Introduction
In the realm of clinical research, the safety and well-being of participants take precedence, making the monitoring and reporting of Serious Adverse Events (SAEs) a critical component of trial management. Defined as significant medical incidents that can result in severe consequences such as death or life-threatening conditions, SAEs can arise from various sources, including investigational products and patient-specific factors.
The complexities surrounding these events necessitate a thorough understanding of regulatory guidelines and best practices to ensure compliance and protect trial participants. With recent studies revealing alarming trends in adverse events across diverse patient demographics, the urgency for effective SAE management has never been more pronounced.
This article delves into the intricacies of SAEs, exploring their definitions, monitoring protocols, regulatory frameworks, and operational best practices that are essential for safeguarding participant safety and enhancing the integrity of clinical trials.
Defining Serious Adverse Events (SAEs) in Clinical Research
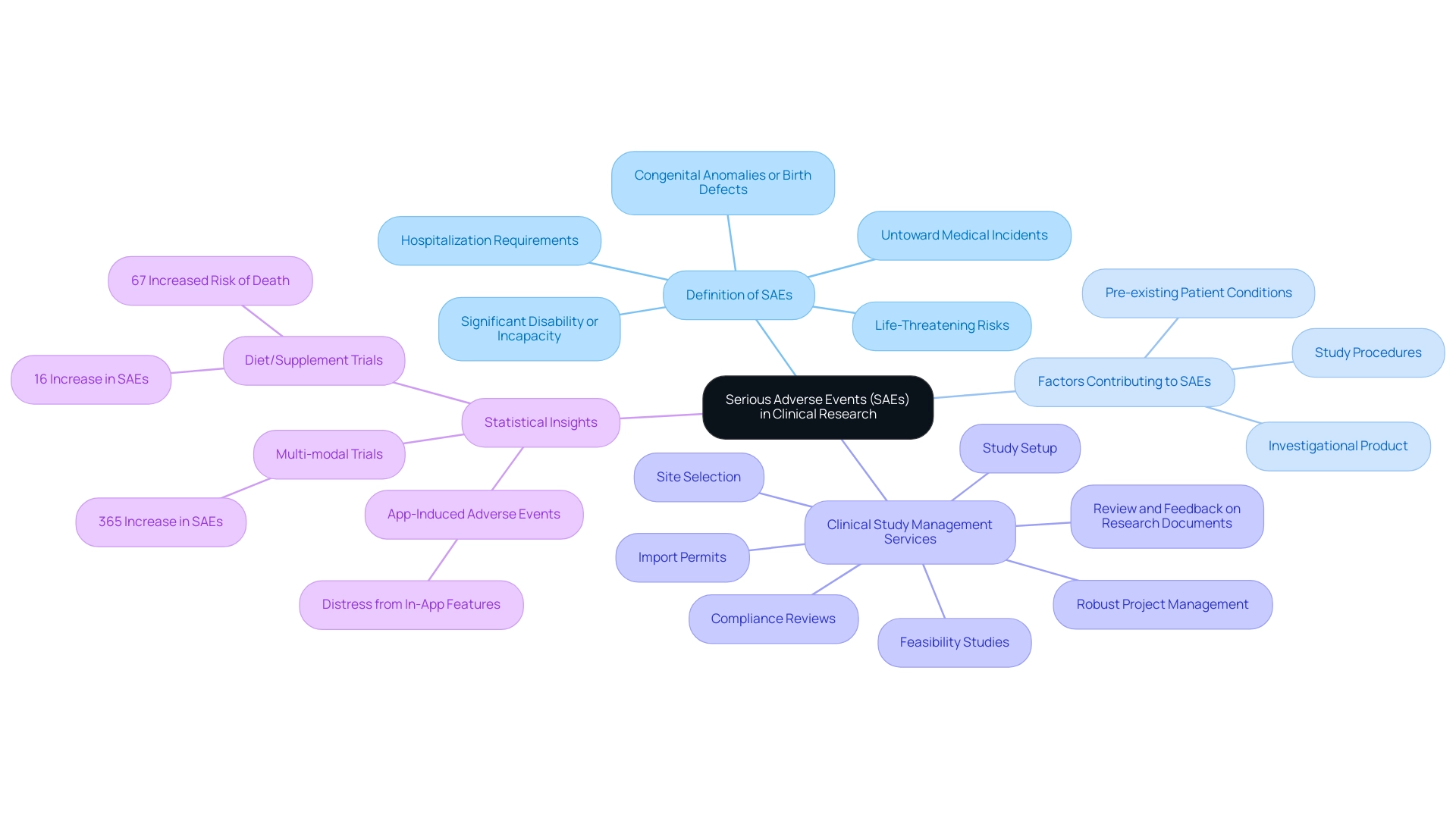
In medical research, what is SAE in clinical research refers to Serious Adverse Events, which are critical occurrences defined as any untoward medical incident that results in death, poses a life-threatening risk, necessitates hospitalization, prolongs existing hospitalization, leads to significant disability or incapacity, or results in a congenital anomaly or birth defect. These events may arise from various factors, including the investigational product, the procedures involved in the study, or pre-existing conditions of the patients. Grasping the dynamics of SAE is crucial not only for safeguarding patient safety but also for maintaining the integrity of research.
