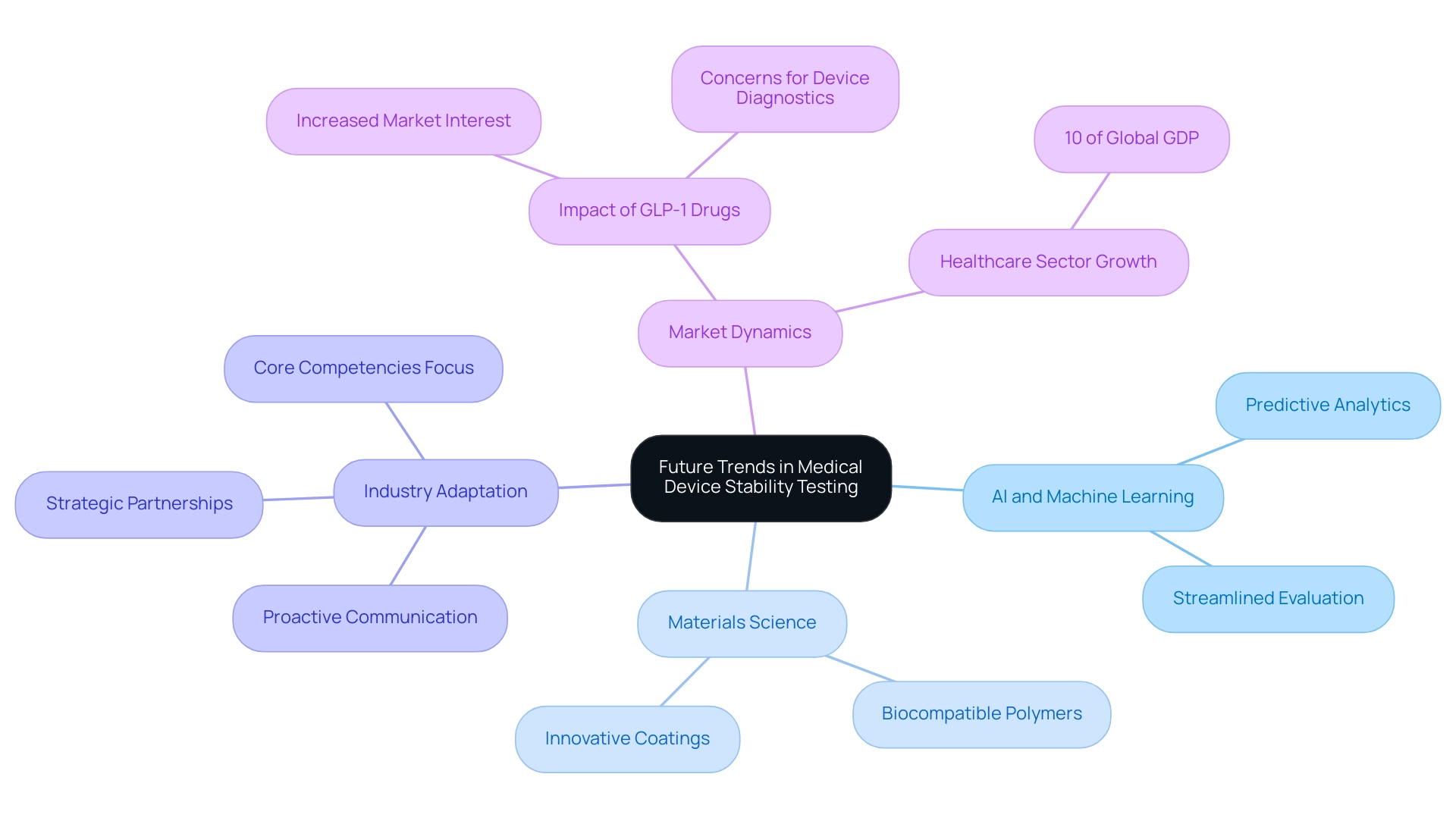
Future Trends in Medical Device Stability Testing
The terrain of healthcare device reliability assessment is on the verge of transformative advancements, significantly shaped by innovative research in medical device stability testing led by experts like Dr. Sergio Alvarado, a Clinical Trial Manager at bioaccess®. His dedication to incorporating artificial intelligence (AI) and machine learning into health research is crucial, as these technologies enhance predictive analytics in medical device stability testing. This approach not only streamlines evaluation processes but also significantly enhances accuracy, culminating in more reliable outcomes essential for patient safety.
