Introduction
In the intricate landscape of medical device regulation, the concept of predicate devices plays a pivotal role in ensuring safety and efficacy. By serving as benchmarks for new devices, these previously approved products facilitate the evaluation process under the 510(k) submission pathway. As the medical technology industry continues to evolve, understanding the nuances of predicate device selection and the implications of substantial equivalence becomes increasingly essential for manufacturers.
With the stakes high—evidenced by the alarming rates of device recalls—navigating this regulatory framework effectively is crucial for safeguarding patient health and promoting innovation. This article delves into the critical aspects of predicate devices, exploring their definition, significance in the approval process, and best practices for selection, while highlighting the insights of industry experts who illuminate the path toward compliance and successful market entry.
Defining Predicate Devices: An Overview
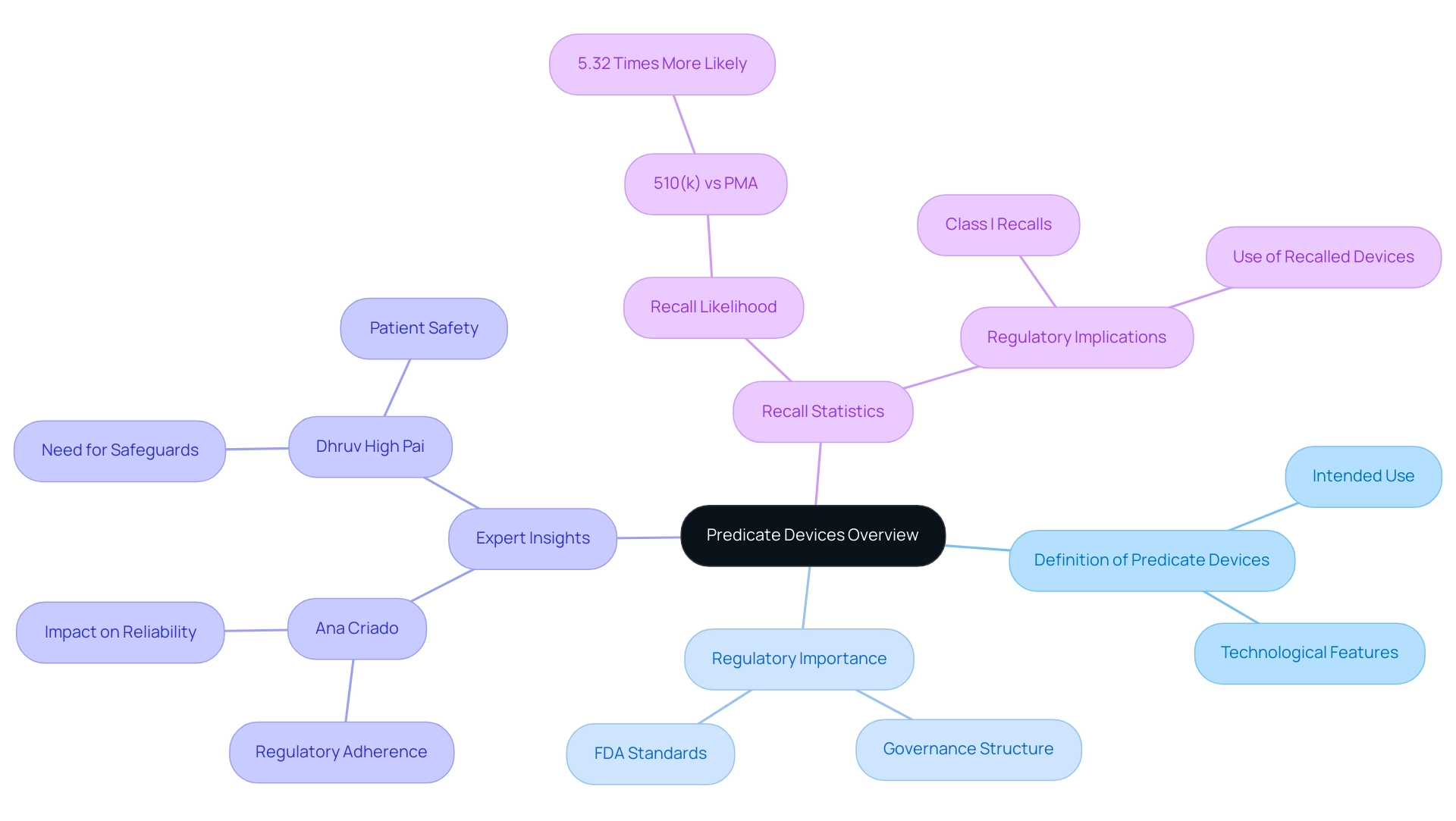
The predicate device definition refers to previously approved medical tools that serve as critical benchmarks for evaluating the safety and effectiveness of new instruments. According to the Federal Food, Drug, and Cosmetic Act, an existing instrument must possess the same intended use and technological features as the new item being submitted for approval. This predicate device definition emphasizes the governance structure that must be followed, enabling a more straightforward route to meeting FDA standards.