Our comprehensive clinical study management services, which encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Study setup
- Review and feedback on research documents
- Import permits
- Robust project management
are designed to ensure diligent monitoring and reporting of what is SAE in clinical research, addressing both serious and non-serious adverse events. This is particularly crucial, as highlighted by recent investigations such as those conducted by Kuhn et al. on a mobile application created to track PTSD symptoms, where the most frequent app-induced negative outcome was distress from specific in-app features, occurring in fewer than 5% of participants throughout studies.
Furthermore, a case study on Diet/Supplement and Multi-modal Assessments of Risk revealed that Diet/Supplement studies had a 16% increase in SAE and a 67% increased risk of death, while Multi-modal assessments exhibited a staggering 365% increase in SAE. As observed by expert Key MN, these discoveries should be carefully evaluated due to the low rate of documentation, but highlight the importance of disclosing research outcomes, improving transparency, and enabling more precise safety evaluations in cognitive aging and lifestyle interventions for older adults. Our dedication to strong SAE oversight not only reduces risks but also enhances the reliability of research findings, ultimately promoting job creation and economic growth in the local healthcare environment.
The Importance of SAE Monitoring and Reporting in Clinical Trials
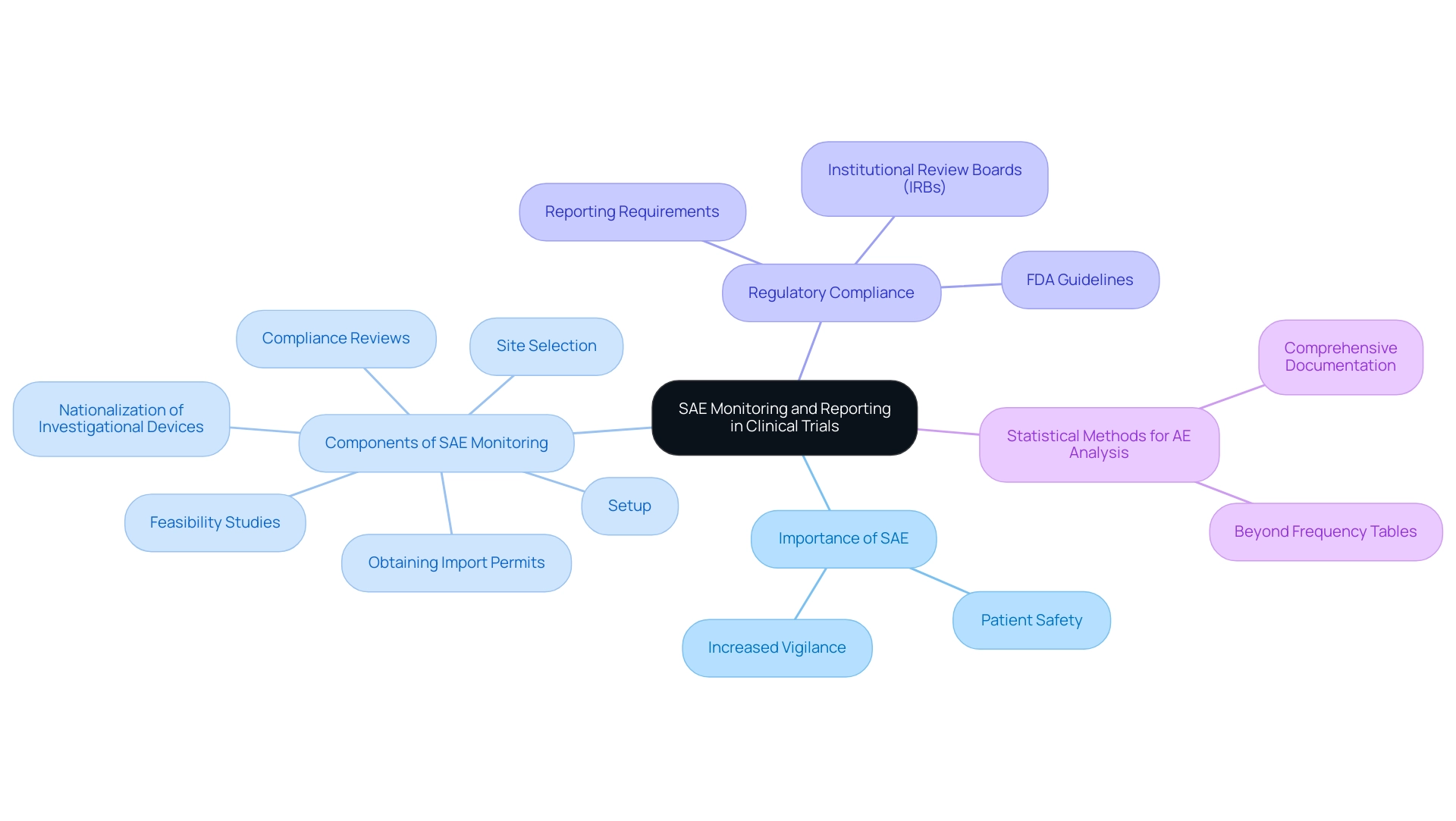
Understanding what is SAE in clinical research is essential for monitoring and reporting Serious Adverse Events (SAEs) in research studies for multiple reasons. Primarily, it safeguards patient safety by identifying and mitigating potential risks associated with investigational products. The recent statistics indicate that adverse event diversities among patients aged 30 to 69 have surged significantly, with an average increase of 77% in each age group.
This alarming trend highlights the urgent necessity for increased vigilance in oversight practices within research studies. Our comprehensive clinical study management services encompass:
- Feasibility studies
- Site selection
- Compliance reviews
- Setup
- Obtaining import permits
- Nationalization of investigational devices
All of which play a crucial role in ensuring that monitoring protocols are robust and effective. Furthermore, timely reporting of both serious and non-serious adverse events is mandated by regulatory authorities, including the FDA and Institutional Review Boards (IRBs), ensuring compliance with established guidelines.
This adherence not only fulfills regulatory obligations but also fosters trust in the research process. Furthermore, knowing what is SAE in clinical research is crucial for efficient oversight of serious adverse events, which can encourage essential adjustments to study protocols, thereby improving both safety and effectiveness. As emphasized in the detailed summary of AE analysis techniques, a mix of statistical methods beyond basic frequency tables can greatly enhance the evaluation of harm results in research.
Expert views highlight that ongoing observation of serious adverse events, which relates to what is SAE in clinical research, is a foundation of ethical research, strengthening the idea that comprehensive documentation is not simply a regulatory obligation but an essential aspect of responsible medical practice. As JL observed, the manuscript was evaluated and endorsed by research specialists, further highlighting the significance of thorough SAE monitoring.