Moreover, progress in materials science, including the creation of biocompatible polymers and innovative coatings, enhances medical device stability testing for the development of more stable healthcare instruments, potentially reducing the necessity for extensive evaluation procedures. The urgency for manufacturers to adopt these innovations is underscored by the fact that the global healthcare sector accounts for 10% of the global GDP. As mentioned by a representative from Benchmark Electronics Inc., 'In the competitive healthcare equipment marketplace, companies cannot afford to lose time.'
This highlights the necessity for proactive adaptation to evolving trends to maintain compliance and ensure product safety. Moreover, methods to accelerate time to market, such as reusing technology and managing strategic partnerships, are critical for manufacturers aiming to thrive in a rapidly changing environment. Notably, the impact of GLP-1 drugs on Medtech illustrates how market dynamics can influence the development and testing of healthcare devices.
Despite the benefits of these therapies, concerns about their potential to reduce the need for device-enabled diagnostics emphasize the importance of communication and adaptation within the industry. Staying informed about these developments is crucial for manufacturers looking to succeed in this competitive landscape while contributing to job creation, economic growth, and improved healthcare through international collaboration. Currently, Dr. Alvarado is working on several projects, including those focused on degenerative disc disease and vascular access technologies, leveraging his extensive background from his previous roles at Novartis and Colsubsidio to drive innovation in the region.
His insights into the potential of innovative medical solutions in Latin America reflect a deep understanding of the unique challenges and opportunities present in these markets.
Conclusion
The importance of stability testing for medical devices cannot be overstated. It serves as a foundational process that not only ensures compliance with regulatory standards but also enhances the safety and effectiveness of medical devices. By employing methodologies such as accelerated aging and real-time testing, manufacturers can proactively identify potential failures and make informed decisions that protect patient health. The rigorous adherence to guidelines set forth by organizations like INVIMA further emphasizes the critical role of stability testing in maintaining the integrity of medical devices throughout their lifecycle.
As the industry evolves, the integration of advanced technologies such as artificial intelligence promises to revolutionize stability testing protocols. These innovations will not only improve predictive analytics but also streamline testing processes, ultimately leading to more reliable outcomes. Additionally, the shift towards digitalization and enhanced materials science will support the development of more stable devices, reducing the need for extensive testing while ensuring compliance with ever-stricter regulations.
Moving forward, the collective focus on stability testing will be vital for manufacturers aiming to thrive in a competitive landscape. The benefits of thorough testing extend beyond regulatory compliance; they bolster consumer confidence and safeguard public health. As the medical device industry continues to navigate complexities and embrace innovation, the commitment to robust stability testing frameworks will remain essential in delivering safe, effective, and reliable medical solutions.
Frequently Asked Questions
What is medical device stability testing?
Medical device stability testing is a structured approach for evaluating the reliability and lifespan of medical equipment under various environmental conditions, ensuring that devices maintain their intended performance and safety throughout their shelf life.
Why is medical device stability testing important?
It is crucial for detecting potential failures before they occur, ensuring compliance with regulatory standards, and protecting patient health by maintaining the integrity of medical equipment.
What role does INVIMA play in medical device stability testing?
INVIMA, Colombia's National Food and Drug Surveillance Institute, oversees the approval and monitoring of health-related products, establishes technical standards, and conducts post-market surveillance to ensure the safety and effectiveness of health products, including medical devices.
What are the two main approaches to evaluating medical equipment reliability?
The two main approaches are accelerated aging assessment and real-time evaluation. Accelerated aging assessment subjects devices to elevated stress conditions to expedite the aging process, while real-time evaluation involves monitoring equipment performance under standard conditions over a prolonged period.
How does accelerated aging assessment work?
It involves exposing medical devices to increased temperatures (up to 125 degrees Celsius) and specific voltage levels for extended periods, allowing manufacturers to predict long-term stability more quickly.
What is the difference between accelerated aging assessment and real-time evaluation?
Accelerated aging assessment provides quicker results by simulating stress conditions, while real-time evaluation offers a more accurate depiction of a device's longevity but requires a longer timeframe to obtain results.
What is the significance of comprehensive documentation in medical equipment evaluation?
Comprehensive documentation is essential for assessing equipment reliability and performance, as it provides a record of testing and compliance, which is critical for regulatory adherence.
What services does bioaccess® offer in relation to clinical studies?
Bioaccess® offers expedited clinical study services in Latin America, including Early-Feasibility Studies (EFS), First-In-Human Studies (FIH), Pilot Studies, Pivotal Studies, and Post-Market Clinical Follow-Up Studies (PMCF), with a tailored approach.
How many batches should manufacturers evaluate to determine suitable expiration dates?
Manufacturers should evaluate at least three batches to ensure reliable data for regulatory adherence and to determine appropriate expiration dates for medical devices.