Specialists such as Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, highlight the significance of comprehending foundational products in the realm of biomedical engineering and health economics. With her vast experience in Regulatory Affairs, Ana emphasizes that for manufacturers, comprehending prior products is crucial as they not only establish a basis for demonstrating regulatory adherence but also impact the overall reliability and effectiveness of new medical technologies. Considering the growing examination of equipment approval routes, especially with the concerning figure that 510(k) product recalls are 5.32 times more probable than those for PMA products, a comprehensive understanding of reference products is crucial for guaranteeing patient welfare and reducing hazards linked to product recalls.
As Dhruv High Pai highlights, 'Safeguards for the 510(k) pathway are needed to prevent troublesome reference selection and ensure patient safety.' Moreover, the case study named 'Regulatory Implications of Class I Recalls in Medical Equipment' demonstrates the potential hazards linked to utilizing recalled items as references, emphasizing the essential requirement for thorough assessment in the approval procedure. Katherine Ruiz, a specialist in Regulatory Affairs for Medical Equipment and In Vitro Diagnostics, offers valuable insights into these regulatory challenges, concentrating on the intricacies of compliance and the implications of recalls in the Colombian regulatory landscape.
The Role of Predicate Devices in the 510(k) Submission Process
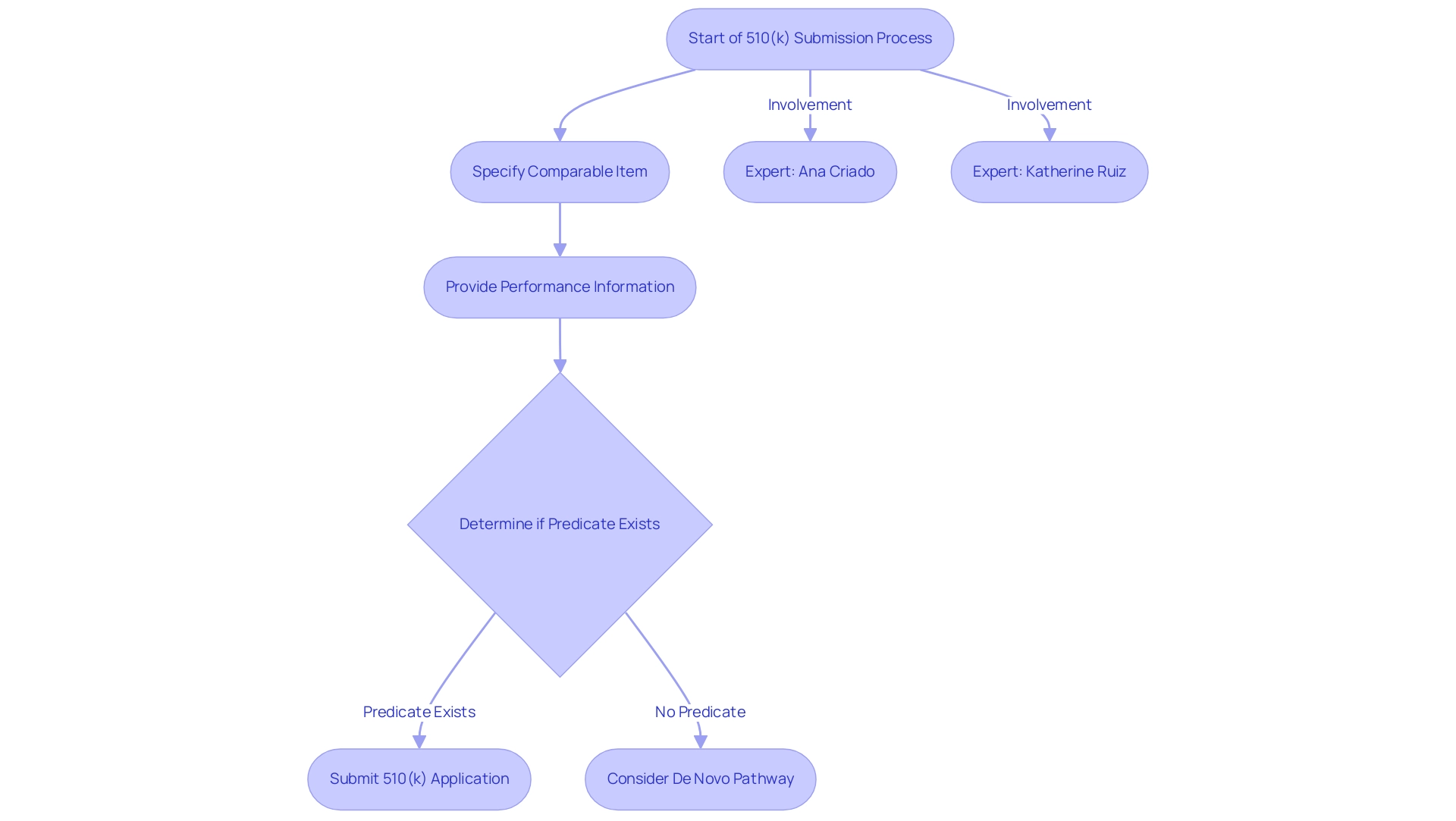
In the 510(k) submission process, manufacturers must specify a comparable item to support their assertion of substantial equivalence. This process entails presenting information that verifies the new apparatus's performance in safety and effectiveness is similar to that of the previous equipment. The 510(k) document control number begins with the letter K followed by 6 digits, serving as a factual reference point for submissions.
The 510(k) pathway is vital for accelerating the approval of medical products that do not introduce significant differences from existing offerings, thus promoting faster patient access to innovative healthcare solutions. Recent FDA guidance documents, including 'Evidentiary Expectations for 510(k) Implant Products,' provide clarity on the suitability of chosen predicate products in relation to the predicate device definition, ensuring manufacturers comply with standards while navigating this essential aspect of product approval.