Regulatory Guidelines for SAE Reporting: FDA and IRB Perspectives
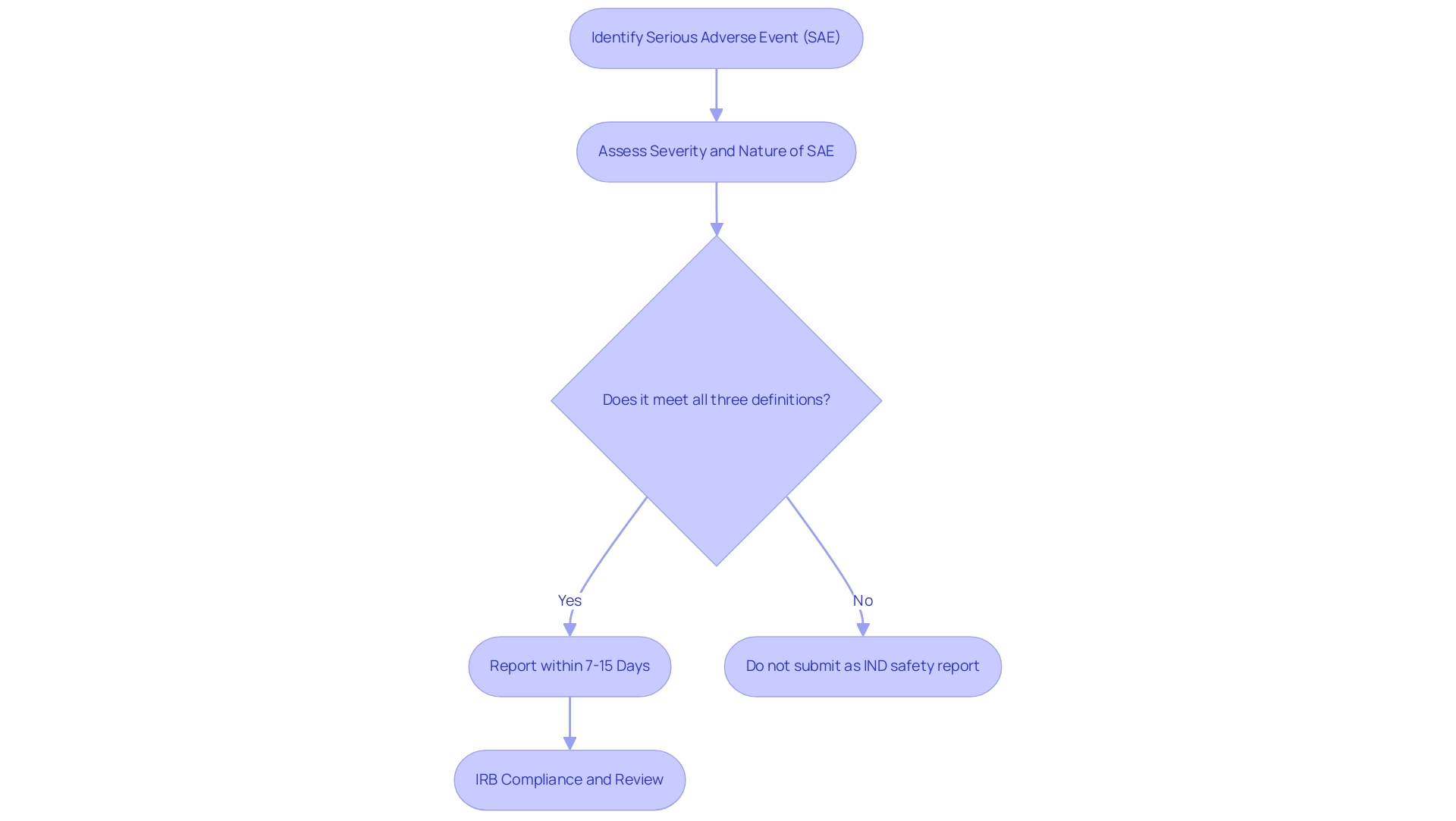
The regulatory framework surrounding serious adverse occurrences, or what is SAE in clinical research, is primarily guided by the FDA and Institutional Review Boards (IRBs), both of which impose stringent documentation requirements. The FDA stipulates that all SAEs, which are critical to understanding what is SAE in clinical research, must be reported within a designated timeframe, typically ranging from 7 to 15 days, contingent upon the severity and nature of the event. This swift documentation is critical, as the U.S. Department of Health and Human Services and FDA state,
If the adverse event does not meet all three of the definitions, it should not be submitted as an IND safety report.