A significant factor emerges when innovative Class II products lack a clear counterpart; in such instances, manufacturers may need to follow the de novo pathway, as emphasized by industry specialists like Ana Criado, Director of Compliance at Mahu Pharma, and Katherine Ruiz, who focuses on compliance for medical products and in vitro diagnostics in Colombia.
- Ana Criado brings a wealth of experience from her previous roles at INVIMA, where she held various executive positions, and her academic background as a professor of biomedical engineering at top Colombian universities.
- Katherine Ruiz adds further depth to the discussion with her expertise in navigating complex legal frameworks.
They emphasize that if your equipment is definitely Class II and there’s really no substantial equivalent at all, you’ll have to go down the de novo route.
Ultimately, the efficient recognition and use of such products not only aid in meeting compliance demands but also increase the chances of successful submissions, leading to better patient outcomes.
Understanding Substantial Equivalence and Its Importance
Substantial equivalence is a critical concept in the regulatory framework of medical equipment, indicating that a new item is as safe and effective as indicated by the predicate device definition. The FDA meticulously evaluates substantial equivalence based on several criteria, including:
- Intended use
- Technological characteristics
- Performance data
Notably, products introduced after May 28, 1976, must demonstrate substantial equivalence to be exempt from pre-market approval, which significantly streamlines the approval process.
If a new product meets these criteria, it can be approved through the 510(k) process, allowing for expedited market entry without the necessity of extensive clinical trials. This efficiency is essential for manufacturers seeking to navigate the complex regulatory landscape. According to recent statistics, a substantial number of instruments have successfully leveraged this pathway to gain approval, emphasizing the significance of understanding the predicate device definition and substantial equivalence for effective product development and market strategy.
As Tom Rish, a recognized expert in the field, states, 'Substantial equivalence is vital for ensuring that new products can swiftly meet market needs while maintaining safety and effectiveness standards.' Furthermore, the concept of substantial equivalence also pertains to preamendment items, which are those legally marketed before May 28, 1976, that have not been significantly altered and do not require a PMA application. These instruments are deemed grandfathered and underscore the continued importance of substantial equivalence in the oversight framework.