The cumulative incidence of suspected unexpected serious adverse reactions is affected by elements like patient demographics and duration of follow-up, which highlights the significance of precise documentation. IRBs similarly emphasize the necessity for prompt reporting to assess what is SAE in clinical research, particularly regarding the potential ramifications on participant safety and the integrity of the study. Recent modifications in IRB requirements have further emphasized the need for adherence, which is essential for maintaining oversight of serious adverse events, particularly in understanding what is SAE in clinical research and enhancing compliance rates among research professionals.
Our extensive research management services encompass:
- Feasibility studies
- Site selection
- Import permits
- Nationalization of investigational devices
This ensures that research sites and principal investigators (PIs) are well-equipped to handle SAEs appropriately. Additionally, we provide thorough compliance reviews and trial setup support to navigate regulatory complexities effectively. For instance, a case study titled 'Impact of Prevalence on Agreement Measures' illustrates how the prevalence of a condition in a study population can affect the agreement measures between a new test and a non-reference standard.
By changing the prevalence from 23.2% to 7%, the overall percent agreement increased to 97.5%, while the positive percent agreement decreased to 76.8%. This variability highlights the need for researchers to fully understand these guidelines and their implications to navigate the complex regulatory landscape and ensure that they meet their ethical and legal obligations effectively. Katherine Ruiz, a specialist in regulatory matters for medical devices and in vitro diagnostics in Colombia, emphasizes the significance of careful project management and documentation to maintain the integrity of research.
Operationalizing SAE Management: Best Practices and Tools
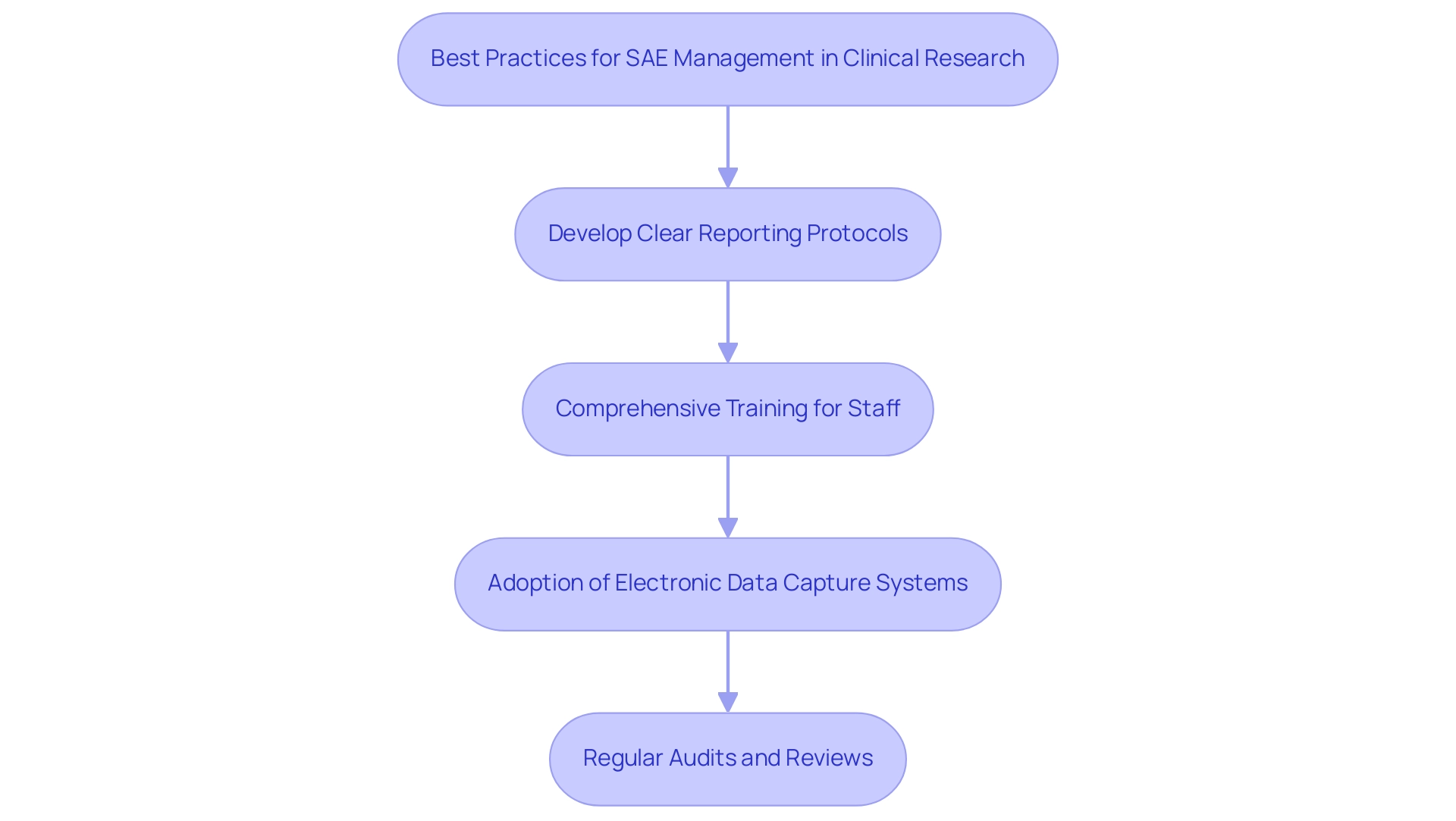
Understanding what is SAE in clinical research is essential for efficient oversight of significant adverse occurrences, which relies on the application of recognized best practices and sophisticated tracking tools. Key strategies involve:
- The development of clear reporting protocols.
- Comprehensive training for staff on what is SAE in clinical research and the identification and reporting of SAEs.
- The adoption of electronic data capture systems that streamline the reporting process.
Such systems not only enhance the accuracy of data collection but also facilitate compliance with regulatory requirements.
Regular audits and reviews are essential for understanding what is SAE in clinical research data, especially considering the statistic that only three studies adjusted for multiplicity of tests in adverse event (AE) data analysis, underscoring the need for meticulous monitoring. This is further supported by the case study titled 'Multiplicity Considerations in QTLs,' which discusses how appropriate adjustments can significantly enhance the accuracy of evaluations and ensure that critical parameters are monitored effectively. The importance of these practices is underscored by a recent discussion on multiplicity considerations, which highlights how appropriate adjustments can improve the accuracy of evaluations and reduce false discovery rates.
Moreover, as Pucker AD, the executive director of medical sciences at Lexitas Pharma Services, aptly states,
As clinicians, it is imperative that we regularly review new research as it becomes available so that we can bring the best and newest treatments to our patients.
By implementing these practices, research teams can significantly improve their response to serious adverse events, addressing what is SAE in clinical research, ultimately resulting in better safety outcomes in studies.