As the oversight landscape changes, remaining aware of the standards and consequences of substantial equivalence is crucial for producers seeking to thrive in the competitive medical equipment market. Ana Criado, with her extensive experience in compliance as the Director of Affairs and her roles in consulting for global companies, provides valuable insights into navigating these guidelines effectively.

Global Perspectives on Predicate Device Identification
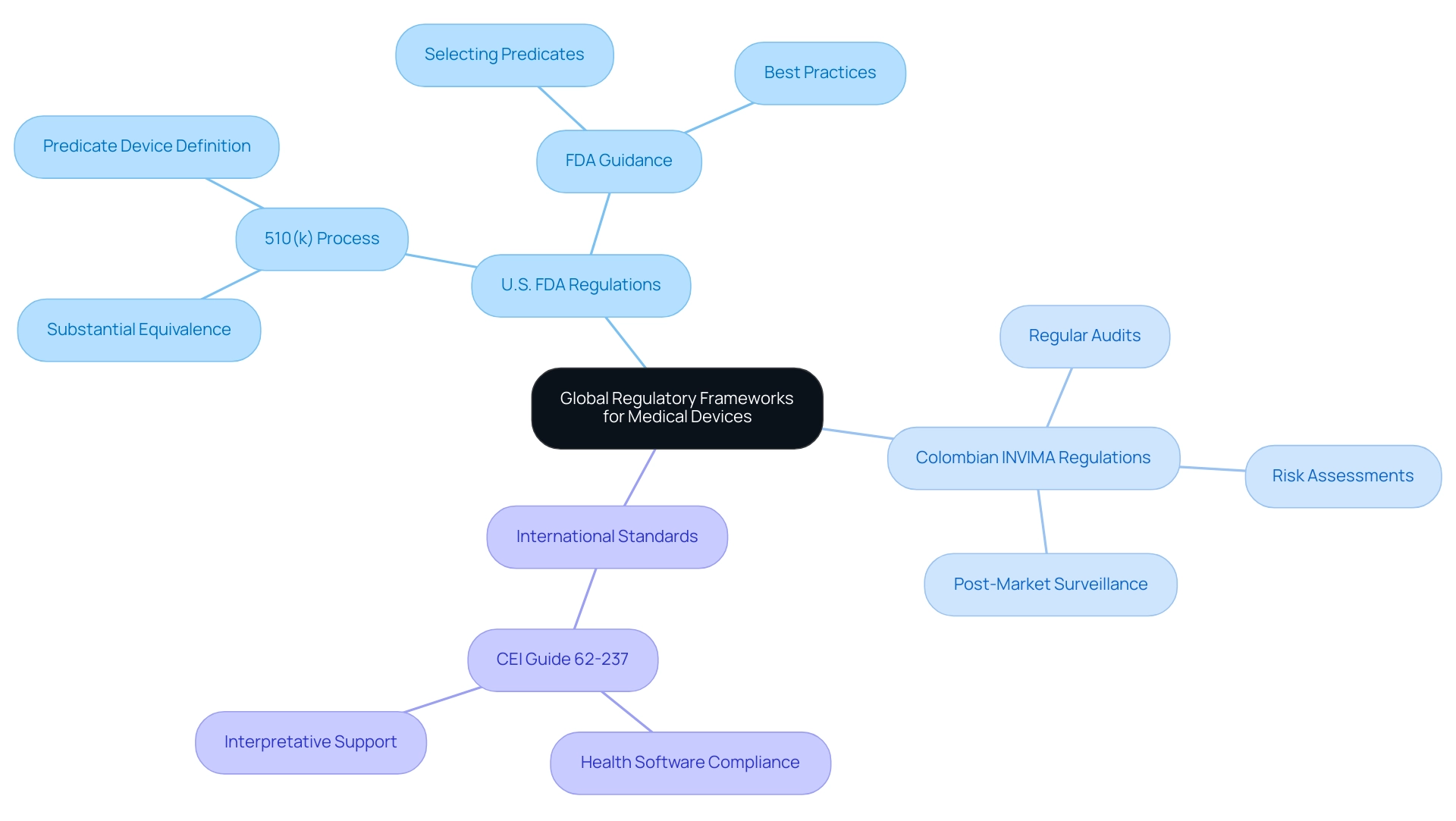
The regulation and identification of similar products exhibit significant variability across different nations, reflecting diverse regulatory frameworks. In the United States, the FDA utilizes the 510(k) process, which requires manufacturers to demonstrate that their product is substantially equivalent to a predicate device definition that is already available on the market. Conversely, many international markets, including Colombia, are regulated by authorities like INVIMA (Instituto Nacional de Vigilancia de Medicamentos y Alimentos), established in 1992.
INVIMA supervises the marketing and production of health products, including medical instruments, ensuring adherence to standards of security and effectiveness as categorized by PAHO/WHO as a Level 4 health authority. This authority monitors compliance of equipment through its Directorate for Medical Devices, which employs specific methodologies such as:
- Risk assessments
- Regular audits
- Post-market surveillance
These processes are essential for ensuring that medical equipment meets required safety and performance criteria.
In areas like Asia, local clinical data and specific criteria for defining elements may differ significantly from U.S. standards. For example, the CEI Guide 62-237, published in February 2015, provides interpretative support for operators and manufacturers, ensuring compliance with European and international regulations regarding health software. Significantly, the Regulatory Data Center (RDC) shows that there are over 5,000 reference products currently acknowledged in the U.S. market, demonstrating the variety of choices accessible for manufacturers.
This worldwide variation in equipment regulations requires a customized regulatory approach for producers seeking to enter international markets. The FDA's recent guidance on choosing references for 510(k) submissions emphasizes the importance of a strategic selection process, focusing on well-established methods and a strong safety performance record. As noted by Prof. Kerstin N Vokinger, 'Understanding the landscape of predicate device definitions is crucial for ensuring compliance and fostering innovation in medical technologies.'
Furthermore, INVIMA's oversight functions have significant implications for manufacturers looking to enter international markets, as understanding and adapting to these regulations is vital for successful market access. This ongoing evolution in international medical equipment approval processes highlights the importance of adapting to these regulatory landscapes to ensure safety and effectiveness in development.
Best Practices for Selecting Predicate Devices
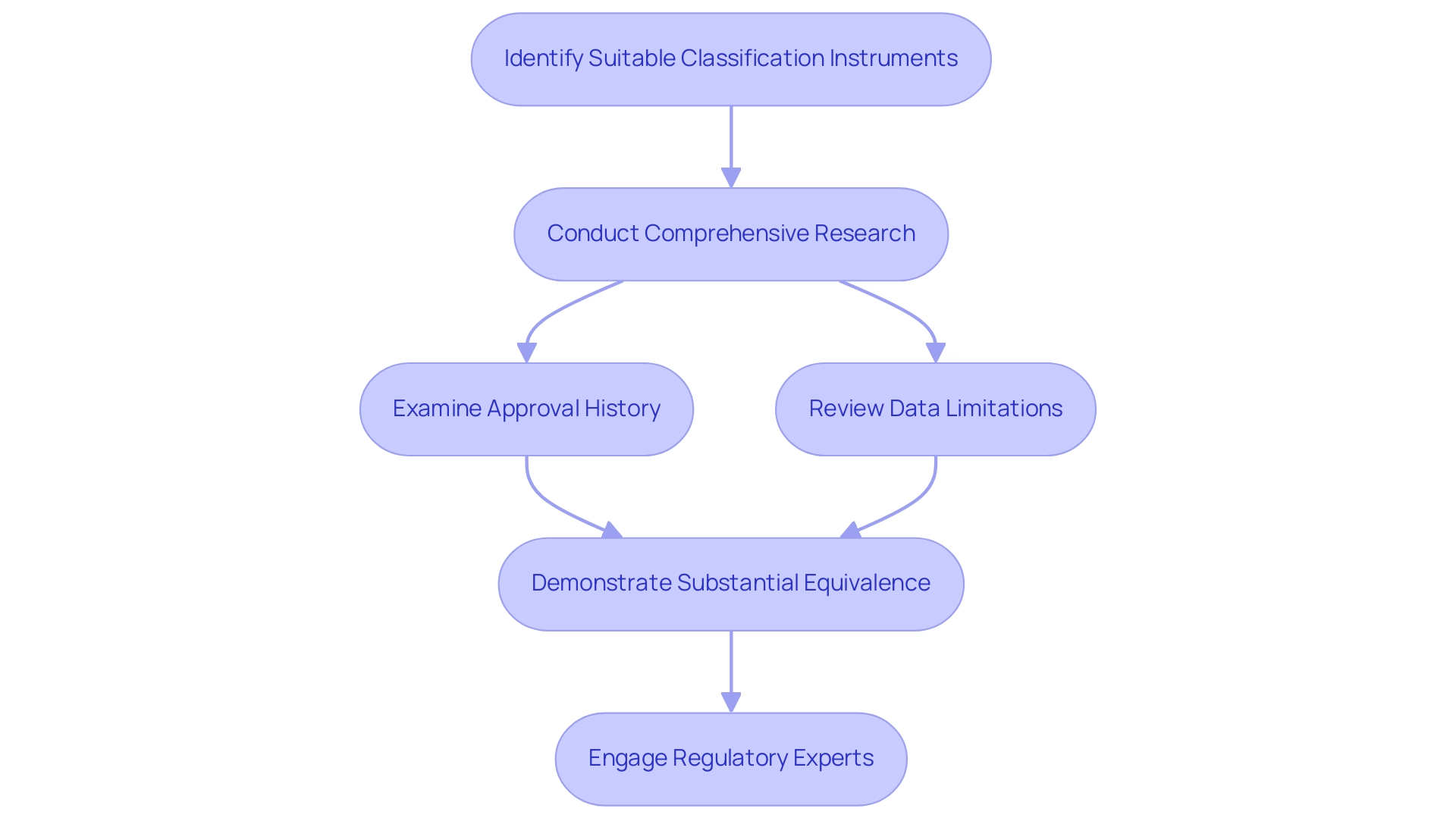
Choosing the suitable classification instrument is crucial for a successful 510(k) submission. Manufacturers must undertake comprehensive research to identify items that share similar intended uses and technological characteristics. A comprehensive examination of the approval history of the referenced item, including post-market monitoring information, is essential for assessing its performance and risk profile.