Common Types of Serious Adverse Events in Clinical Trials
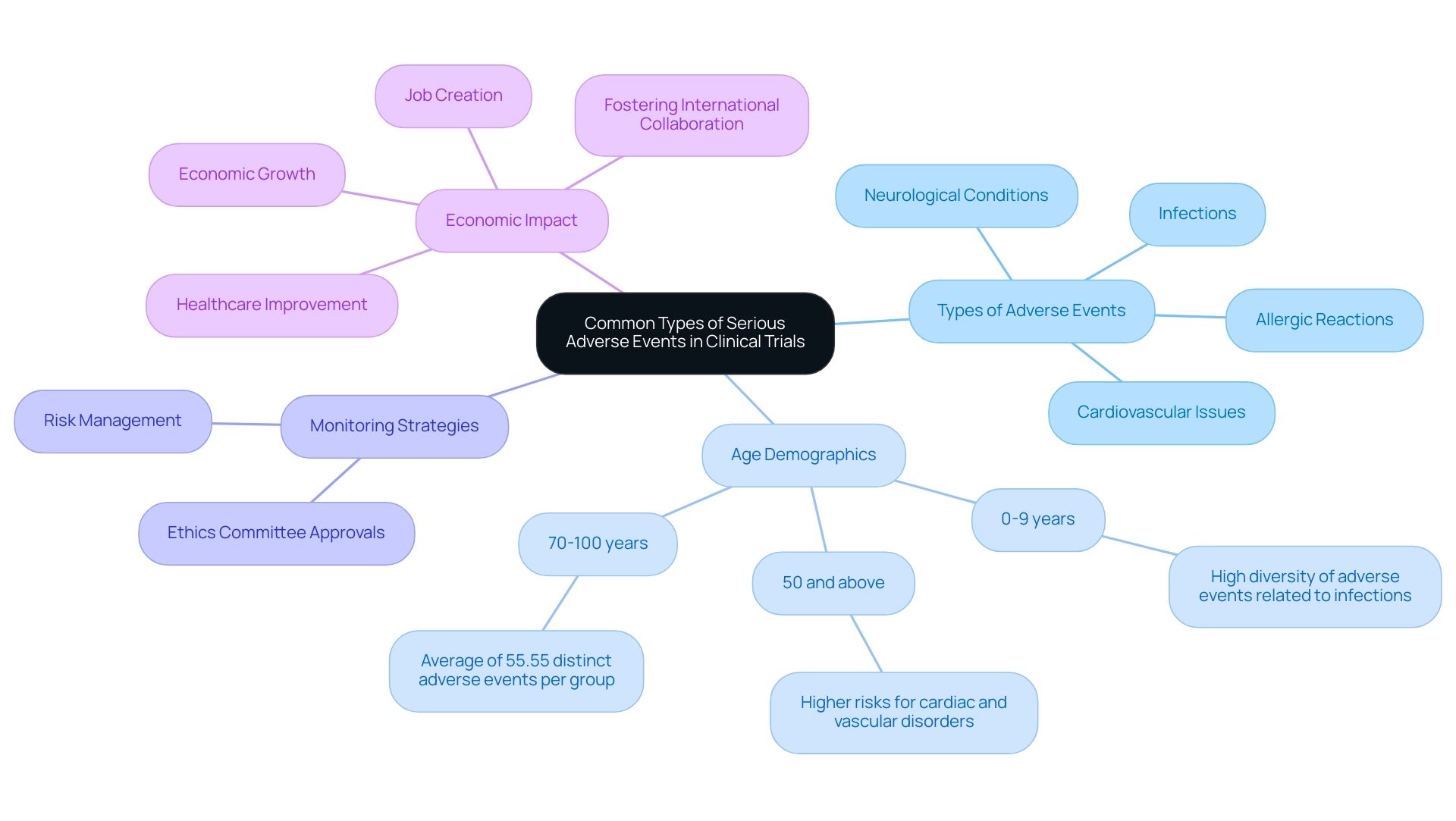
In clinical studies, significant adverse occurrences often present as intense allergic responses, cardiovascular issues, infections, and neurological conditions. Notably, the 0-9 years-old group exhibits a high diversity of adverse events related to infections, highlighting the varying risks across different age demographics. For example, what is SAE in clinical research can be demonstrated if a participant suffers a severe allergic reaction to an investigational drug, which would require immediate medical attention.
The analysis of adverse events categorized by organ system illustrates that older patients, particularly those aged 50 and above, face a notably increased diversity of Sales. Findings indicate that individuals in the 70-100 years age group experience an average of 55.55 distinct adverse events per group, underscoring the necessity for heightened vigilance in monitoring these participants. Our comprehensive clinical study management services encompass feasibility studies, meticulous site selection, and rigorous compliance reviews to ensure adherence to country requirements, all designed to mitigate these risks effectively.
The research setup process includes obtaining necessary approvals from ethics committees and health ministries, as well as managing import permits and the nationalization of investigational devices. As Eric D. Vidoni notes, understanding what is SAE in clinical research helps researchers to better anticipate risks and establish robust monitoring strategies, ultimately ensuring the safety of participants throughout the study process. This is particularly crucial given the reported probability of death in intervention groups at 2.98%, compared to 2.33% in control groups, reinforcing the imperative for comprehensive monitoring.
Furthermore, effective management of clinical trials contributes positively to local economies through job creation, economic growth, healthcare improvement, and fostering international collaboration.
Conclusion
The management of Serious Adverse Events (SAEs) is a cornerstone of clinical research, ensuring the safety and well-being of participants while upholding the integrity of studies. By comprehensively defining SAEs, monitoring their occurrence, and adhering to regulatory guidelines, researchers can effectively mitigate risks associated with investigational products. The alarming trends in adverse events across diverse patient demographics underscore the urgent need for vigilant SAE monitoring and reporting, reinforcing the necessity of robust clinical trial management practices.
Regulatory frameworks established by the FDA and Institutional Review Boards (IRBs) provide critical guidance, mandating timely reporting and compliance to enhance participant safety. Understanding these regulations is essential for clinical researchers to navigate the complexities of SAE management effectively. Furthermore, operationalizing best practices, such as developing clear reporting protocols and utilizing advanced reporting tools, plays a vital role in minimizing risks and improving safety outcomes.
In conclusion, the commitment to diligent monitoring and reporting of SAEs not only safeguards participants but also enhances the reliability of clinical findings. As the landscape of clinical research continues to evolve, the importance of effective SAE management cannot be overstated. By prioritizing participant safety and adhering to regulatory requirements, researchers contribute to the advancement of medical knowledge and the overall improvement of healthcare outcomes.
Frequently Asked Questions
What does SAE stand for in clinical research?
SAE stands for Serious Adverse Events, which are critical occurrences defined as any untoward medical incident that results in death, poses a life-threatening risk, necessitates hospitalization, prolongs existing hospitalization, leads to significant disability or incapacity, or results in a congenital anomaly or birth defect.
What factors can lead to Serious Adverse Events (SAEs)?
SAEs may arise from various factors, including the investigational product, the procedures involved in the study, or pre-existing conditions of the patients.
Why is understanding SAE important in clinical research?
Understanding SAE is crucial for safeguarding patient safety and maintaining the integrity of research. It helps in identifying and mitigating potential risks associated with investigational products.
What services are included in comprehensive clinical study management related to SAE?
Comprehensive clinical study management services include feasibility studies, site selection, compliance reviews, study setup, review and feedback on research documents, import permits, and robust project management.
What recent findings highlight the importance of monitoring SAEs?
Recent investigations revealed that certain studies, such as those on Diet/Supplement and Multi-modal Assessments of Risk, showed significant increases in SAEs and risks of death, underscoring the need for careful evaluation and transparency in research outcomes.
How does the trend of adverse events among patients aged 30 to 69 impact clinical research?
There has been an alarming 77% average increase in adverse events among patients aged 30 to 69, highlighting the urgent necessity for increased vigilance in oversight practices within research studies.
What regulatory authorities mandate the reporting of SAEs?
Regulatory authorities, including the FDA and Institutional Review Boards (IRBs), mandate the timely reporting of both serious and non-serious adverse events to ensure compliance with established guidelines.
How does ongoing observation of SAEs contribute to ethical research?
Ongoing observation of SAEs is foundational to ethical research, reinforcing that comprehensive documentation is not merely a regulatory obligation but an essential aspect of responsible medical practice.