As highlighted by Dylan A Mordaunt, 'The suggestion from the reviewer is an excellent one; however, the TPLC database provided by the FDA does not have temporal resolution within the complaints to allow the establishment of causal relation between the product recalls and the complaints.' This underscores the importance of scrutinizing available data critically, as understanding the limitations of the data can significantly impact the selection process. Insights from the case study titled 'Mapping the Genealogy of Medical Equipment Predecessors in the United States' reveal the importance of demonstrating substantial equivalence in safety and effectiveness to prior models, further emphasizing the need for careful predecessor selection.
Furthermore, the recent FDA guidelines on choosing products in line with the predicate device definition offer manufacturers a framework for adherence to legal standards, increasing the significance of this discussion. Engaging with regulatory experts, such as:
- Ana Criado, Director of Regulatory Affairs and CEO of Mahu Pharma, who holds a degree in chemical pharmacology and has extensive experience at INVIMA
- Katherine Ruiz, an expert in Regulatory Affairs for Medical Devices and In Vitro Diagnostics in Colombia
can provide invaluable insights into the complexities of the submission process and ensure compliance with relevant regulations. By implementing these best practices, manufacturers can enhance their chances of a successful 510(k) submission and contribute to the overall safety and quality of medical devices.
Conclusion
The intricate landscape of medical device regulation highlights the vital importance of predicate devices in ensuring both safety and efficacy. Throughout this discussion, it has been established that predicate devices serve as essential benchmarks for new medical technologies, particularly within the 510(k) submission process. By demonstrating substantial equivalence to these previously approved devices, manufacturers can navigate the regulatory framework more effectively, thereby expediting patient access to innovative healthcare solutions.
Key insights from industry experts emphasize that a thorough understanding of predicate devices is not only crucial for compliance but also for minimizing the risks associated with device recalls. The statistics surrounding 510(k) device recalls underscore the urgency for manufacturers to carefully select predicates, ensuring that their devices align closely with established safety and performance standards. This vigilance is further supported by the evolving global regulatory landscape, where different countries impose varying requirements for predicate identification.
Ultimately, the article has underscored that best practices in selecting predicate devices—such as comprehensive research and engagement with regulatory experts—are essential for successful market entry. By adhering to these practices, manufacturers can enhance the safety and quality of their medical devices, fostering innovation while safeguarding patient health. As the medical technology industry continues to advance, the importance of predicate devices will remain a cornerstone of effective regulatory strategy and compliance.




